Synergistic Fermentation with Functional Microorganisms Improves Safety and Quality of Traditional Chinese Fermented Foods
Abstract
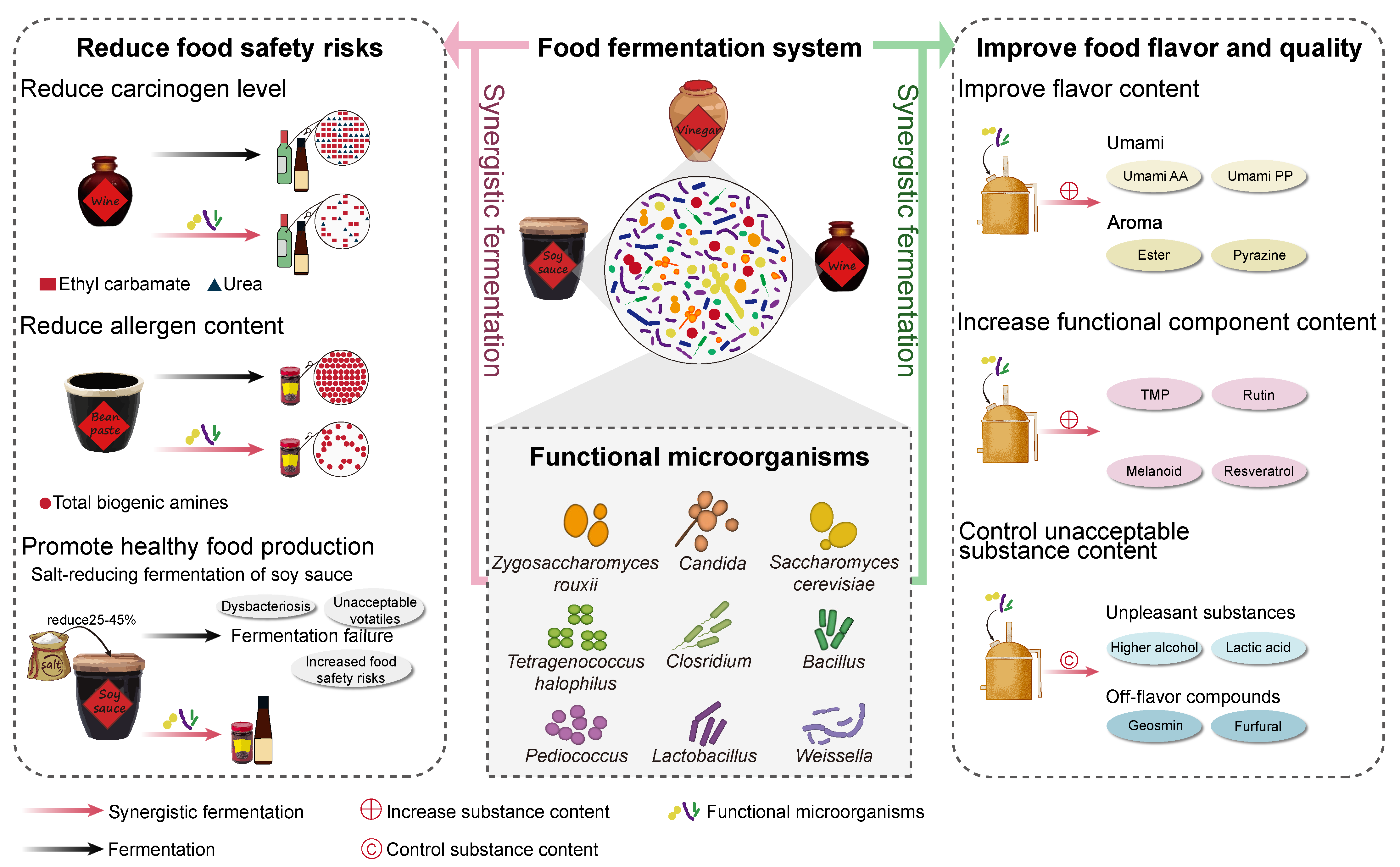
:1. Introduction
2. Microbial Community and Functions of Food Microbiota in the Process of Food Fermentation
| Figure | Role | Origin | References | |
|---|---|---|---|---|
| Yeast | Saccharomyces | Synthesize alcohols | Baijiu | [1,2] |
| Saccharomyces cerevisiae | Synthesize volatiles (higher alcohols, phenols) | Baijiu, vinegar | [1,2,41,45] | |
| Issatchenkia | Synthesize alcohols | Baijiu | [1,2] | |
| Rhizopus | Produce amylase | Baijiu | [1,18] | |
| Saccharomycopsis | Produce amylase and protease | Baijiu, vinegar | [1,18,44,45] | |
| Hansenula | Synthesize alcohols, produce esterase | Baijiu | [1,2,23,24] | |
| Candida | Synthesize alcohols, produce esterase | Baijiu | [1,2,23,24] | |
| Pichia | Produce esterase | Baijiu | [23,24] | |
| Brettanomyces | Produce esterase | Baijiu | [23] | |
| Zygosaccharomyces rouxii | Synthesize volatiles (ethanol, 4-EG, pyrazines, phenylethyl alcohol, 3-methyl butanol, ethyl acetate, and phenethyl acetate) | Soy sauce, jiang (bean paste) | [3,7,12,35,36] | |
| Filamentous fungi | Aspergillus | Produce protease, glucoamylase, amylase, and lipase | Baijiu, soy sauce, jiang (bean paste) | [1,2,4,5,16,33,44] |
| Mucor | Produce amylase, lipase, and protease | Vinegar | [5,42,43,44] | |
| Absidia | Produce amylase, lipase, and protease | Vinegar | [5,42,43,44] | |
| Functional bacteria | Clostridium | Synthesize butyric acid and acetic acid | Baijiu | [11,55] |
| Bacillus | Synthesize organic acids, produce amylase, peptidase, and protease | Baijiu, soy sauce, jiang (bean paste) | [1,4,18,29] | |
| Bacillus subtilis | Synthesize pyrazines, produce peptidase and transaminase | Baijiu, jiang (bean paste) | [16,19,20] | |
| Bacillus licheniformis | Synthesize pyrazines, produce peptidase and transaminase | Baijiu, jiang (bean paste) | [16,19,20] | |
| Bacillus pumilus | Produce peptidase and transaminase | Jiang (bean paste) | [16] | |
| Staphylococcus | Synthesize organic acids, produce peptidase | Baijiu, soy sauce | [25,29] | |
| Tetragenococcus | Synthesize organic acids, produce peptidase | Soy sauce | [29] | |
| Pseudomonas | Synthesize volatiles (3-methyl butyraldehyde, 2-methyl butyraldehyde, and 5-methyl-2-phenyl-2-hexenal), degrade peptides | Jiang (bean paste) | [4,33] | |
| Acetobacter | Synthesize acetic acid, amino acids, esters | Vinegar | [5,46] | |
| Acetobacter pasteurianus | Produce the enzyme that converts diacetyl to acetoin | Vinegar | [46,53,54] | |
| LAB | Lactobacillus | Synthesize organic acids, produce peptidase, proteases, and aminopeptidases | Baijiu, vinegar | [25,42,46,47,48,49,50] |
| Levilactobacillus brevis | Produce enzymes that are responsible for synthesizing acetoin | Vinegar | [53] | |
| Limosilactobacillus fermentum | Produce enzymes that are responsible for synthesizing acetoin | Vinegar | [53] | |
| Pediococcus | Synthesize organic acids, produce peptidase, proteases, and aminopeptidases | Baijiu, soy sauce, vinegar | [25,29,42,46,47,48,49,50] | |
| Weissella | Synthesize organic acids, produce peptidase, proteases, and aminopeptidases | Soy sauce, vinegar | [29,42,46,47,48,49,50] | |
| Leuconostoc | Synthesize organic acids, produce proteases and aminopeptidases | Vinegar | [42,46,47,48,49,50] | |
3. Functions of Synergistic Fermentation in Maintaining Food Safety
| Functional Microorganisms | Function | Reduction of Biohazard Compounds | Food | Reference | |
|---|---|---|---|---|---|
| Compounds | Reduction Rate (%) | ||||
| Bacillus amyloliquefaciens JP21 | Produce urease | Urea | 50.05 | Baijiu | [9] |
| EC | 30.16 | ||||
| Bacillus licheniformis DX530 | Degrade urea and citrulline | Citrulline | 11 | Baijiu | [68] |
| Urea | 10 | ||||
| EC | 16 | ||||
| Bacillus amyloliquefaciens JY06 | Control the synthesis of citrulline | Citrulline | 80.9 | Soy sauce | [65] |
| EC | 82.5 | ||||
| Lysinibacillus sphaericus MT33 | Produce urease | Urea | 28.15 | Baijiu | [10] |
| EC | 41.77 | ||||
| Saccharomyces cerevisiae N14 | Low urea-producing capacity | Urea | 16.8 | Huangjiu | [64] |
| Saccharomyces cerevisiae 5–11C | Utilize urea | Urea | 50.6 | Huangjiu | [78] |
| Lactobacillus brevis 2–34 | Utilize citrulline | Citrulline | 58.2 | Huangjiu | [79] |
| EC | 29.6 | ||||
| Lactobacillus plantarum HM24 | Degrade BAs | Total BAs | 35.79 | Soybean paste | [80] |
| Staphylococcus piscifermentans CGMCC 18053, Zygosaccharomyces rouxii CICC 1417, and Torulopsis candida CICC 1019 | Degrade BAs | Total BAs | 63.25 | Soy sauce | [81] |
4. Improvement in Food Flavor and Quality by Synergistic Fermentation with Functional Microorganisms
5. Selection and Mutagenesis of Functional Microorganisms for Food Fermentation
5.1. Selection and Mutagenesis of Microorganisms to Reduce Biohazard Compounds in Fermented Foods
5.2. Selection and Mutagenesis of Microorganisms to Improve the Quality of Fermented Foods
6. Conclusions
7. Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Gao, L.; Zhou, J.; He, G. Effect of microbial interaction on flavor quality in Chinese baijiu fermentation. Front. Nutr. 2022, 9, 960712. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Cao, X.; Cheng, J.; Li, L.; Zhang, T.; Wu, Q.; Xiang, P.; Shen, C.; Li, Q. Chinese Baijiu: The perfect works of microorganisms. Front. Microbiol. 2022, 13, 919044. [Google Scholar] [CrossRef] [PubMed]
- Devanthi, P.V.P.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Res. Int. 2019, 120, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rui, J.; Li, X.; Li, J.; Dong, L.; Huang, Q.; Huang, C.; Wang, Z.; Li, L.; Xuan, P.; et al. Bacterial community succession and metabolite changes during doubanjiang-meju fermentation, a Chinese traditional fermented broad bean (Vicia faba L.) paste. Food Chem. 2017, 218, 534–542. [Google Scholar] [CrossRef]
- Huang, T.; Lu, Z.; Peng, M.; Liu, Z.; Chai, L.; Zhang, X.; Shi, J.; Li, Q.; Xu, Z. Combined effects of fermentation starters and environmental factors on the microbial community assembly and flavor formation of Zhenjiang aromatic vinegar. Food Res. Int. 2022, 152, 110900. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Yan, D.H.; Liu, Y.; Wang, C.; Guo, C. Bacterial and fungal communities of traditional fermented Chinese soybean paste (Doujiang) and their properties. Food Sci. Nutr. 2021, 9, 5457–5466. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, M.; Zhang, Y.; Zhang, X.; Zhang, X.; Zhao, X.; Xu, H.; Huang, Y. Correlation between bacterial diversity and flavor substances in Longgang soy sauce. Biosci. Biotechnol. Biochem. 2023, 87, 541–554. [Google Scholar] [CrossRef]
- Ai, M.; Qiu, X.; Huang, J.; Wu, C.; Jin, Y.; Zhou, R. Characterizing the microbial diversity and major metabolites of Sichuan bran vinegar augmented by Monascus purpureus. Int. J. Food Microbiol. 2019, 292, 83–90. [Google Scholar] [CrossRef]
- Fang, F.; Ding, X.; Li, Q.; Liu, F.; Zhou, X.; Chen, X.; Chen, J.; Du, G. Method for simultaneously reducing urethane and its precursors levels during Chinese liquor production process. US 10253285 B2, 9 April 2019. [Google Scholar]
- Cui, K.; Wu, Q.; Xu, Y. Biodegradation of ethyl carbamate and urea with Lysinibacillus sphaericus MT33 in Chinese liquor fermentation. J. Agric. Food Chem. 2018, 66, 1583–1590. [Google Scholar] [CrossRef]
- Qian, W.; Lu, Z.M.; Chai, L.; Zhang, X.; Li, Q.; Wang, S.; Shen, C.; Shi, J.; Xu, Z. Cooperation within the microbial consortia of fermented grains and pit mud drives organic acid synthesis in strong-flavor Baijiu production. Food Res. Int. 2021, 147, 110449. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, M.; Xie, N.; Huang, M.; Feng, Y. Community structure of yeast in fermented soy sauce and screening of functional yeast with potential to enhance the soy sauce flavor. Int. J. Food Microbiol. 2022, 370, 109652. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Hussain, R.; Farid Khan, Q.; Zhang, H. Functional microbiota in Chinese traditional Baijiu and Mijiu Qu (starters): A review. Food Res. Int. 2020, 138, 109830. [Google Scholar] [CrossRef]
- Musatti, A.; Mapelli, C.; Foschino, R.; Picozzi, C.; Rollini, M. Unconventional bacterial association for dough leavening. Int. J. Food Microbiol. 2016, 237, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Hu, M.; Li, X.; Li, X.; Pan, Z.; Li, M.; Li, L.; Wang, Y.; Zheng, Z. Microbial community and metabolite dynamics during soy sauce koji making. Front. Microbiol. 2022, 13, 841529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, C.; Li, S.; Wang, X.; Yao, Y. Exploring the flavor formation mechanism under osmotic conditions during soy sauce fermentation in Aspergillus oryzae by proteomic analysis. Food Funct. 2020, 11, 640–648. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, C.; Shan, W.; Zheng, F.; Liu, C.; Wang, J.; Li, Q. Physicochemical, flavor and microbial dynamic changes during low-salt doubanjiang (broad bean paste) fermentation. Food Chem. 2021, 351, 128454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Che, Z.; Xu, W.; Yue, P.; Li, R.; Li, Y.; Pei, X.; Zeng, P. Dynamics of physicochemical factors and microbial communities during ripening fermentation of Pixian Doubanjiang, a typical condiment in Chinese cuisine. Food Microbiol. 2020, 86, 103342. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhao, S.; Chen, S.; Han, X.; Yang, Q.; Zhang, L.; Xia, X.; Tu, J.; Hu, Y. Tetramethylpyrazine in Chinese baijiu: Presence, analysis, formation, and regulation. Front. Nutr. 2022, 9, 1004435. [Google Scholar] [CrossRef]
- Zhang, W.; Si, G.; Du, H.; Li, J.; Zhou, P.; Ye, M. Directional design of a starter to assemble the initial microbial fermentation community of baijiu. Food Res. Int. 2020, 134, 109255. [Google Scholar] [CrossRef]
- You, L.; Zhao, D.; Zhou, R.; Tan, Y.; Wang, T.; Zheng, J. Distribution and function of dominant yeast species in the fermentation of strong-flavor baijiu. World J. Microbiol. Biotechnol. 2021, 37, 26. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Q.; Chen, D.; Fu, B.; Zhang, Y.; Zhang, Y.; Xia, X.; Peng, N.; Liang, Y.; Zhao, S. Study on microbial communities and higher alcohol formations in the fermentation of Chinese Xiaoqu Baijiu produced by traditional and new mechanical technologies. Food Res. Int. 2021, 140, 109876. [Google Scholar] [CrossRef]
- He, M.; Jin, Y.; Zhou, R.; Zhao, D.; Zheng, J.; Wu, C. Dynamic succession of microbial community in Nongxiangxing daqu and microbial roles involved in flavor formation. Food Res. Int. 2022, 159, 111559. [Google Scholar] [CrossRef] [PubMed]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Studies on acetate ester production by non-Saccharomyces wine yeasts. Int. J. Food Microbiol. 2001, 70, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Zhao, C.; Luo, H. Diversity and function of microbial community in Chinese strong-flavor baijiu ecosystem: A review. Front. Microbiol. 2018, 9, 671. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, Y.; Jin, L.; He, L.; Ao, X.; Liu, S.; Yang, Y.; Liu, A.; Chen, S.; Zou, L. Analyzing bacterial community in pit mud of Yibin Baijiu in China using high throughput sequencing. PeerJ 2020, 8, e9122. [Google Scholar] [CrossRef]
- Xiao, C.; Yang, Y.; Lu, Z.; Chai, L.; Zhang, X.; Wang, S.; Shen, C.; Shi, J.; Xu, Z. Daqu microbiota exhibits species-specific and periodic succession features in Chinese baijiu fermentation process. Food Microbiol. 2021, 98, 103766. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Zhu, Y.; Huang, Z.; Zhang, M.; Zhu, X. Research advances of flavor enhancement of high-salt dilute soy sauce by yeast. Food Ferment. Ind. 2022, 1–8. [Google Scholar]
- Hu, C.; Li, Q.; Zhou, C.; Li, T.; Chen, J.; Du, G.; Fang, F. Functional analysis of bacteria isolated from soy sauce moromi. Microbiol. China 2017, 44, 1899–1907. [Google Scholar]
- Guo, J.; Luo, W.; Wu, X.M.; Fan, J.; Zhang, W.X.; Suyama, T. Improving RNA content of salt-tolerant Zygosaccharomyces rouxii by atmospheric and room temperature plasma (ARTP) mutagenesis and its application in soy sauce brewing. World J. Microbiol. Biotechnol. 2019, 35, 180. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Ye, X.; Zhao, X.; Liu, Y.; Zhang, M.; Luo, Y.; Xiong, Y.; Liu, Y.; Che, Z.; Lin, H.; et al. Fermentation characteristics of Pixian broad bean paste in closed system of gradient steady-state temperature field. Food Chem. 2022, 374, 131560. [Google Scholar] [CrossRef]
- Lu, Y.; Tan, X.; Lv, Y.; Yang, G.; Chi, Y.; He, Q. Physicochemical properties and microbial community dynamics during Chinese horse bean-chili-paste fermentation, revealed by culture-dependent and culture-independent approaches. Food microbiol. 2020, 85, 103309. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, L.; Zheng, P.; Chen, H.; Huang, J.; Xu, Y. Diversity and source tracking of microbial community in Pixian broad bean paste. Acta Microbiol. Sin. 2020, 60, 2555–2571. [Google Scholar]
- Li, Z.; Dong, L.; Huang, Q.; Wang, X. Bacterial communities and volatile compounds in Doubanjiang, a Chinese traditional red pepper paste. J. Appl. Microbiol. 2016, 120, 1585–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, R.; Lv, G.; Guo, B.; Li, Y.; Ai, M.; He, B.; Wan, R. Physiological and transcriptomic analyses revealed the change of main flavor substance of Zygosaccharomyces rouxii under salt treatment. Front. Nutr. 2022, 9, 990380. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, X.; Zhao, J.; Qin, L.; Shi, L.; Zhou, T.; Liu, S.; Wang, C. Effects of salinity on the synthesis of 3-methylthiopropanol, 2-phenylethanol, and isoamyl acetate in Zygosaccharomyces rouxii and Z. rouxii 3-2. Bioprocess Biosyst. Eng. 2020, 43, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, Z.; Zhang, X.; Mao, J.; Shi, J.; Xu, Z. Microbial ecology of cereal vinegar fermentation: Insights for driving the ecosystem function. Curr. Opin. Biotechnol. 2018, 49, 88–93. [Google Scholar] [CrossRef]
- Huang, T.; Lu, Z.M.; Peng, M.; Chai, L.; Zhang, X.; Shi, J.; Li, Q.; Xu, Z. Constructing a defined starter for multispecies vinegar fermentation via evaluation of the vitality and dominance of functional microbes in an autochthonous starter. Appl. Environ. Microbiol. 2022, 88, e0217521. [Google Scholar] [CrossRef]
- Xie, Z.; Koysomboon, C.; Zhang, H.; Lu, Z.; Zhang, X.; Chen, F. Vinegar volatile organic compounds: Analytical methods, constituents, and formation processes. Front. Microbiol. 2022, 13, 907883. [Google Scholar] [CrossRef]
- Li, N.; Fu, J.; Zhang, G.; Liu, J.; Li, Z.; Luo, R.; Li, L. Investigating the mechanism of the flavor formation in Sichuan sun vinegar based on flavor-orientation and metagenomics. Curr. Res. Food Sci. 2023, 6, 100460. [Google Scholar] [CrossRef]
- Fu, J.; Feng, J.; Zhang, G.; Liu, J.; Li, N.; Xu, H.; Zhang, Y.; Cao, R.; Li, L. Role of bacterial community succession in flavor formation during Sichuan sun vinegar grain (Cupei) fermentation. J. Biosci. Bioeng. 2023, 135, 109–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Zhu, W.; Chen, J.; Liu, Y.; Xu, H. Analysis of microbial community structure in Sichuan bran vinegar fermentation based on illumina miseq high-throughput sequencing technology. J. Chin. Inst. Food Sci. Technol. 2022, 22, 299–306. [Google Scholar]
- Zhu, Y.; Zhang, F.; Zhang, C.; Yang, L.; Fan, G.; Xu, Y.; Sun, B.; Li, X. Dynamic microbial succession of Shanxi aged vinegar and its correlation with flavor metabolites during different stages of acetic acid fermentation. Sci. Rep. 2018, 8, 8612. [Google Scholar] [CrossRef] [PubMed]
- Kou, R.; Li, M.; Xing, J.; He, Y.; Wang, H.; Fan, X. Exploring of seasonal dynamics of microbial community in multispecies fermentation of Shanxi mature vinegar. J. Biosci. Bioeng. 2022, 133, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lv, X.; Zhang, C.; Zheng, Y.; Zheng, B.; Duan, X.; Tian, Y. Microbial dynamics and flavor formation during the traditional brewing of Monascus vinegar. Food Res. Int. 2019, 125, 108531. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Zheng, Y.; Xie, S.; Zhang, X.; Song, J.; Xia, M.; Wang, M. Unraveling the correlation between microbiota succession and metabolite changes in traditional Shanxi aged vinegar. Sci. Rep. 2017, 7, 9240. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, X.; Yan, Y.; Liu, Y.; Zhao, X.; Xu, H.; He, L.; Huang, Y. Relationship between flavor compounds and changes of microbial community in the solid fermented vinegar. Biosci. Biotechnol. Biochem. 2022, 86, 1581–1589. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.; Liu, F.; Zhang, C.; Ma, H.; Wang, L.; Zhao, S. Effects of ultrasonic treatment on the maturation of Zhenjiang vinegar. Ultrason. Sonochem. 2017, 39, 272–280. [Google Scholar] [CrossRef]
- Jiang, R.; Xu, J.; Zhang, Y.; Zhu, X.; Liu, J.; Tan, Y. Ligustrazine alleviate acute lung injury through suppressing pyroptosis and apoptosis of alveolar macrophages. Front. Pharmacol. 2021, 12, 680512. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, X.; Xiao, Y.; Sheng, Q.; Tu, L.; Chen, F.; Yan, Y.; Zheng, Y.; Wang, M. Interaction of acetic acid bacteria and lactic acid bacteria in multispecies solid-state fermentation of traditional Chinese cereal vinegar. Front. Microbiol. 2022, 13, 964855. [Google Scholar] [CrossRef]
- He, H.; Wang, L.; Qiao, Y.; Zhou, Q.; Yang, B.; Yin, L.; Yin, D.; He, M. Vinegar/Tetramethylpyrazine induces nutritional preconditioning protecting the myocardium mediated by VDAC1. Oxid. Med. Cell. Longev. 2021, 2021, 6670088. [Google Scholar] [CrossRef]
- Kandylis, P.; Bekatorou, A.; Dimitrellou, D.; Plioni, I.; Giannopoulou, K. Health promoting properties of cereal vinegars. Foods 2021, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Chai, L.; Zhong, X.; Jiang, Y. Deciphering the succession patterns of bacterial community and their correlations with environmental factors and flavor compounds during the fermentation of Zhejiang rosy vinegar. Int. J. Food Microbiol. 2021, 341, 109070. [Google Scholar] [CrossRef]
- Yun, J.; Zhao, F.; Zhang, W.; Yan, H.; Zhao, F.; Ai, D. Monitoring the microbial community succession and diversity of Liangzhou fumigated vinegar during solid-state fermentation with next-generation sequencing. Ann. Microbiol. 2019, 69, 279–289. [Google Scholar] [CrossRef]
- Wu, L.; Fan, J.; Chen, J.; Fang, F. Chemotaxis of Clostridium strains isolated from pit mud and its application in Baijiu fermentation. Foods 2022, 11, 3639. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Zhang, M. Ethyl carbamate in Chinese liquor (Baijiu): Presence, analysis, formation, and control. Appl. Microbiol. Biotechnol. 2021, 105, 4383–4395. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, J.; Zhou, J.; Zhou, Z.; Li, T.; Lu, L.; Zeng, W.; Du, G.; Chen, J. Accumulation of citrulline by microbial arginine metabolism during alcoholic fermentation of soy sauce. J. Agric. Food Chem. 2018, 66, 2108–2113. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Xu, Y. Determination of the microbial origin of geosmin in Chinese liquor. J. Agric. Food Chem. 2012, 60, 2288–2292. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Huang, J.; Zhou, R.; Jin, Y.; Wu, C. Abating biogenic amines and improving the flavor profile of Cantonese soy sauce via co-culturing Tetragenococcus halophilus and Zygosaccharomyces rouxii. Food Microbiol. 2022, 106, 104056. [Google Scholar] [CrossRef]
- Abt, E.; Incorvati, V.; Robin, L.P.; Redan, B.W. Occurrence of ethyl carbamate in foods and beverages: Review of the formation mechanisms, advances in analytical methods, and mitigation strategies. J. Food Prot. 2021, 84, 2195–2212. [Google Scholar] [CrossRef]
- Jung, J.; Kang, M.; Hwang, H.; Baek, K.; Seo, S. Reduction of ethyl carbamate in an alcoholic beverage by CRISPR/Cas9-based genome editing of the wild yeast. Foods 2022, 12, 102. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Q.; Zhou, C.; Lu, L.; Du, G.; Chen, J.; Fang, F. Reduction of ethyl carbamate in soy sauce by Bacillus amyloliquefaciens mutants. Acta Microbiol. Sin. 2017, 57, 1817–1826. [Google Scholar]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Cheng, Y.; Li, Y.; Du, G.; Xie, G.; Zou, H.; Zhou, J.; Chen, J. Adaptive evolution relieves nitrogen catabolite repression and decreases urea accumulation in cultures of the Chinese rice wine yeast strain Saccharomyces cerevisiae XZ-11. J. Agric. Food Chem. 2018, 66, 9061–9069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, G.; Chen, J.; Fang, F. Characterization of a Bacillus amyloliquefaciens strain for reduction of citrulline accumulation during soy sauce fermentation. Biotechnol. Lett. 2016, 38, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wen, X.; Wen, Z.; Chen, S.; Wang, L.; Wei, X. Evaluation of the biogenic amines formation and degradation abilities of Lactobacillus curvatus from Chinese bacon. Front. Microbiol. 2018, 9, 1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Song, Z.; Xu, Y. Ethyl Carbamate formation regulated by lactic acid bacteria and nonconventional yeasts in solid-state fermentation of Chinese moutai-flavor liquor. J. Agric. Food Chem. 2018, 66, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, Q.; Liu, F.; Zeng, W.; Chen, J.; Du, G.; Fang, F. lsolation of microbial strains for degradation of ethyl carbamate in Luzhou-flavour Baijiu and characterization of corresponding enzymes. Food Ferment. Ind. 2018, 44, 29–36. [Google Scholar]
- Di, Y.; Li, J.; Chen, J.; Zhao, X.; Du, G. Simulation and control of the formation of ethyl carbamate during the fermentation and distillation processes of Chinese Baijiu. Foods 2023, 12, 821. [Google Scholar] [CrossRef]
- Tian, S.; Zeng, W.; Zhou, J.; Du, G. Correlation between the microbial community and ethyl carbamate generated during Huzhou rice wine fermentation. Food Res. Int. 2022, 154, 111001. [Google Scholar] [CrossRef]
- Saito, T.; Takano, Y. QM/MM molecular dynamics simulations revealed catalytic mechanism of urease. J. Phys. Chem. B 2022, 126, 2087–2097. [Google Scholar] [CrossRef]
- Fang, F.; Ding, X.; Chen, J.; Zhou, X.; Chen, X.; Du, G.; Gan, G. A Strain of Bacillus licheniformis Degrading Ethyl Carbamate and Its Precursors. CN107502574B, 8 October 2019. [Google Scholar]
- Zhang, J.; Fang, F.; Chen, J.; Du, G. The arginine deiminase pathway of koji bacteria is involved in ethyl carbamate precursor production in soy sauce. FEMS Microbiol Lett 2014, 358, 91–97. [Google Scholar] [CrossRef]
- Liang, Z.; Su, H.; Ren, X.; Lin, X.; He, Z.; Li, X.; Zheng, Y. Analysis of key genes responsible for low urea production in Saccharomyces cerevisiae JH301. Front. Microbiol. 2022, 13, 894661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, G.; Zhou, J.; Chen, J. Regulation of sensing, transportation, and catabolism of nitrogen sources in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2018, 82, e00040-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ter Schure, E.; van Riel, N.W.; Verrips, C.T. The role of ammonia metabolism in nitrogen catabolite repression in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2000, 24, 67–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Lu, J.; Yu, W.; Cai, G. A Lactobacillus brevis Strain that Can Efficiently Utilize Citrulline and Its Application. CN106978371B, 2 July 2019. [Google Scholar]
- Chen, Y.; Du, G.; Zhou, J.; Chen, J. High-throughput screening of mutant strain to reduce the accumulation of ethyl carbamate precursor in rice wine fermentation. Acta Microbiol. Sin. 2017, 57, 1517–1526. [Google Scholar]
- Yu, W.; Li, X.; Lu, J.; Xie, G. Citrulline production by lactic acid bacteria in Chinese rice wine. J. I. Brewing 2018, 124, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Du, X.; Feng, L.; Mu, G.; Tuo, Y. The microbial community, biogenic amines content of soybean paste, and the degradation of biogenic amines by Lactobacillus plantarum HM24. Food Sci. Nutr. 2021, 9, 6458–6470. [Google Scholar] [CrossRef]
- Guo, J.; Luo, W.; Fan, J.; Suyama, T.; Zhang, W. Co-inoculation of Staphylococcus piscifermentans and salt-tolerant yeasts inhibited biogenic amines formation during soy sauce fermentation. Food Res. Int. 2020, 137, 109436. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Li, L.; Chen, S.; Wu, Y.; Qi, B. Microbial community changes induced by a newly isolated salt-tolerant Tetragenococcus muriaticus improve the volatile flavor formation in low-salt fish sauce. Food Res. Int. 2022, 156, 111153. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Liu, X.; Zhang, C.; Zhao, Z.; Li, X.; Sun, B. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chem. 2022, 369, 130920. [Google Scholar] [CrossRef]
- Fan, G.; Sun, B.; Fu, Z.; Xia, Y.; Huang, M.; Xu, C.; Li, X. Analysis of physicochemical indices, volatile flavor components, and microbial community of a light-flavor daqu. J. Am. Soc. Brew. Chem. 2018, 76, 209–218. [Google Scholar] [CrossRef]
- Tie, Y.; Zhu, W.; Zhang, C.; Yin, L.; Li, L.; Liu, J. Effect of temperature on chemical compounds of Cupei (precursor of bran vinegar) during in-situ aging and revelation of functional microorganisms in the process. LWT-Food Sci. Technol. 2023, 182, 114912. [Google Scholar] [CrossRef]
- Chai, L.; Qiu, T.; Lu, Z.; Deng, Y.; Zhang, X.; Shi, J.; Xu, Z. Modulating microbiota metabolism via bioaugmentation with Lactobacillus casei and Acetobacter pasteurianus to enhance acetoin accumulation during cereal vinegar fermentation. Food Res. Int. 2020, 138, 109737. [Google Scholar] [CrossRef]
- Pan, F.; Qiu, S.; Lv, Y.; Li, D. Exploring the controllability of the Baijiu fermentation process with microbiota orientation. Food Res. Int. 2023, 91, 113249. [Google Scholar] [CrossRef]
- Tan, Y.; Zhong, H.; Zhao, D.; Du, H.; Xu, Y. Succession rate of microbial community causes flavor difference in strong-aroma Baijiu making process. Int. J. Food Microbiol. 2019, 311, 108350. [Google Scholar] [CrossRef]
- Luo, L.; Song, L.; Han, Y.; Zhen, P.; Han, D.; Zhao, X.; Zhou, X.; Wei, Y.; Yu, H.; Han, P.; et al. Microbial communities and their correlation with flavor compound formation during the mechanized production of light-flavor Baijiu. Food Res. Int. 2023, 172, 113139. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Xu, C.; Zhu, G.; Chen, J. Domestication of caproic acid-producing and lactic acid-utilizing microbe in the pit muds and its application in Baijiu fermentation. Food Ferment. Ind. 2020, 46, 167–174. [Google Scholar]
- Meng, Q.; Zhou, X.; Chen, X.; Du, G.; Chen, J.; Fang, F. Reduction of urea in fermented grains during Chinese liquor brewing process. China Brew. 2017, 43, 33–39. [Google Scholar]
- Zeng, X.; Mo, Z.; Zheng, J.; Wei, C.; Dai, Y.; Yan, Y.; Qiu, S. Effects of biofilm and co-culture with Bacillus velezensis on the synthesis of esters in the strong flavor Baijiu. Int. J. Food Microbiol. 2023, 394, 110166. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Luo, W.; Gong, R.; Xue, X.; Guan, X.; Song, L.; Guo, X.; Xiao, D. Improved ethyl caproate production of Chinese liquor yeast by overexpressing fatty acid synthesis genes with OPI1 deletion. J. Ind. Microbiol. Biotechnol. 2016, 43, 1261–1270. [Google Scholar] [CrossRef]
- Fan, G.; Teng, C.; Xu, D.; Fu, Z.; Liu, P.; Wu, Q.; Yang, R.; Li, X. Improving ethyl acetate production in baijiu manufacture by Wickerhamomyces anomalus and Saccharomyces cerevisiae mixed culture fermentations. Biomed Res. Int. 2019, 2019, 1470543. [Google Scholar] [CrossRef] [Green Version]
- Zhi, Y.; Wu, Q.; Du, H.; Xu, Y. Biocontrol of geosmin-producing Streptomyces spp. by two Bacillus strains from Chinese liquor. Int. J. Food Microbiol. 2016, 231, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Y.; Du, H. Analysis of the geosmin-inhibiting microorganisms in Daqu under low water content. Food Ferment. Ind. 2022, 48, 55–62. [Google Scholar]
- Luo, H.; Zeng, X.; Chen, L.; Yang, F.; Du, H.; Huang, X. Microbial intervention to reduce lactic acid in Maotai-flavor liquor brewing. Food Ferment. Ind. 2022, 1–9. [Google Scholar]
- Deng, N.; Du, H.; Xu, Y. Cooperative response of Pichia kudriavzevii and Saccharomyces cerevisiae to lactic acid stress in baijiu fermentation. J. Agric. Food Chem. 2020, 68, 4903–4911. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, S.; Yang, Q.; Han, X.; Zhou, Z.; Mao, J. Saccharomyces cerevisiae strains with low-yield higher alcohols and high-yield acetate esters improve the quality, drinking comfort and safety of huangjiu. Food Res. Int. 2022, 161, 111763. [Google Scholar] [CrossRef] [PubMed]
- Gou, W.; Fang, F. Control and reduction of n-butanol synthesis during Baijiu fermentation interfered with Clostridia in pit mud. Food Ferment. Indu. 2021, 47, 43–49. [Google Scholar]
- Cui, R.; Zheng, J.; He, F.; Zhang, L.; Yang, S.; Huang, J.; Wu, C.; Zhou, R. Effect of the enhancing technique of halophilic LAB and yeasts on the quality of high-salt dilute-state soy sauce moromi. Food Ferment. Ind. 2013, 34, 197–201. [Google Scholar]
- Kijima, K.; Suzuki, H. Improving the umami taste of soy sauce by the addition of bacterial γ-glutamyltranspeptidase as a glutaminase to the fermentation mixture. Enzyme Microb. Tech. 2007, 41, 80–84. [Google Scholar] [CrossRef]
- Wang, P.; Kan, Q.; Yang, L.; Huang, W.; Wen, L.; Fu, J.; Liu, Z.; Lan, Y.; Huang, Q.; Ho, C.T.; et al. Characterization of the key aroma compounds in soy sauce by gas chromatography-mass spectrometry-olfactometry, headspace-gas chromatography-ion mobility spectrometry, odor activity value, and aroma recombination and omission analysis. Food Chem. 2023, 419, 135995. [Google Scholar] [CrossRef]
- Fang, J.; Liu, J.; Peng, Y.; Yao, Y.; Fang, F. Effect of enrichment of Tetragenococcus halophilus on simulated fermentation of low-salt soy sauce. China Brew. 2020, 39, 146–152. [Google Scholar]
- Duan, W.; Xia, T.; Zhang, B.; Li, S.; Zhang, C.; Zhao, C.; Song, J.; Wang, M. Changes of physicochemical, bioactive compounds and antioxidant capacity during the brewing process of Zhenjiang aromatic vinegar. Molecules 2019, 24, 3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Wang, Q.; Zhou, S.; Xu, S.; Yao, K. Tetramethylpyrazine: A review on its mechanisms and functions. Biomed. Pharmacother. 2022, 150, 113005. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Shen, J.; Meng, Z.; Zhao, J.Y.; Xiao, Z. Tetramethylpyrazine production from edible materials by the probiotic Bacillus coagulans. Prep. Biochem. Biotechnol. 2020, 50, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sun, J.; Zhang, H.; Ren, X.; Guo, X.; Zhang, X.; Xu, N. Effects of fermentation with excellent strains combination on synthesis of ligustrazine and its precursor in Cupei. China Brew. 2022, 41, 55–60. [Google Scholar]
- Lei, Q.; Wang, B.; Du, G.; Fang, F. Enhancemment of arginine and citrulline utilization ability of Tetragenococcus halophilus by mutation breeding. Food Ferment. Ind. 2018, 44, 30–36. [Google Scholar]
- Fang, J.; Li, J.; Wang, B.; Fang, F. Role of multiple copies of arc operon genes in arginine metabolism of Tetragenococcus halophilus. Microbiol. China 2022, 49, 4766–4777. [Google Scholar]
- Zhang, C.; Pu, C.; Bai, C.; Tang, J.; Long, Y.; Li, Z.; Zhang, L. Screening and identification of Moutai flavor producing bacteria from high-temperature daqu and lts aroma producing characteristics. Food Sci. Technol. 2022, 47, 1–6. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.; Zhang, L.; Wang, H.; Huang, D.; Luo, H. Effect of Bacillus velezensis on the microbial community structure and volatile flavor compounds of fermented grains(jiupei) for Nongxiangxing Baijiu. Food Sci. 2022, 43, 175–182. [Google Scholar]
- Xu, X.; Niu, C.; Liu, C.; Li, Q. Unraveling the mechanisms for low-level acetaldehyde production during alcoholic fermentation in Saccharomyces pastorianus lager yeast. J. Agric. Food Chem. 2019, 67, 2020–2027. [Google Scholar] [CrossRef]
- Guan, J.; Liu, C.; Li, Q.; He, X.; Xie, X.; Guo, L.; Wang, J. Screening of low-acetaldehyde beer yeast with flavor advantages. J. Anhui Agric. Univ. 2021, 48, 511–517. [Google Scholar]
- Sun, K.; Yin, H.; Zhao, X.; Chen, L.; Li, J.; Hou, X.; Du, G. Rapid screening of industrial brewer’s yeast with low acetaldehyde yield by multi-round ARTP mutagenesis. Food Ferment. Ind. 2021, 47, 56–62. [Google Scholar]
- Fan, J.; Liu, S.; Lu, X.; Chen, J. Breeding Aspergillus oryzae with high-productive protease and its influence on soy sauce fermentation. Food Ferment. Ind. 2021, 47, 1–8. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Qu, G.; Wang, D.; Chen, J.; Du, G.; Fang, F. Synergistic Fermentation with Functional Microorganisms Improves Safety and Quality of Traditional Chinese Fermented Foods. Foods 2023, 12, 2892. https://doi.org/10.3390/foods12152892
Fan J, Qu G, Wang D, Chen J, Du G, Fang F. Synergistic Fermentation with Functional Microorganisms Improves Safety and Quality of Traditional Chinese Fermented Foods. Foods. 2023; 12(15):2892. https://doi.org/10.3390/foods12152892
Chicago/Turabian StyleFan, Jingya, Guanyi Qu, Datao Wang, Jian Chen, Guocheng Du, and Fang Fang. 2023. "Synergistic Fermentation with Functional Microorganisms Improves Safety and Quality of Traditional Chinese Fermented Foods" Foods 12, no. 15: 2892. https://doi.org/10.3390/foods12152892





