Thermal Characterisation and Isoconversional Kinetic Analysis of Osmotically Dried Pork Meat Proteins Longissimus dorsi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Osmotic Dehydration of Pork Meat in Sugar Beet Molasses
2.3. Determination of Proximate Composition and Water Activity (aw)
2.4. Thermal Analysis
Kinetics of Meat Protein Thermal Denaturation
2.5. Statistics
3. Results
3.1. Proximate Composition of Pork Meat (Longissimus dorsi) and Sugar Beet Molasses
3.2. Thermal Analysis of Pork Meat Proteins Longissimus dorsi
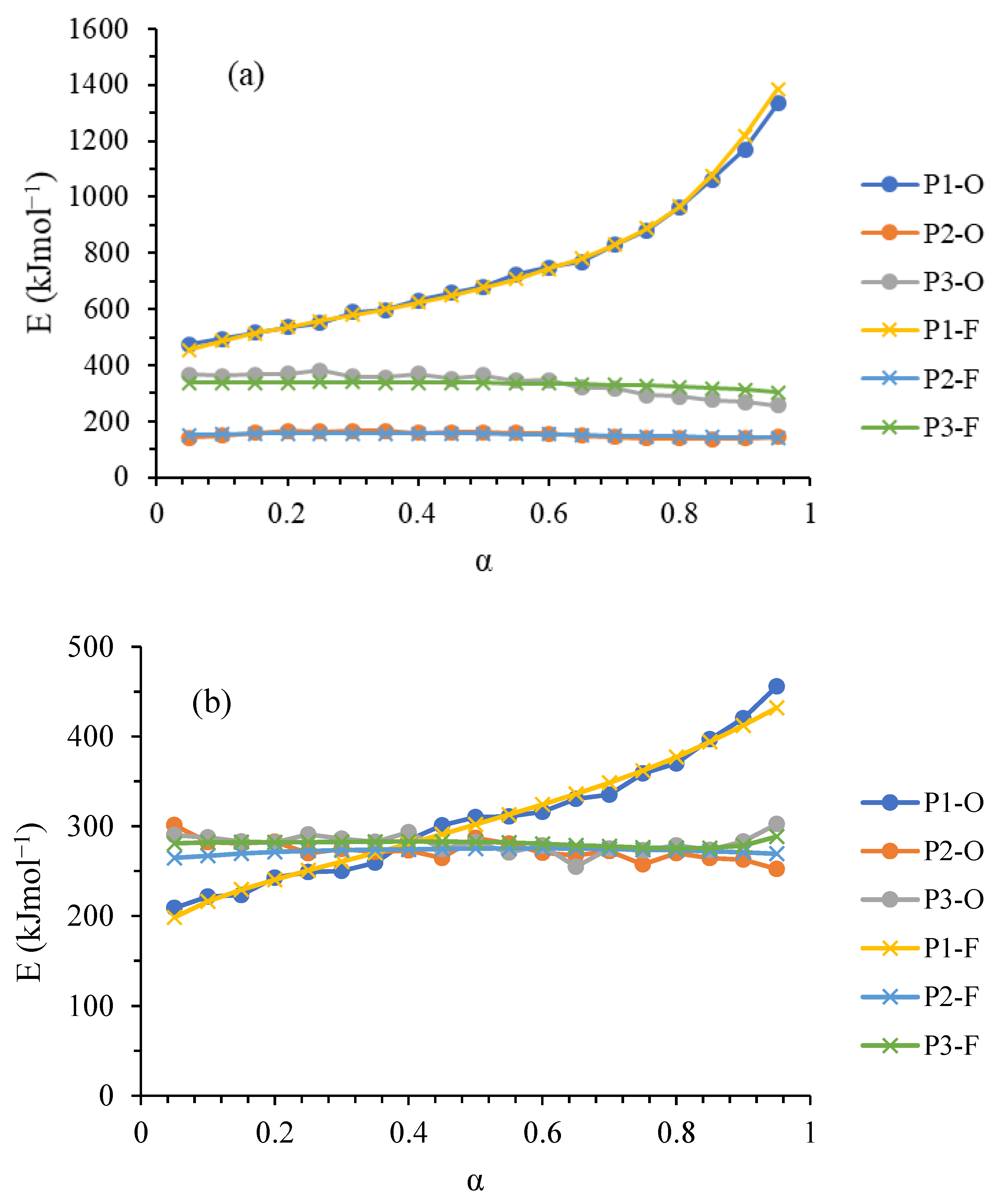
Kinetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berk, Z. Dehydration. In Food Process Engineering and Technology; Academic Press: London, UK, 2018; pp. 513–566. [Google Scholar] [CrossRef]
- Leroy, F.; Smith, N.W.; Adesogan, A.T.; Beal, T.; Iannotti, L.; Moughan, P.J.; Mann, N. The Role of Meat in the Human Diet: Evolutionary Aspects and Nutritional Value. Anim. Front. 2023, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, R. A Review on Nutritional Advantages of Edible Mushrooms and Its Industrialization Development Situation in Protein Meat Analogues. J. Future Foods 2023, 3, 1–7. [Google Scholar] [CrossRef]
- Mediani, A.; Hamezah, H.S.; Jam, F.A.; Mahadi, N.F.; Chan, S.X.Y.; Rohani, E.R.; Che Lah, N.H.; Azlan, U.K.; Khairul Annuar, N.A.; Azman, N.A.F.; et al. A Comprehensive Review of Drying Meat Products and the Associated Effects and Changes. Front. Nutr. 2022, 9, 1057366. [Google Scholar] [CrossRef]
- Suput, D.; Lazic, V.L.; Pezo, L.; Levic, L.B.; Gubic, J.; Hromis, N.M.; Šojić, B. Modified Atmosphere Packaging and Osmotic Dehydration Effect on Pork Quality and Stability. Rom. Biotechnol. Lett. 2013, 18, 8160–8169. [Google Scholar]
- Lončar, B.; Nićetin, M.; Filipović, V.; Knežević, V.; Pezo, L.; Šuput, D.; Kuljanin, T. Osmotic Dehydration in Sugar Beet Molasses-Food Safety and Quality Benefits. J. Hyg. Eng. Des. 2021, 34, 15–20. [Google Scholar]
- Šarić, L.Ć.; Filipčev, B.V.; Šimurina, O.D.; Plavšić, D.V.; Šarić, B.M.; Lazarević, J.M.; Milovanović, I.L. Sugar Beet Molasses: Properties and Applications in Osmotic Dehydration of Fruits and Vegetables. Food Feed Res. 2016, 43, 135–144. [Google Scholar] [CrossRef]
- Sjölin, M.; Thuvander, J.; Wallberg, O.; Lipnizki, F. Purification of Sucrose in Sugar Beet Molasses by Utilizing Ceramic Nanofiltration and Ultrafiltration Membranes. Membranes 2019, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Wani, A.K.; Rahayu, F.; Fauziah, L.; Suhara, C. Advances in Safe Processing of Sugarcane and Bagasse for the Generation of Biofuels and Bioactive Compounds. J. Agric. Food Res. 2023, 12, 100549. [Google Scholar] [CrossRef]
- Mišljenović, N.M.; Koprivica, G.B.; Pezo, L.L.; Lević, L.B.; Ćurčić, B.L.; Filipović, V.S.; Nićetin, M.R. Optimization of the Osmotic Dehydration of Carrot Cubes in Sugar Beet Molasses. Therm. Sci. 2012, 16, 43–52. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Brekke, C.J.; Leung, H.K. Thermal Denaturation of Muscle Proteins From Different Species and Muscle Types as Studied by Differential Scanning Calorimetry. Can. Inst. Food Sci. Technol. J. 1987, 20, 357–362. [Google Scholar] [CrossRef]
- Naveena, B.M.; Sen, A.R.; Vaithiyanathan, S.; Babji, Y.; Kondaiah, N. Comparative Efficacy of Pomegranate Juice, Pomegranate Rind Powder Extract and BHT as Antioxidants in Cooked Chicken Patties. Meat Sci. 2008, 80, 1304–1308. [Google Scholar] [CrossRef]
- Sanchez-Ruiz, J.M. Protein Kinetic Stability. Biophys. Chem. 2010, 148, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ishiwatari, N.; Fukuoka, M.; Sakai, N. Effect of Protein Denaturation Degree on Texture and Water State of Cooked Meat. J. Food Eng. 2013, 117, 361–369. [Google Scholar] [CrossRef]
- Zhang, Y.; Puolanne, E.; Ertbjerg, P. Mimicking Myofibrillar Protein Denaturation in Frozen-Thawed Meat: Effect of PH at High Ionic Strength. Food Chem. 2021, 338, 128017. [Google Scholar] [CrossRef]
- Yang, N.; Liang, X.; Cao, J.; Zhang, Q.; Tan, Y.; Xu, B.; Yang, Y.; Wang, Y.; Yang, Q.; Liu, H.; et al. Denaturation Manner of Sarcoplasmic Proteins in Pale, Soft and Exudative Meat Determines Their Positive Impacts on Myofibrillar Water-Holding Capacity. Meat Sci. 2022, 185, 108723. [Google Scholar] [CrossRef] [PubMed]
- Bampi, M.; Sereno, A.M.; Schmidt, F.C.; Laurindo, J.B. Evaluation of Different Software Tools for Deconvolving Differential Scanning Calorimetry Thermograms of Salted Beef. Food Sci. Technol. 2016, 36, 694–700. [Google Scholar] [CrossRef] [Green Version]
- Istrate, D.; Popescu, C.; Möller, M. Non-Isothermal Kinetics of Hard Alpha-Keratin Thermal Denaturation. Macromol. Biosci. 2009, 9, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Vyazovkin, S.; Vincent, L.; Sbirrazzuoli, N. Thermal Denaturation of Collagen Analyzed by Isoconversional Method. Macromol. Biosci. 2007, 7, 1181–1186. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of Thermal Degradation of Char-Forming Plastics from Thermogravimetry. Application to a Phenolic Plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Ortega, A. A Simple and Precise Linear Integral Method for Isoconversional Data. Thermochim. Acta 2008, 474, 81–86. [Google Scholar] [CrossRef]
- ISO 1442: 1998; Meat and Meat Products—Determination of Moisture Content (Reference Method). Institute for Standardization of Serbia: Belgrade, Serbia, 1998. Available online: https://iss.rs/en/project/show/iss:proj:15442 (accessed on 21 June 2023).
- ISO 1871: 1992; Agricultural Food Products—General Directions for the Determination of Nitrogen by the Kjeldahl Method. Institute for Standardization of Serbia: Belgrade, Serbia, 1992. Available online: https://iss.rs/en/project/show/iss:proj:12764 (accessed on 21 June 2023).
- ISO 936: 1999; Meat and Meat Products—Determination of Total Ash. Institute for Standardization of Serbia: Belgrade, Serbia, 1999. Available online: https://iss.rs/en/project/show/iss:proj:15818 (accessed on 21 June 2023).
- ISO 1443: 1992; Meat and meat products—Determination of Total Fat Content. Institute for Standardization of Serbia: Belgrade, Serbia, 1992. Available online: https://iss.rs/en/project/show/iss:proj:13848 (accessed on 21 June 2023).
- Della Gatta, G.; Richardson, M.J.; Sarge, S.M.; Stølen, S. Standards, Calibration, and Guidelines in Microcalorimetry. Part 2. Calibration for Differential Scanning Calorimetry (IUPAC Technical Report). Pure Appl. Chem. 2006, 78, 1455–1476. [Google Scholar] [CrossRef] [Green Version]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee Recommendations for Collecting Experimental Thermal Analysis Data for Kinetic Computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes; Springer International Publishing: Cham, Switzerland, 2015; ISBN 9783319141749. [Google Scholar]
- Kawakami, H.; Morita, J.I.; Takahashi, K.; Yasui, T. Thermal Denaturation of Myosin, Heavy Meromyosin and Subfragment 1. J. Biochem. 1971, 70, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Konno, K. Myosin Denaturation Study for the Quality Evaluation of Fish Muscle-Based Products. Food Sci. Technol. Res. 2017, 23, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Markov, D.I.; Zubov, E.O.; Nikolaeva, O.P.; Kurganov, B.I.; Levitsky, D.I. Thermal Denaturation and Aggregation of Myosin Subfragment 1 Isoforms with Different Essential Light Chains. Int. J. Mol. Sci. 2010, 11, 4194–4226. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Puolanne, E.; Ertbjerg, P. Temperature Induced Denaturation of Myosin: Evidence of Structural Alterations of Myosin Subfragment-1. Meat Sci. 2014, 98, 124–128. [Google Scholar] [CrossRef]
- Stabursvik, E.; Martens, H. Thermal Denaturation of Proteins in Post Rigor Muscle Tissue as Studied by Differential Scanning Calorimetry. J. Sci. Food Agric. 1980, 31, 1034–1042. [Google Scholar] [CrossRef]
- Bozec, L.; Odlyha, M. Thermal Denaturation Studies of Collagen by Microthermal Analysis and Atomic Force Microscopy. Biophys. J. 2011, 101, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Tornberg, E. Effects of Heat on Meat Proteins—Implications on Structure and Quality of Meat Products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef]
- Kajitani, S.; Fukuoka, M.; Sakai, N. Kinetics of Thermal Denaturation of Protein in Cured Pork Meat. Jpn. J. Food Eng. 2011, 12, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Bischof, J.C.; He, X. Thermal Stability of Proteins. Ann. N. Y. Acad. Sci. 2005, 1066, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.W. Scanning Calorimetry of Complex Biological Structures. Trends Biochem. Sci. 1984, 9, 340–344. [Google Scholar] [CrossRef]
- Atuonwu, J.C.; Ray, J.; Stapley, A.G.F. A Kinetic Model for Whey Protein Denaturation at Different Moisture Contents and Temperatures. Int. Dairy J. 2017, 75, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Sablani, S.S.; Rahman, M.S.; Al-Busaidi, S.; Guizani, N.; Al-Habsi, N.; Al-Belushi, R.; Soussi, B. Thermal Transitions of King Fish Whole Muscle, Fat and Fat-Free Muscle by Differential Scanning Calorimetry. Thermochim. Acta 2007, 462, 56–63. [Google Scholar] [CrossRef]
- Shi, Q.L.; Zhao, Y.; Chen, H.H.; Li, Z.J.; Xue, C.H. Glass Transition and State Diagram for Freeze-Dried Horse Mackerel Muscle. Thermochim. Acta 2009, 493, 55–60. [Google Scholar] [CrossRef]
- Phan-Xuan, T.; Bogdanova, E.; Sommertune, J.; Fureby, A.M.; Fransson, J.; Terry, A.E.; Kocherbitov, V. The Role of Water in the Reversibility of Thermal Denaturation of Lysozyme in Solid and Liquid States. Biochem. Biophys. Rep. 2021, 28, 101184. [Google Scholar] [CrossRef] [PubMed]
- Vaskoska, R.; Ha, M.; Ong, L.; Chen, G.; White, J.; Gras, S.; Warner, R. Myosin Sensitivity to Thermal Denaturation Explains Differences in Water Loss and Shrinkage during Cooking in Muscles of Distinct Fibre Types. Meat Sci. 2021, 179, 108521. [Google Scholar] [CrossRef]
- Lee, J.C.; Timasheff, S.N. The Stabilization of Proteins by Sucrose. J. Biol. Chem. 1981, 256, 7193–7201. [Google Scholar] [CrossRef]
- Semenova, M.G.; Antipova, A.S.; Belyakova, L.E. Food Protein Interactions in Sugar Solutions. Curr. Opin. Colloid Interface Sci. 2002, 7, 438–444. [Google Scholar] [CrossRef]
- Ferreira, L.A.; Fedotoff, O.; Uversky, V.N.; Zaslavsky, B.Y. Effects of Osmolytes on Protein–Solvent Interactions in Crowded Environments: Study of Sucrose and Trehalose Effects on Different Proteins by Solvent Interaction Analysis. RSC Adv. 2015, 5, 27154–27162. [Google Scholar] [CrossRef]
- Cao, Y.; Park, S.J.; Im, W. A Systematic Analysis of Protein-Carbohydrate Interactions in the Protein Data Bank. Glycobiology 2021, 31, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, M.; Karbstein, H.P.; Emin, M.A. Denaturation Behavior and Kinetics of Single- and Multi-Component Protein Systems at Extrusion-Like Conditions. Polymers 2020, 12, 2145. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yu, Z.; Wang, B.; Chiou, B.-S. Changes in Structures and Properties of Collagen Fibers during Collagen Casing Film Manufacturing. Foods 2023, 12, 1847. [Google Scholar] [CrossRef] [PubMed]

| Heat Rate (°Cmin−1) | P1 | P2 | P3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tdo1 (°C) | Tdp1 (°C) | ΔHd1 (Jg−1) | Tdo2 (°C) | Tdp2 (°C) | ΔHd2 (Jg−1) | Tdo3 (°C) | Tdp3 (°C) | ΔHd3 (Jg−1) | |
| FM | |||||||||
| 0.5 | 48.0 ± 0.0 A | 51.2 ± 0.1 A | 0.38 ± 0.01 A | 54.3 ± 0.1 B | 57.8 ± 0.3 B | 0.22 ± 0.03 A | 69.4 ± 0.2 B | 72.3 ± 0.1 B | 0.21 ± 0.02 A |
| 1.5 | 50.1 ± 0.4 B | 54.2 ± 0.1 B | 0.69 ± 0.20 A | 62.7 ± 0.9 B | 65.5 ± 0.2 B | 0.09 ± 0.06 A | 72.1 ± 0.6 B | 75.2 ± 0.6 B | 0.19 ± 0.04 A |
| 2 | 51.1 ± 0.1 B | 56.0 ± 0.3 B | 0.69 ± 0.14 A | 63.4 ± 0.4 B | 66.1 ± 0.7 B | 0.09 ± 0.08 A | 73.1 ± 0.2 B | 75.6 ± 0.0 B | 0.15 ± 0.02 A |
| 3 | 51.2 ± 1.0 B | 55.6 ± 1.3 B | 0.26 ± 0.12 A | 63.4 ± 0.1 B | 66.5 ± 0.8 B | 0.07 ± 0.04 A | 74.1 ± 0.4 B | 76.6 ± 0.2 B | 0.13 ± 0.06 A |
| 5 | 52.3 ± 0.1 B | 56.0 ± 0.3 B | 0.09 ± 0.03 A | 63.0 ± 0.1 B | 66.7 ± 0.8 B | 0.09 ± 0.05 A | 75.5 ± 0.6 B | 78.1 ± 0.2 B | 0.13 ± 0.06 A |
| 10 | 56.3 ± 0.2 B | 59.0 ± 0.7 B | 0.13 ± 0.09 A | 64.4 ± 2.1 B | 70.2 ± 0.3 B | 0.09 ± 0.04 A | 77.5 ± 0.8 B | 79.7 ± 0.6 B | 0.12 ± 0.02 A |
| DM | |||||||||
| 0.5 | 43.3 ± 0.5 B | 49.2 ± 0.2 B | 0.20 ± 0.02 A | 66.0 ± 0.0 A | 66.8 ± 0.1 A | 0.02 ± 0.01 A | 74.3 ± 0.3 A | 78.0 ± 0.3 A | 0.35 ± 0.02 A |
| 1.5 | 58.8 ± 0.1 A | 60.7 ± 0.3 A | 0.01 ± 0.00 A | 68.6 ± 0.2 A | 70.7 ± 0.2J A | 0.03 ± 0.00 A | 77.8 ± 0.3 A | 80.9 ± 0.3 A | 0.35 ± 0.01 A |
| 2 | 54.0 ± 1.5 A | 60.0 ± 0.3 A | 0.28 ± 0.14 A | 68.6 ± 0.2 A | 70.9 ± 0.0J A | 0.06 ± 0.04 A | 76.2 ± 1.1 A | 81.3 ± 0.9 A | 0.46 ± 1.16 A |
| 3 | 55.0 ± 1.5 A | 60.9 ± 0.5 A | 0.27 ± 0.21 A | 68.8 ± 0.8 A | 71.5 ± 1.6 A | 0.05 ± 0.04 A | 78.4 ± 1.1 A | 82.0 ± 0.4 A | 0.40 ± 0.11 A |
| 5 | 59.8 ± 0.7 A | 64.1 ± 0.2 A | 0.03 ± 0.01 A | 70.6 ± 0.7 A | 74.0 ± 0.5 A | 0.06 ± 0.04 A | 79.2 ± 1.6 A | 82.8 ± 0.5 A | 0.39 ± 0.16 A |
| 10 | 60.4 ± 2.5 A | 62.3 ± 1.1 A | 0.11 ± 0.18 A | 72.8 ± 0.6 A | 75.9 ± 0.5 A | 0.18 ± 0.11 A | 82.1 ± 0.5 A | 85.9 ± 0.5 A | 0.74 ± 0.15 A |
| E (kJ·mol−1) | Ln (A/min−1) | k0.5 (min−1) | |||
|---|---|---|---|---|---|
| FM | Friedman | P1 | 751.1 ± 256.4 A | 273.6 ± 93.2 A | 3.86 |
| P2 | 152.1 ± 5.2 C | 53.6 ± 1.8 C | 1.26 | ||
| P3 | 331.2 ± 10.4 B | 113.8 ± 3.6 B | 2.85 | ||
| Ortega | P1 | 747.6 ± 240.7 A | 272.4 ± 87.5 A | 3.87 | |
| P2 | 152.5 ± 10.9 C | 53.8 ± 3.8 C | 1.28 | ||
| P3 | 334.8 ± 39.5 B | 115.0 ± 13.5 B | 2.92 | ||
| DM | Friedman | P1 | 307.2 ± 68.0 B | 110.2 ± 24.4 B | 1.54 |
| P2 | 272.4 ± 3.0 B | 94.6 ± 1.0 BC | 2.01 | ||
| P3 | 280.8 ± 3.2 B | 94.8 ± 1.1 BC | 1.91 | ||
| Ortega | P1 | 307.6 ± 70.2 B | 110.4 ± 25.2 B | 1.57 | |
| P2 | 272.9 ± 11.2 B | 94.7 ± 3.9 BC | 2.04 | ||
| P3 | 281.1 ± 10.2 B | 94.9 ± 3.4 BC | 1.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostojić, S.; Micić, D.; Zlatanović, S.; Lončar, B.; Filipović, V.; Pezo, L. Thermal Characterisation and Isoconversional Kinetic Analysis of Osmotically Dried Pork Meat Proteins Longissimus dorsi. Foods 2023, 12, 2867. https://doi.org/10.3390/foods12152867
Ostojić S, Micić D, Zlatanović S, Lončar B, Filipović V, Pezo L. Thermal Characterisation and Isoconversional Kinetic Analysis of Osmotically Dried Pork Meat Proteins Longissimus dorsi. Foods. 2023; 12(15):2867. https://doi.org/10.3390/foods12152867
Chicago/Turabian StyleOstojić, Sanja, Darko Micić, Snežana Zlatanović, Biljana Lončar, Vladimir Filipović, and Lato Pezo. 2023. "Thermal Characterisation and Isoconversional Kinetic Analysis of Osmotically Dried Pork Meat Proteins Longissimus dorsi" Foods 12, no. 15: 2867. https://doi.org/10.3390/foods12152867







