Effects of Debranching Conditions and Annealing Treatment on the Formation of Starch Nanoparticles and Their Physicochemical Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rice Starch Preparation
2.3. Optimization of Enzymatic Debranching for Starch Nanoparticle Production
2.4. Combined Modification with Annealing Treatments
2.5. Percent Recovery of Crystalline SNPs
2.6. Measurements of Amylose, Total Starch, and Crude Protein Contents
2.7. Gel Permeation Chromatography
2.8. Measurement of Color Attributes
2.9. Measurement of Pasting Properties
2.10. Scanning Electron Microscopy
2.11. In Vitro Digestibility
2.12. Statistical Analysis
3. Results and Discussions
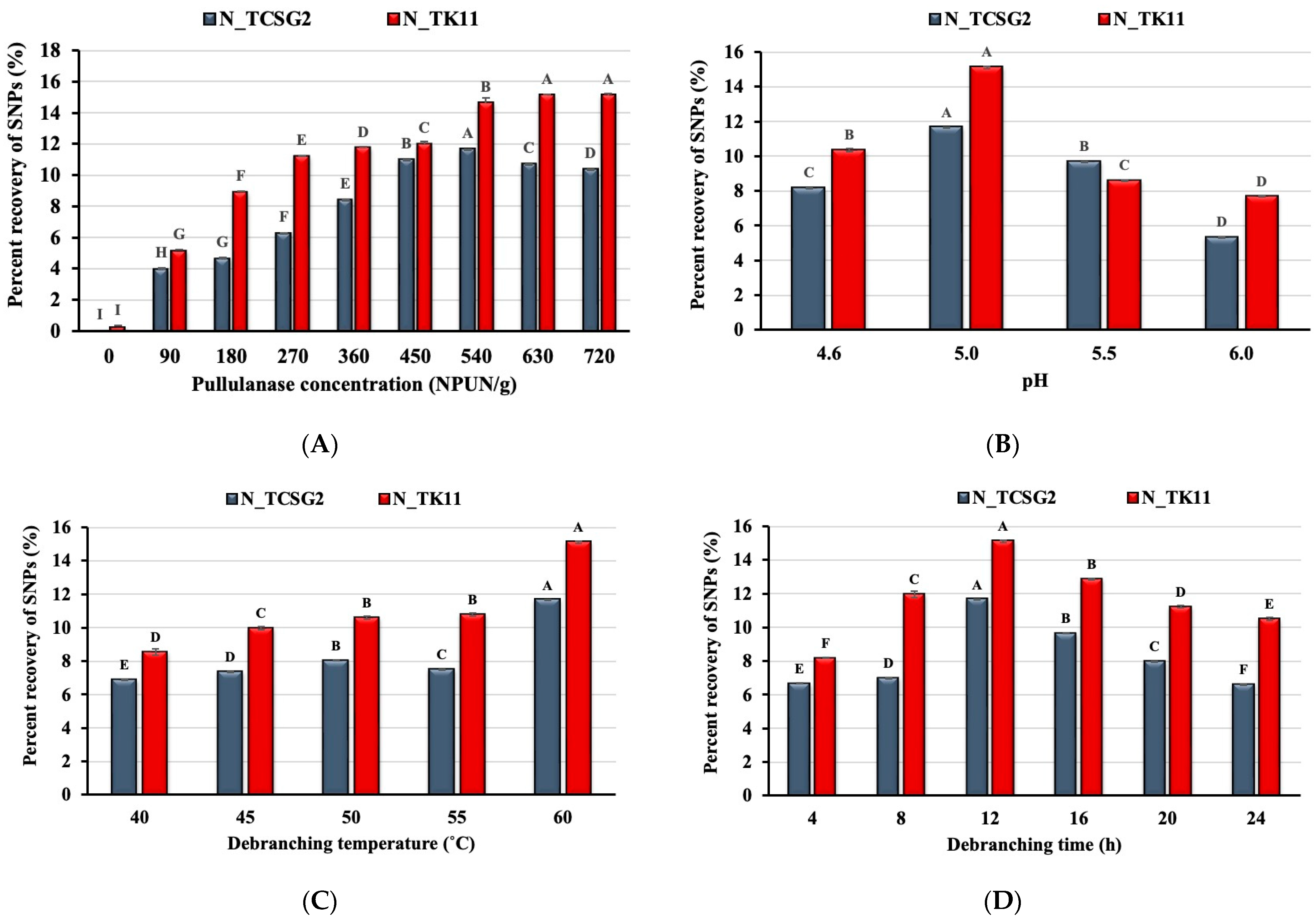
3.1. Effect of Enzyme Concentration and Debranching Conditions on Starch Nanoparticle Formation
3.2. Effect of Debranching Combined with Annealing Treatment on Starch Nanoparticles Formation
3.3. Total Starch and Crude Protein Contents
3.4. Weight-Average Molecular Weight, Weight-Average Degree of Polymerization, and Polydispersity Index
3.5. Color Parameters
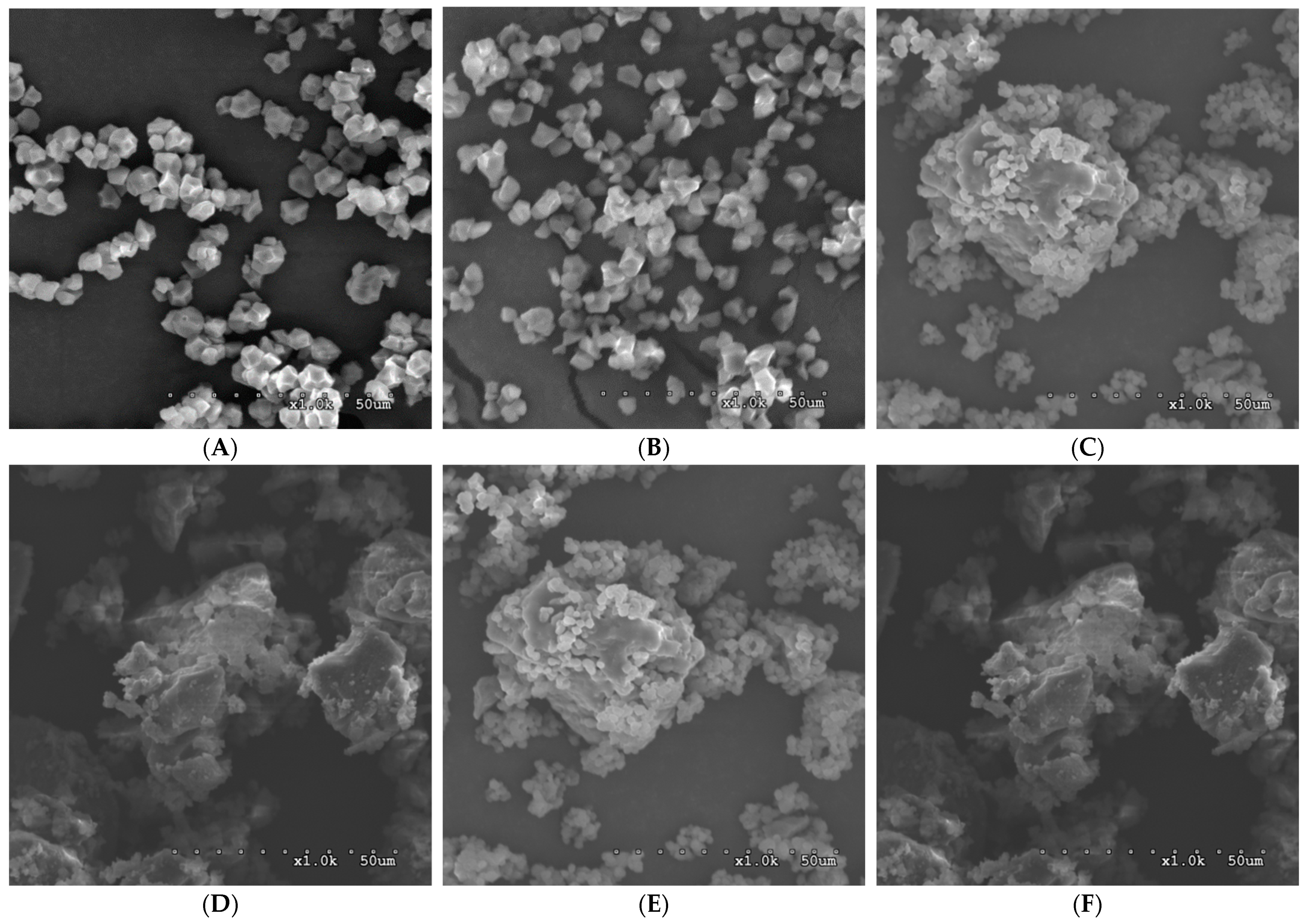
3.6. Scanning Electron Microscopy
3.7. Pasting Properties
3.8. In Vitro Digestion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miao, M.; Hamaker, B.R. Food matrix effects for modulating starch bioavailability. Annu. Rev. Food Sci. Technol. 2021, 12, 169–191. [Google Scholar] [CrossRef]
- Yulianti, R.; Liao, H.-J. V-amylose nanocarriers complexed with debranched sweet potato starch: Structural characteristics and digestibility. Food Biophys. 2023, in press. Available online: https://link.springer.com/article/10.1007/s11483-023-09782-x (accessed on 2 March 2023).
- Huang, H.-H.; Liao, H.-J. Digestion kinetics and molecular structural evolution during in vitro digestion of green banana (cv. Giant Cavendish) starch nanoparticles. Food Res. Int. 2023, 170, 113016. [Google Scholar] [CrossRef]
- Wang, C.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. Resistant starch and its nanoparticles: Recent advances in their green synthesis and application as functional food ingredients and bioactive delivery systems. Trends Food Sci. 2022, 119, 90–100. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.; Chen, Z.; Zhong, Q. Structural basis for the low digestibility of starches recrystallized from side chains of amylopectin modified by amylosucrase to different chain lengths. Carbohydr. Polym. 2020, 241, 116352. [Google Scholar] [CrossRef]
- Campelo, P.H.; Sant’Ana, A.S.; Clerici, M.T.P.S. Starch nanoparticles: Production methods, structure, and properties for food applications. Curr. Opin. Food Sci. 2020, 33, 136–140. [Google Scholar] [CrossRef]
- Kumari, S.; Yadav, B.S.; Yadav, R.B. Synthesis and modification approaches for starch nanoparticles for their emerging food industrial applications: A review. Food Res. Int. 2020, 128, 108765. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.; Sinhmar, A.; Nehra, M.; Thory, R.; Pathera, A.K.; Sundarraj, A.A.; Nain, V. Impact on various properties of native starch after synthesis of starch nanoparticles: A review. Food Chem. 2021, 364, 130416. [Google Scholar] [CrossRef]
- Torres, F.G.; De-la-Torre, G.E. Synthesis, characteristics, and applications of modified starch nanoparticles: A review. Int. J. Biol. Macromol. 2022, 194, 289–305. [Google Scholar] [CrossRef]
- Miao, T.; Xiong, K.; Ji, N.; Xiong, L.; Sun, C.; Li, X.; Ma, A.; Sun, Q. Resistant starch nanoparticles prepared from debranched starch by medium-temperature recrystallization. Int. J. Biol. Macromol. 2020, 155, 598–604. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, S.; Han, Z.; Xiong, L.; Sun, Q. In vitro digestion of nanoscale starch particles and evolution of thermal, morphological, and structural characteristics. Food Hydrocoll. 2016, 61, 344–350. [Google Scholar] [CrossRef]
- Cardoso, M.B.; Putaux, J.-L.; Samios, D.; da Silveira, N.P. Influence of alkali concentration on the deproteinization and/or gelatinization of rice starch. Carbohydr. Polym. 2007, 70, 160–165. [Google Scholar] [CrossRef]
- Liao, H.-J.; Lai, P.Y.; Koh, Y.C.; Hung, C.C. Physicochemical characteristics and in vitro digestibility of indica, japonica, and waxy type rice flours and their derived resistant starch type III products. Starke 2016, 68, 462–468. [Google Scholar] [CrossRef]
- Liao, H.-J.; Hung, C.-C. Chemical composition and in vitro starch digestibility of green banana (cv. Giant Cavendish) flour and its derived autoclaved/debranched powder. LWT 2015, 64, 639–644. [Google Scholar] [CrossRef]
- Liao, H.-J.; Hung, C.-C. Functional, Thermal and Structural Properties of Green Banana Flour (cv. Giant Cavendish) by De-astringency, Enzymatic and Hydrothermal Treatments. Plant Foods Hum. Nutr. 2023, 78, 52–60. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Park, D.J.; Kim, J.-Y.; Lim, S.-T. Preparation of crystalline starch nanoparticles using cold acid hydrolysis and ultrasonication. Carbohydr. Polym. 2013, 98, 295–301. [Google Scholar] [CrossRef]
- AACC American Association of Cereal Chemists. Approved Methods of the AACC, 10th ed.; The Association: St. Paul, MN, USA, 2000. [Google Scholar]
- Van Hung, P.; Maeda, T.; Miskelly, D.; Tsumori, R.; Morita, N. Physicochemical characteristics and fine structure of high-amylose wheat starches isolated from Australian wheat cultivars. Carbohydr. Polym. 2008, 71, 656–663. [Google Scholar] [CrossRef]
- Yu, W.; Zhou, X.; Li, C. Application of first-order kinetics modeling to reveal the nature of starch digestion characteristics. Food Funct. 2021, 12, 6652–6663. [Google Scholar] [CrossRef]
- Shi, M.; Chen, Y.; Yu, S.; Gao, Q. Preparation and properties of RS III from waxy maize starch with pullulanase. Food Hydrocoll. 2013, 33, 19–25. [Google Scholar] [CrossRef]
- Shi, M.-M.; Gao, Q.-Y. Physicochemical properties, structure and in vitro digestion of resistant starch from waxy rice starch. Carbohydr. Polym. 2011, 84, 1151–1157. [Google Scholar] [CrossRef]
- Shah, A.; Zhang, G.; Hamaker, B.; Campanella, O. Rheological properties of a soluble self-assembled complex from starch, protein and free fatty acids. J. Food Eng. 2011, 105, 444–452. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Z. Preparation of products rich in resistant starch from maize starch by an enzymatic method. Carbohydr. Polym. 2011, 86, 1610–1614. [Google Scholar] [CrossRef]
- González-Soto, R.A.; Mora-Escobedo, R.; Hernández-Sánchez, H.; Sanchez-Rivera, M.; Bello-Pérez, L.A. The influence of time and storage temperature on resistant starch formation from autoclaved debranched banana starch. Food Res. Int. 2007, 40, 304–310. [Google Scholar] [CrossRef]
- Kiatponglarp, W.; Tongta, S.; Rolland-Sabaté, A.; Buléon, A. Crystallization and chain reorganization of debranched rice starches in relation to resistant starch formation. Carbohydr. Polym. 2015, 122, 108–114. [Google Scholar] [CrossRef]
- Pyrgiotakis, G.; Blattmann, C.O.; Pratsinis, S.; Demokritou, P. Nanoparticle–nanoparticle interactions in biological media by atomic force microscopy. Langmuir 2013, 29, 11385–11395. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. npj Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Li, M.; Chang, R.; Xiong, L.; Sun, Q. In vitro inhibition of pancreatic α-amylase by spherical and polygonal starch nanoparticles. Food Funct. 2018, 9, 355–363. [Google Scholar] [CrossRef]
- Haralampu, S.G. Resistant starch—A review of the physical properties and biological impact of RS3. Carbohydr. Polym. 2000, 4, 285–292. [Google Scholar] [CrossRef]
- Pongjanta, J.; Utaipattanaceep, A.; Naivikul, O.; Piyachomkwan, K. Debranching enzyme concentration effected on physicochemical properties and α-amylase hydrolysis rate of resistant starch type III from amylose rice starch. Carbohydr. Polym. 2009, 78, 5–9. [Google Scholar] [CrossRef]
- Reddy, C.K.; Suriya, M.; Haripriya, S. Physico-chemical and functional properties of Resistant starch prepared from red kidney beans (Phaseolus vulgaris. L) starch by enzymatic method. Carbohydr. Polym. 2013, 95, 220–226. [Google Scholar] [CrossRef]
- Duyen, T.T.M.; Van Hung, P. Morphology, crystalline structure and digestibility of debranched starch nanoparticles varying in average degree of polymerization and fabrication methods. Carbohydr. Polym. 2021, 256, 117424. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Z. Preparation of resistant starch by hydrolysis of maize starch with pullulanase. Carbohydr. Polym. 2011, 83, 865–867. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Li, W.; Wang, X.; Peng, Q.; Wang, M. Changes in physicochemical properties and in vitro digestibility of common buckwheat starch by heat-moisture treatment and annealing. Carbohydr. Polym. 2015, 132, 237–244. [Google Scholar] [CrossRef]
- Simsek, S.; El, S.N. Production of resistant starch from taro (Colocasia esculenta L. Schott) corm and determination of its effects on health by in vitro methods. Carbohydr. Polym. 2012, 90, 1204–1209. [Google Scholar] [CrossRef]
- Varatharajan, V.; Hoover, R.; Liu, Q.; Seetharaman, K. The impact of heat-moisture treatment on the molecular structure and physicochemical properties of normal and waxy potato starches. Carbohydr. Polym. 2010, 81, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.-J.; Shin, D.-H.; Lim, S.-T. In vitro starch digestibility and estimated glycemic index of chemically modified corn starches. Food Res. Int. 2008, 41, 579–585. [Google Scholar] [CrossRef]
- Frei, M.; Siddhuraju, P.; Becker, K. Studies on the in vitro starch digestibility and the glycemic index of six different indigenous rice cultivars from the Philippines. Food Chem. 2003, 83, 395–402. [Google Scholar] [CrossRef]
- Bello-Perez, L.A.; Flores-Silva, P.C.; Agama-Acevedo, E.; Tovar, J. Starch digestibility: Past, present, and future. J. Sci. Food Agric. 2020, 100, 5009–5016. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Huang, S.; Chen, L.; Zhang, Y.; Li, L.; Miao, S. Basic principles in starch multi-scale structuration to mitigate digestibility: A review. Trends Food Sci. 2021, 109, 154–168. [Google Scholar] [CrossRef]




| TS (%) | CP (%) | L* | a* | b* | ∆E | WI | |
|---|---|---|---|---|---|---|---|
| N-TCSG2 | 92.36 ± 1.06 a | 6.22 ± 0.04 c | 96.91 ± 0.01 a | -0.42 ± 0.006 e | 0.95 ± 0.006 f | N.D. | 96.74 ± 0.01 a |
| 1-TCSG2 | 90.21 ± 0.62 a | 6.69 ± 0.04 a | 92.03 ± 0.02 c | 0.020 ± 0.012 d | 8.41 ± 0.015 d | 6.32 ± 0.015 d | 88.42 ± 0.00 c |
| 5-TCSG2 | 90.28 ± 1.13 a b | 6.61 ± 0.12 b | 89.67 ± 0.01 d | 0.210 ± 0.012 c | 10.45 ± 0.01 c | 9.39 ± 0.013 c | 85.30 ± 0.00 d |
| N-TK11 | 90.38 ± 1.13 a | 6.14 ± 0.04 c | 96.85 ± 0.02 b | -0.39 ± 0.025 e | 1.11 ± 0.012 e | N.D. | 96.63 ± 0.01 b |
| 1-TK11 | 90.96 ± 2.32 ab | 6.45 ± 0.27 b | 88.34 ± 0.01 f | 0.58 ± 0.012 a | 11.98 ± 0.006 a | 11.32 ± 0.014 a | 83.28 ± 0.00 f |
| 5-TK11 | 87.41 ± 0.97 b | 6.65 ± 0.16 ab | 88.42 ± 0.02 e | 0.37 ± 0.020 b | 11.22 ± 0.030 b | 10.67 ± 0.003 b | 83.87 ± 0.02 e |
| Sample | Mw (kDa) | DPw | PDI |
|---|---|---|---|
| N-TCSG2 | N.D. | N.D. | N.D. |
| 1-TCSG2 | 7.15 ± 0.08 b | 44 ± 0 a | 1.7 |
| 5-TCSG2 | 7.13 ± 0.06 b | 44 ± 0 a | 1.7 |
| N-TK11 | N.D. | N.D. | N.D. |
| 1-TK11 | 92.76 ± 0.18 a | 572 ± 1 a | 4.4 |
| 5-TK11 | 92.69 ± 0.74 a | 572 ± 5 a | 4.4 |
| Sample | PT (°C) | PV (RVU) | TV (RVU) | BV (RVU) | FV (RVU) | SV (RVU) |
|---|---|---|---|---|---|---|
| N-TCSG2 | 66.2 ± 0.2 a | 1389 ± 23 a | 585 ± 9 b | 804 ± 7 a | 737 ± 5 b | 152 ± 2 b |
| 1-TCSG2 | N.D. | N.D. | N.D. | N.D. | 15 ± 1 | N.D. |
| 5-TCSG2 | N.D. | N.D. | N.D. | N.D. | 27 ± 2 | N.D. |
| N-TK11 | 66.0 ± 0.1 a | 696 ± 2 b | 601 ± 5 a | 95 ± 1 b | 1006 ± 30 a | 405 ± 2 a |
| 1-TK11 | N.D. | N.D. | N.D. | N.D. | 22 ± 1 | N.D. |
| 5-TK11 | N.D. | N.D. | N.D. | N.D. | 40 ± 2 | N.D. |
| C∞ (%) | K (min−1) | HI | eGI | RDS (%) | SDS (%) | RS (%) | |
|---|---|---|---|---|---|---|---|
| N-TCSG2 | 85.32 ± 0.58 b | 0.03 ± 0.00 a | 94.17 ± 1.24 a | 91.41 ± 0.68 a | 75.87 ± 1.30 a | 4.48 ± 0.04 | 19.65 ± 0.54 e |
| 1-TCSG2 | 62.02 ± 0.60 d | 0.02 ± 0.00 b | 60.85 ± 2.19 c | 73.11 ± 1.20 b | 65.80 ± 0.54 c | 8.97 ± 0.05 b | 25.21 ± 0.14 d |
| 5-TCSG2 | 42.41 ± 0.30 e | 0.02 ± 0.00 b | 44.43 ± 3.41 e | 64.10 ± 1.87 c | 58.57 ± 0.89 d | 8.07 ± 0.01 b | 33.34 ± 0.22 c |
| N-TK11 | 82.21 ± 0.00 c | 0.03 ± 0.00 a | 92.22 ± 2.30 b | 90.34 ± 1.26 a | 70.86 ± 1.26 b | 7.77 ± 0.12c | 21.37 ± 0.45 |
| 1-TK11 | 40.92 ± 0.10 f | 0.02 ± 0.00 b | 43.19 ± 3.00 d | 63.42 ± 1.65 c | 54.77 ± 1.34 e | 7.87 ± 0.04 c | 37.36 ± 0.89 b |
| 5-TK11 | 37.85 ± 0.21 g | 0.02 ± 0.00 b | 35.04 ± 4.01 f | 58.94 ± 0.86 d | 53.5 ± 1.24 f | 6.07 ± 0.09 d | 40.43 ± 0.67 a |
| WB | 97.29 ± 0.21 a | 0.028 ± 0.001 a | 100 | 100 | 71.17 ± 1.54 b | 8.31 ± 0.07 a | 20.50 ± 1.25 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, Y.-C.; Liao, H.-J. Effects of Debranching Conditions and Annealing Treatment on the Formation of Starch Nanoparticles and Their Physicochemical Characteristics. Foods 2023, 12, 2890. https://doi.org/10.3390/foods12152890
Koh Y-C, Liao H-J. Effects of Debranching Conditions and Annealing Treatment on the Formation of Starch Nanoparticles and Their Physicochemical Characteristics. Foods. 2023; 12(15):2890. https://doi.org/10.3390/foods12152890
Chicago/Turabian StyleKoh, Yen-Chun, and Hung-Ju Liao. 2023. "Effects of Debranching Conditions and Annealing Treatment on the Formation of Starch Nanoparticles and Their Physicochemical Characteristics" Foods 12, no. 15: 2890. https://doi.org/10.3390/foods12152890





