Physiochemical Quality, Microbial Diversity, and Volatile Components of Monascus-Fermented Hairtail Surimi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Total Volatile Base Nitrogen (T-VBN)
2.3. Texture Profile Analysis (TPA)
2.4. Water Holding Capacity (WHC)
2.5. Scanning Electron Microscope (SEM)
2.6. Low-Field-Nuclear Magnetic Resonance (LF-NMR)
2.7. Total Sulfhydryl Content
2.8. Volatile Flavor
2.9. Electronic Nose
2.10. Sodium Dodecyl Sulfate-Polyacrylamide Electrophoresis (SDS-PAGE)
2.11. Raman Spectrum
2.12. Analysis of Fungal Microbial Community Succession during Fermentation
2.13. Statistics of Data
3. Results and Discussion
3.1. Physicochemical Properties
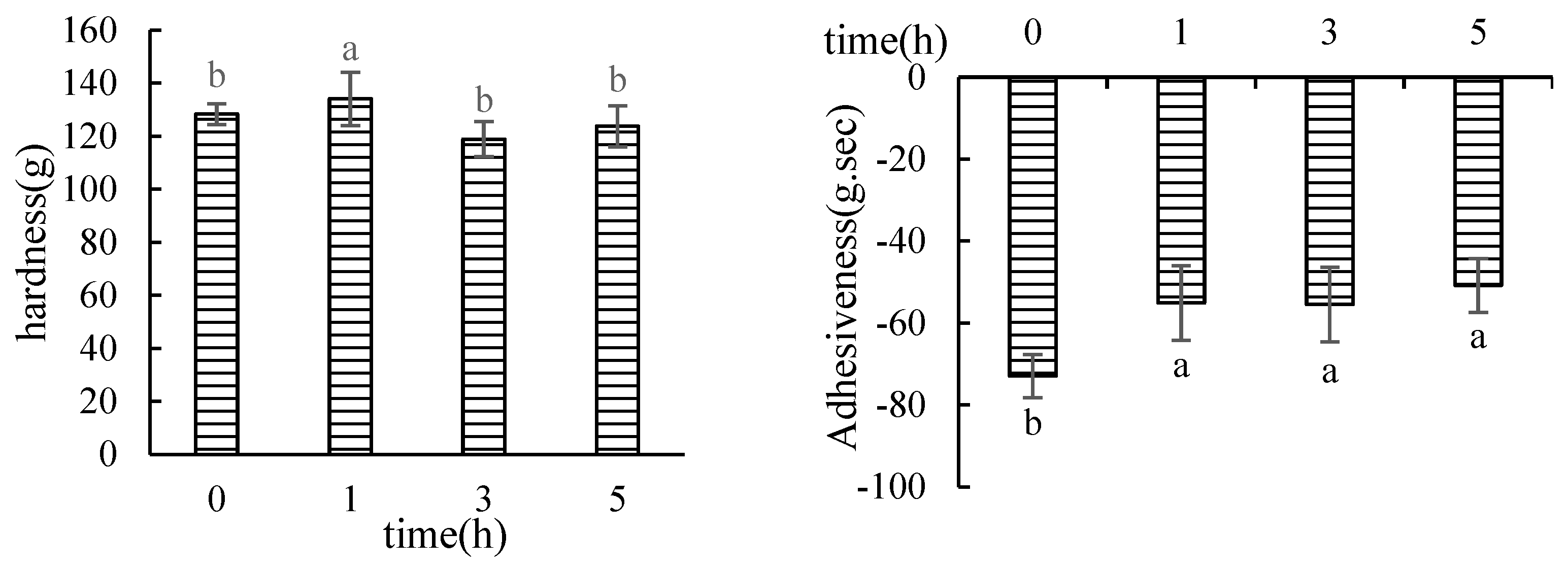
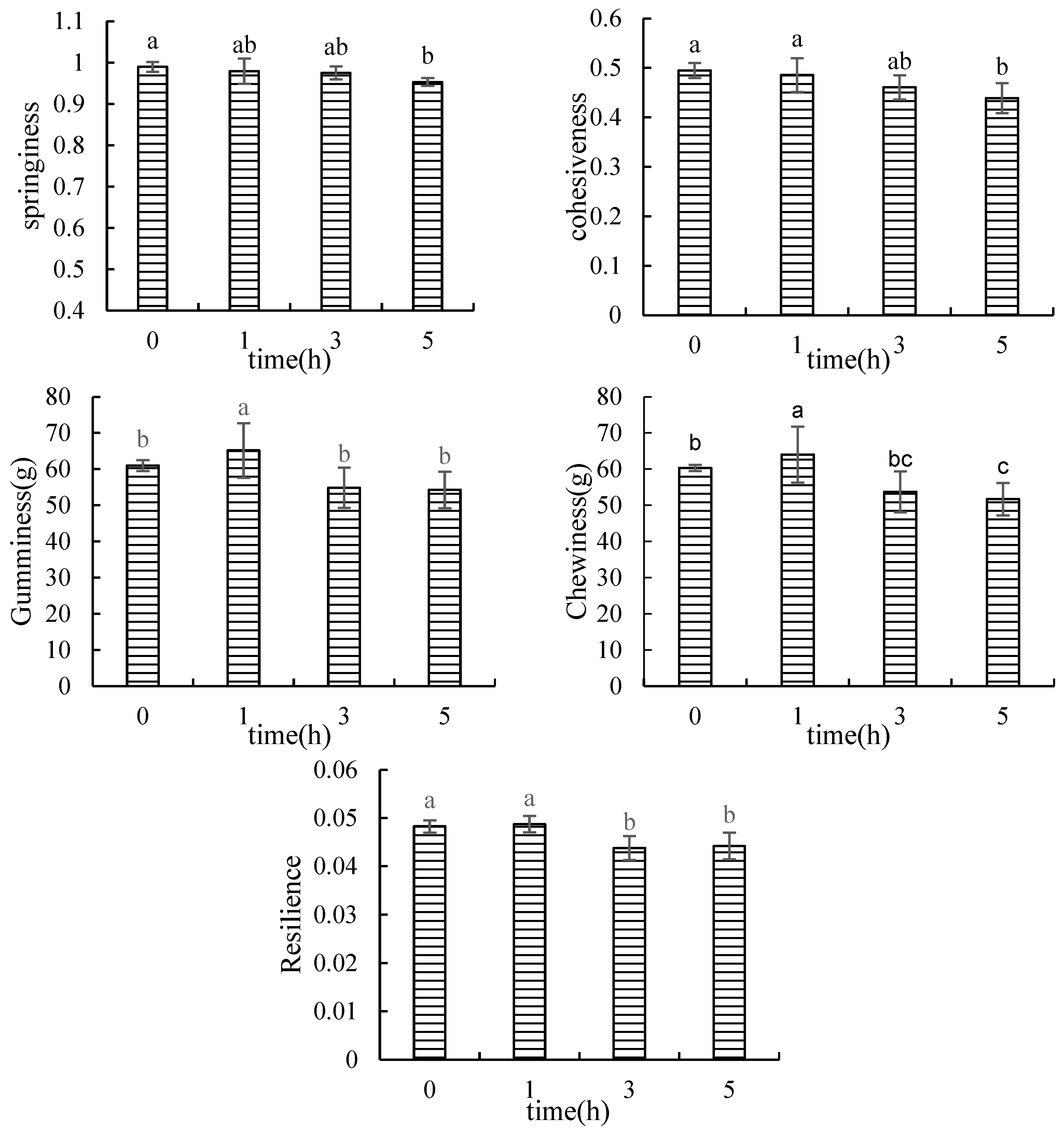
3.1.1. Texture Profile Analysis (TPA)
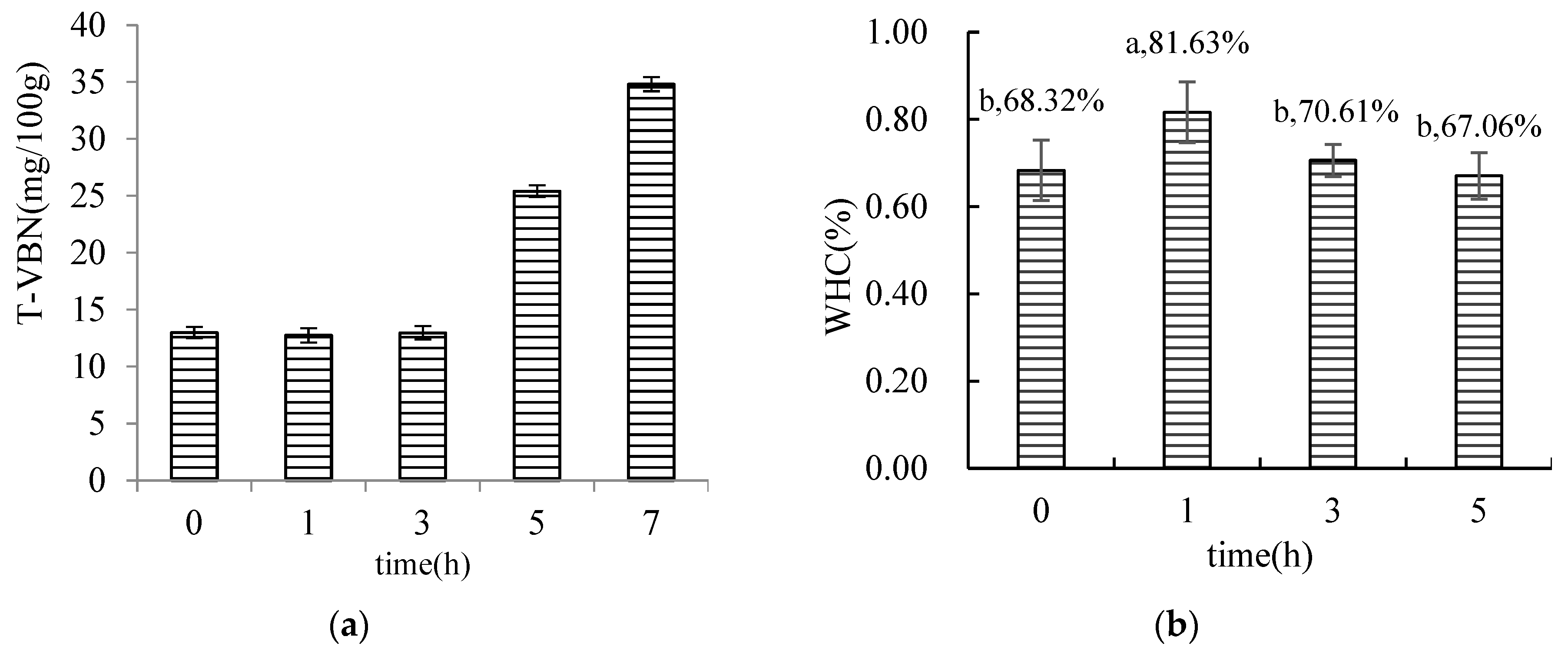
3.1.2. Results of the Determination of T-VBN in MFHS
3.1.3. Water Holding Capacity (WHC)
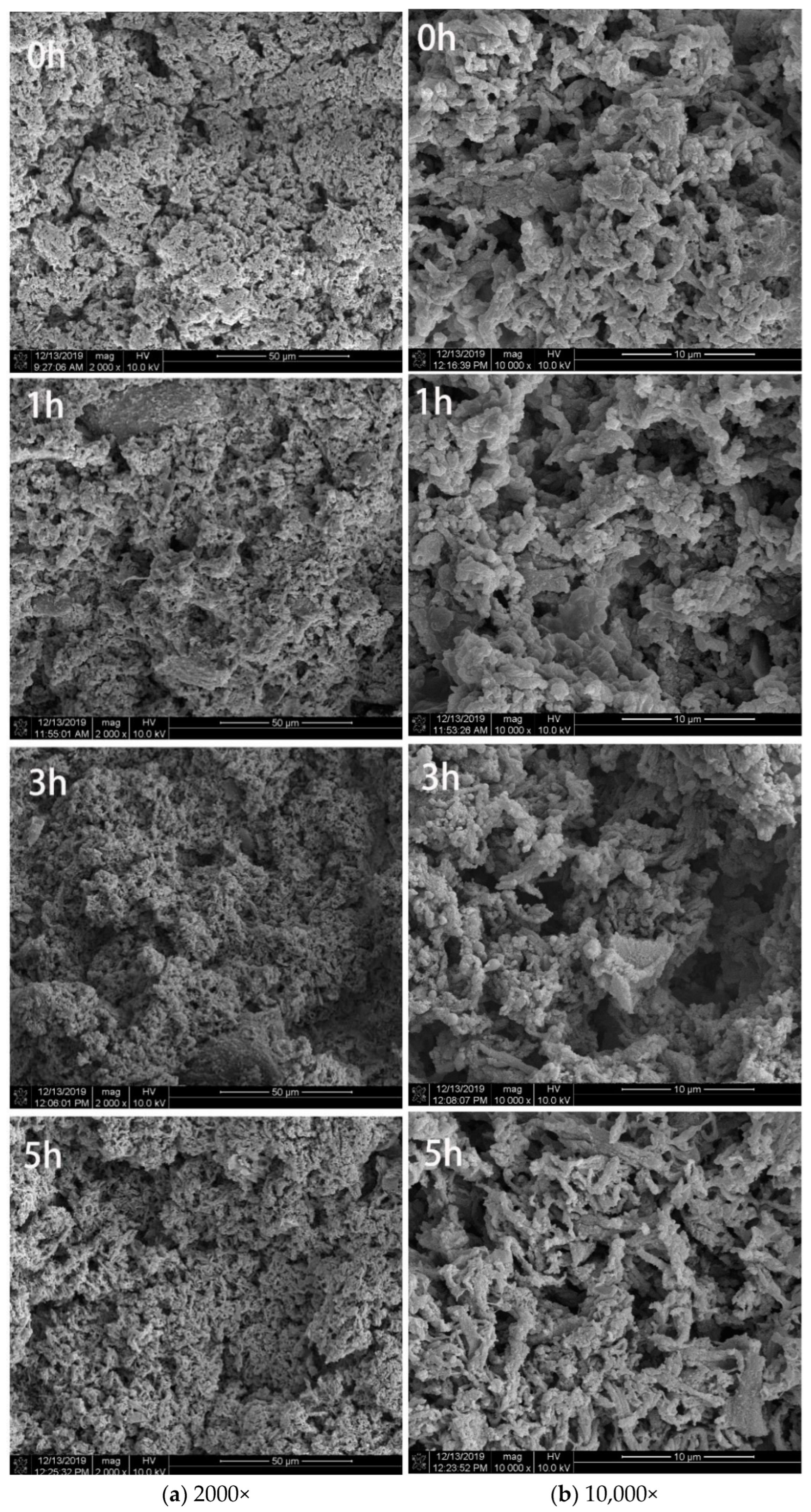
3.1.4. Microstructure Observation of Surimi Gel
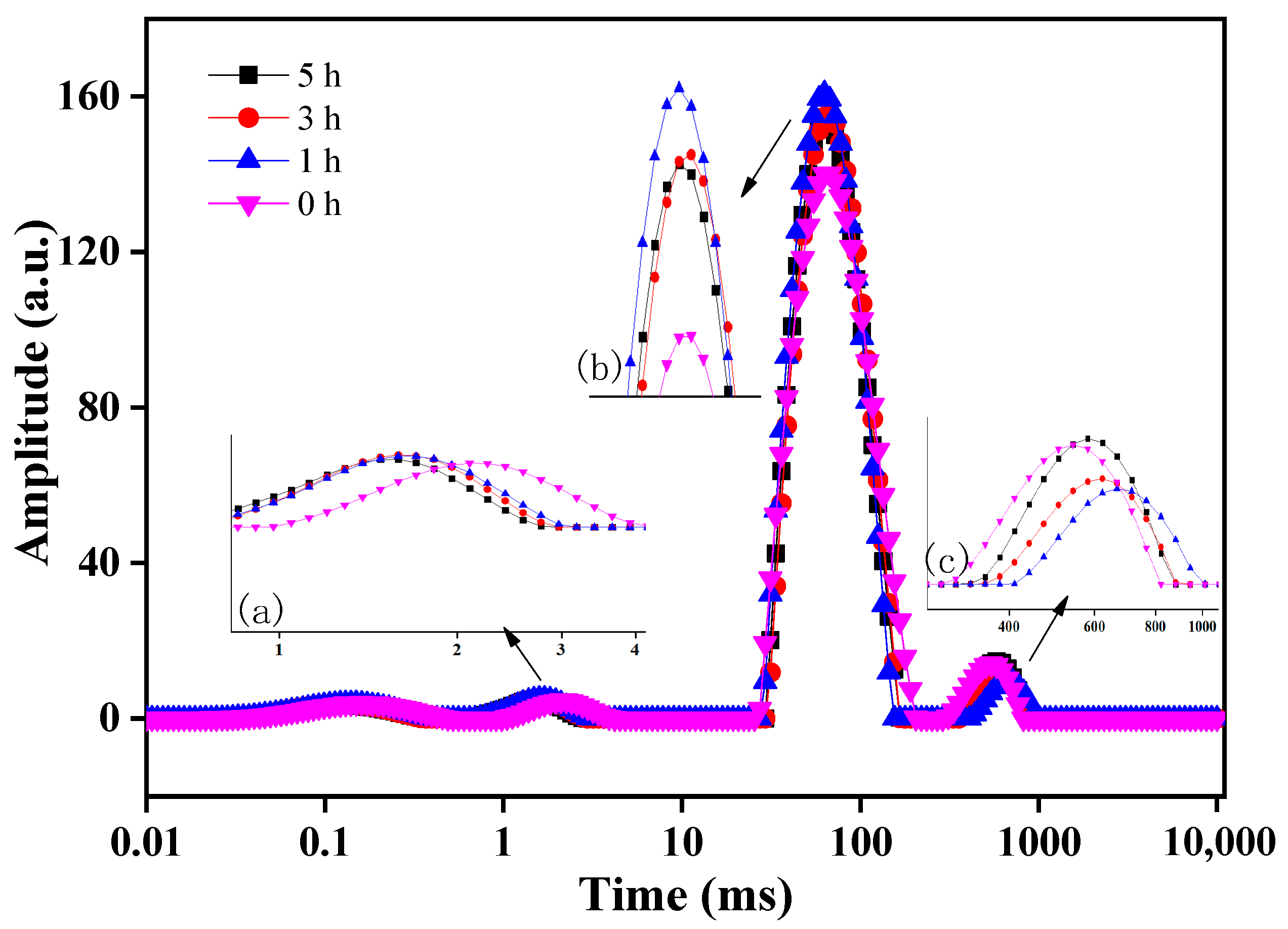
3.1.5. Determination of Water Distribution in Surimi Gel
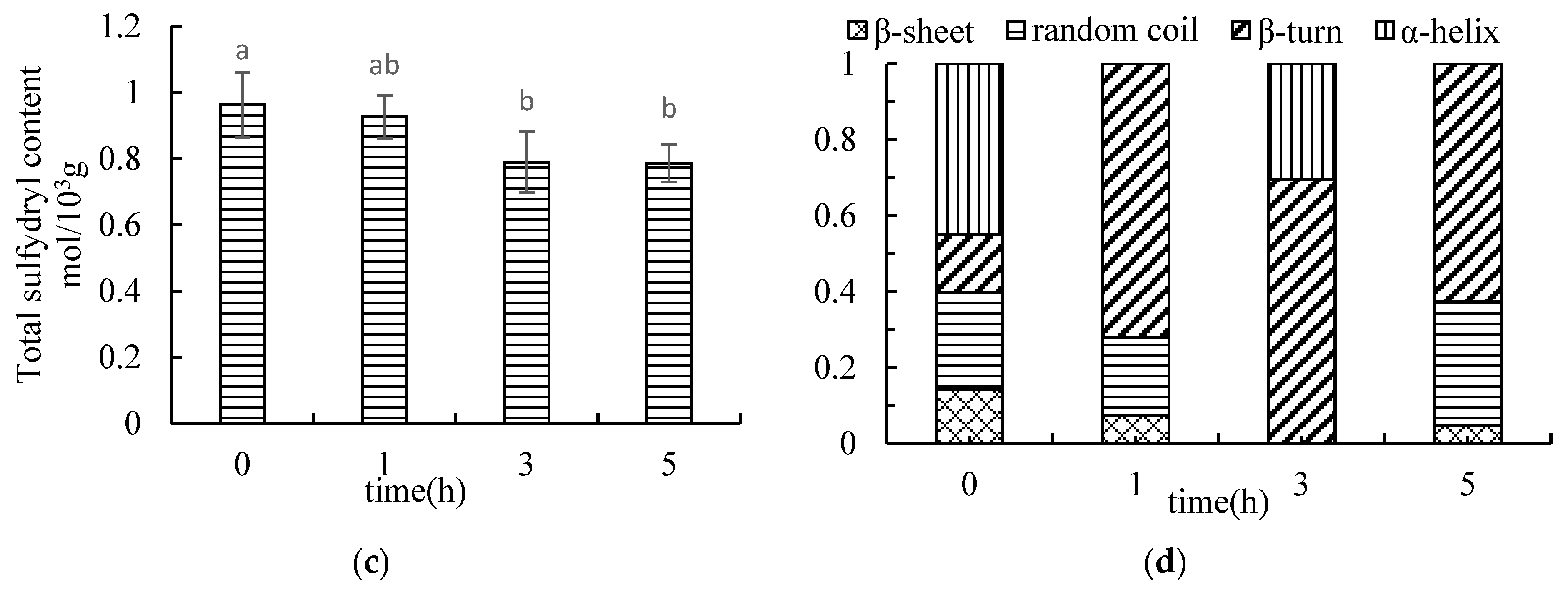
3.1.6. Determination of Total Sulfhydryl Content in MFHS
3.1.7. Determination Result of SDS-PAGE
3.1.8. Determination of Myosin Secondary Structure in Surimi
3.2. Determination of Volatile Substances
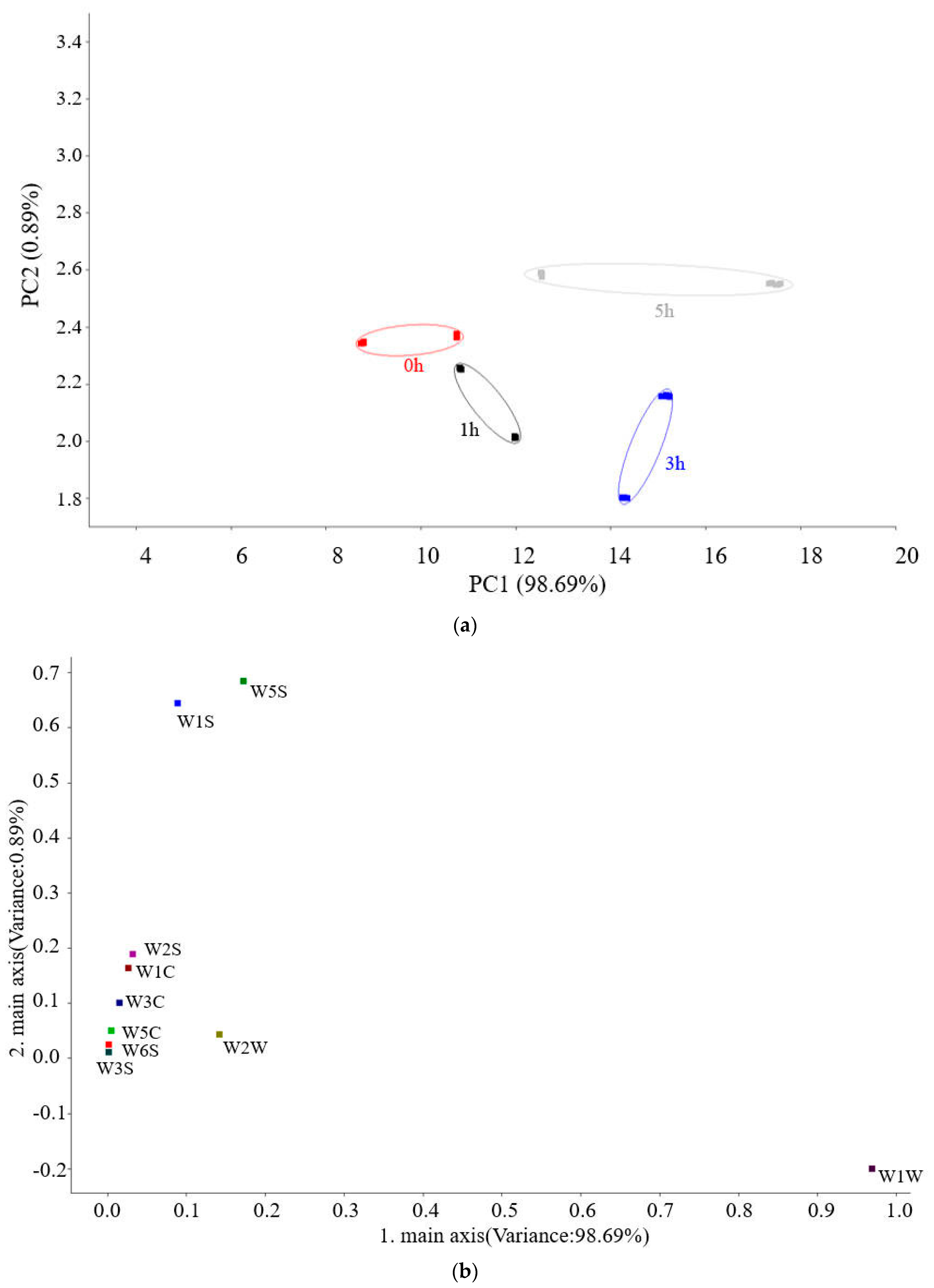
3.2.1. Determination of Volatile Substances in Surimi Gel by Electronic Nose
3.2.2. Results of GC-MS Measured Volatile Substances in Hairtail Surimi
3.3. Microbial Diversity
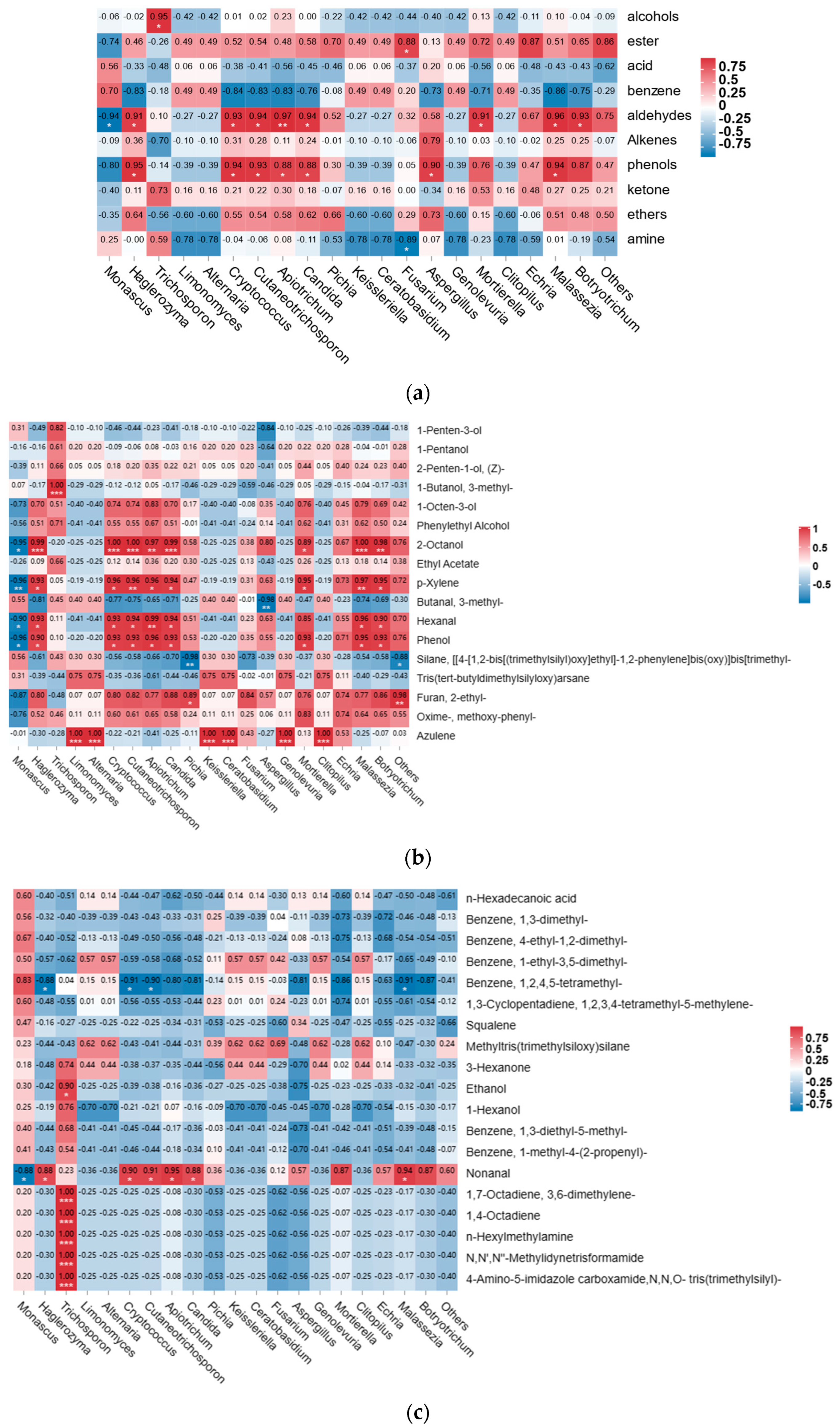
3.4. Correlation Analysis between Fungal Microbiota and Texture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2022. [Google Scholar]
- Somjid, P.; Panpipat, W.; Cheong, L.-Z.; Chaijan, M. Reduced Washing Cycle for Sustainable Mackerel (Rastrelliger Kanagurta) Surimi Production: Evaluation of Bio-Physico-Chemical, Rheological, and Gel-Forming Properties. Foods 2021, 10, 2717. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, H.; Zhu, S.; Xu, X.; Lyu, F.; Ding, Y. Textural, Rheological and Chemical Properties of Surimi Nutritionally-Enhanced with Lecithin. Food Sci. Technol. 2020, 122, 108984. [Google Scholar] [CrossRef]
- Yingchutrakul, M.; Wasinnitiwong, N.; Benjakul, S.; Singh, A.; Zheng, Y.; Mubango, E.; Luo, Y.; Tan, Y.; Hong, H. Asian Carp, an Alternative Material for Surimi Production: Progress and Future. Foods 2022, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2020. [Google Scholar]
- Fernandes, P.A.R.; Moreira, S.A.; Fidalgo, L.G.; Santos, M.D.; Queirós, R.P.; Delgadillo, I.; Saraiva, J.A. Food Preservation under Pressure (Hyperbaric Storage) as a Possible Improvement/Alternative to Refrigeration. Food Eng. Rev. 2015, 7, 1–10. [Google Scholar] [CrossRef]
- Guan, W.; Ren, X.; Li, Y.; Mao, L. The Beneficial Effects of Grape Seed, Sage and Oregano Extracts on the Quality and Volatile Flavor Component of Hairtail Fish Balls during Cold Storage at 4 °C. Food Sci. Technol. 2019, 101, 25–31. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, R.; Rong, J.; Xiong, S. Heat-Induced Denaturation and Aggregation of Actomyosin and Myosin from Yellowcheek Carp during Setting. Food Chem. 2014, 149, 237–243. [Google Scholar] [CrossRef]
- Anwar, J.; Shafique, U.; Waheed-uz-Zaman; Rehman, R.; Salman, M.; Dar, A.; Anzano, J.M.; Ashraf, U.; Ashraf, S. Microwave Chemistry: Effect of Ions on Dielectric Heating in Microwave Ovens. Arab. J. Chem. 2015, 8, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xue, Y.; Xu, J.; Li, Z.; Xue, C. Effects of High-Temperature Treatment (≥100 °C) on Alaska Pollock (Theragra Chalcogramma) Surimi Gels. J. Food Eng. 2013, 115, 115–120. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, A.; Yang, R.; Jia, R.; Zhang, J.; Xu, D.; Yang, W. Chemical Interactions and Rheological Properties of Hairtail (Trichiurus Haumela) Surimi: Effects of Chopping and Pressure. Food Biosci. 2020, 38, 100781. [Google Scholar] [CrossRef]
- Lee, N.-H.; Takeuchi, A.; Konno, K. Inhibition of Transglutaminase and Microbial Transglutaminase Activity by Garlic. Food Sci. Biotechnol. 2007, 16, 223–227. [Google Scholar]
- Liu, Q.; Lin, Z.; Chen, X.; Chen, J.; Wu, J.; Chen, H.; Zeng, X. Characterization of Structures and Gel Properties of Ultra-High-Pressure Treated-Myofibrillar Protein Extracted from Mud Carp (Cirrhinus Molitorella) and Quality Characteristics of Heat-Induced Sausage Products. Food Sci. Technol. 2022, 165, 113691. [Google Scholar] [CrossRef]
- Uzzan, M.; Kesselman, E.; Ramon, O.; Kopelman, I.J.; Mizrahi, S. Mechanism of Textural Changes Induced by Microwave Reheating of a Surimi Shrimp Imitation. J. Food Eng. 2006, 74, 279–284. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Tang, X.; Chen, Y.; You, Y. Chemical Forces and Water Holding Capacity Study of Heat-Induced Myofibrillar Protein Gel as Affected by High Pressure. Food Chem. 2015, 188, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, W.; Yuan, C.; Morioka, K.; Chen, S.; Liu, D.; Ye, X. Enhancement of the Gelation Properties of Hairtail (Trichiurus Haumela) Muscle Protein with Curdlan and Transglutaminase. Food Chem. 2015, 176, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shao, Y.; Wu, C.; Yuan, C.; Ishimura, G.; Liu, W.; Chen, S. Gamma-PGA and MTGase Improve the Formation of Epsilon-(Gamma-Glutamyl) Lysine Cross-Links within Hairtail (Trichiurus Haumela) Surimi Protein. Food Chem. 2018, 242, 330–337. [Google Scholar] [CrossRef]
- Wasinnitiwong, N.; Benjakul, S.; Hong, H. Effects of Kappa-Carrageenan on Gel Quality of Threadfin Bream (Nemipterus spp.) Surimi Containing Salted Duck Egg White Powder. Int. J. Biol. Macromol. 2022, 221, 61–70. [Google Scholar] [CrossRef]
- Yoon, W.B.; Park, J.W.; Jung, H. Effects of Potato Protein Isolated Using Ethanol on the Gelation and Anti-Proteolytic Properties in Pacific Whiting Surimi. Foods 2022, 11, 3114. [Google Scholar] [CrossRef]
- Hoque, M.d.S.; Benjakul, S.; Prodpran, T. Effects of Partial Hydrolysis and Plasticizer Content on the Properties of Film from Cuttlefish (Sepia Pharaonis) Skin Gelatin. Food Hydrocoll. 2011, 25, 82–90. [Google Scholar] [CrossRef]
- Hossain, M.; Morioka, K.; Shikha, F.; Itoh, Y. Effect of Preheating Temperature on the Microstructure of Walleye Pollack Surimi Gels under the Inhibition of the Polymerisation and Degradation of Myosin Heavy Chain. J. Sci. Food Agric. 2011, 91, 247–252. [Google Scholar] [CrossRef]
- Kakatkar, A.; Sharma, A.; Venugopal, V. Hydration of Muscle Proteins of Bombay Duck (Harpodon Nehereus) during Acetic Acid-Induced Gelation and Characteristics of the Gel Dispersion. Food Chem. 2003, 83, 99–106. [Google Scholar] [CrossRef]
- Ngapo, T.M.; Wilkinson, B.H.P.; Chong, R. The Unexpected Behaviour of 1,5-Glucono-δ-Lactone-Induced Myosin Gels upon Dialysis. Food Chem. 1996, 55, 271–279. [Google Scholar] [CrossRef]
- Riebroy, S.; Benjakul, S.; Visessanguan, W. Properties and Acceptability of Som-Fug, a Thai Fermented Fish Mince, Inoculated with Lactic Acid Bacteria Starters. Food Sci. Technol. 2008, 41, 569–580. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Wang, Y.; Li, L.; Li, C.; Zhao, Y.; Yang, S. Contribution of Autochthonous Microbiota Succession to Flavor Formation during Chinese Fermented Mandarin Fish (Siniperca Chuatsi). Food Chem. 2021, 348, 129107. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.J.G.; Quanyou, L.Z.Y. Microbial Community Diversity Analysis during Spontaneous Fermentation of Aromatic Lees Made from Huang Jiu (Yellow Rice Wine) Lees by High-Throughput Sequencing. Food Sci. 2023, 44. [Google Scholar]
- Gao, P.; Wang, W.; Jiang, Q.; Xu, Y.; Xia, W. Effect of Autochthonous Starter Cultures on the Volatile Flavour Compounds of Chinese Traditional Fermented Fish (Suan Yu). Int. J. Food Sci. Technol. 2016, 51, 1630–1637. [Google Scholar] [CrossRef]
- Sheng-Lang, Y.-X.W.J. Effect of Ultra High Pressure on the Changes of Bacterial Community Structure and Quality of Stinky Mandarin Fish. Food Sci. 2022, 43, 81–87. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, W.; Yang, F.; Kim, J.; Nie, X. Effect of Fermentation Temperature on the Microbial and Physicochemical Properties of Silver Carp Sausages Inoculated with Pediococcus Pentosaceus. Food Chem. 2010, 118, 512–518. [Google Scholar] [CrossRef]
- Nie, X.; Lin, S.; Zhang, Q. Proteolytic Characterisation in Grass Carp Sausage Inoculated with Lactobacillus Plantarum and Pediococcus Pentosaceus. Food Chem. 2014, 145, 840–844. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Li, C.; Li, L.; Yang, X.; Wu, Y.; Chen, S.; Zhao, Y. Novel Insight into Physicochemical and Flavor Formation in Naturally Fermented Tilapia Sausage Based on Microbial Metabolic Network. Food Res. Int. 2021, 141, 110122. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Zhang, M.-L.; Li, Z.-H.; Zhang, J.; He, L.; Hou, F. Fermentation of Bighead Carp (Aristichthys Nobilis) Surimi and the Characteristics of Fermented Bighead Carp Surimi Products. J. Sci. Food Agriculture 2009, 89, 511–516. [Google Scholar] [CrossRef]
- Yoshioka, M.; Matsuo, Y.; Nemoto, Y.; Ogushi, M.; Onodera, M.; Yoshie-Stark, Y. Development of Fermented Surimi Products Using Simulated Tofuyo Processing: Associated Changes in Chemical Composition, Antioxidant Activities and Angiotensin-Converting Enzyme Inhibition. Fish. Sci. 2020, 86, 215–229. [Google Scholar] [CrossRef]
- Zhao, D.; Lyu, F.; Gu, S.; Ding, Y.; Zhou, X. Physicochemical Characteristics, Protein Hydrolysis and Textual Properties of Surimi during Fermentation with Actinomucor Elegans. Int. J. Food Prop. 2017, 20, 538–548. [Google Scholar] [CrossRef] [Green Version]
- Suryaningrum, T.D.; Ikasari, D. Syamdidi Nutrition and Sensory Evaluation on Corned Fish from Mackerel Tuna (Euthynus sp.) Processed with Red Fermented Rice and Nitrite Salt. In Proceedings of the 3rd International Symposium on Marine and Fisheries Research (3rd Ismfr); Isnansetyo, A., Jayanti, A.D., Sari, D.W.K., Puspita, I.D., Putra, M.M.P., Huda, N., Bhujel, R.C., Adharini, R.I., Eds.; EDP Sciences: Cedex A, Côte d’Ivoire, 2020; Volume 147, p. 03002. [Google Scholar]
- Luo, H.; Guo, C.; Lin, L.; Si, Y.; Gao, X.; Xu, D.; Jia, R.; Yang, W. Combined Use of Rheology, LF-NMR, and MRI for Characterizing the Gel Properties of Hairtail Surimi with Potato Starch. Food Bioprocess Technol. 2020, 13, 637–647. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Liu, X.; Qiao, M.; Yi, S.; Li, X.; Li, J. Effects of Chickpea and Peanut Protein Isolates on the Gelling Properties of Hairtail (Trichiurus Haumela) Myosin. Food Sci. Technol. 2022, 163, 113562. [Google Scholar] [CrossRef]
- Tian, Z.; Ameer, K.; Shi, Y.; Yi, J.; Zhu, J.; Kang, Q.; Lu, J.; Zhao, C. Characterization of Physicochemical Properties, Microbial Diversity and Volatile Compounds of Traditional Fermented Soybean Paste in Henan Province of China. Food Biosci. 2022, 50, 102045. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H. Effects of calcium ion on gel properties and gelation of tilapia (Oreochromis niloticus) protein isolates processed with pH shift method. Food Chem. 2019, 277, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Nannan, Y.; Xu, Y.; Jiang, Q.; Xia, W. Molecular Forces Involved in Heat-Induced Freshwater Surimi Gel: Effects of Various Bond Disrupting Agents on the Gel Properties and Protein Conformation Changes. Food Hydrocoll. 2017, 69, 193–201. [Google Scholar] [CrossRef]
- ZHANG Da-wei, Z.J. Optimization of Process Conditions of Fermented Golden Pomfret Surimi Production and Analysis of Quality Changes in Fermentation in Process. Mod. Food Technol. 2020, 36, 211–218. [Google Scholar] [CrossRef]
- Gassem, M.A. Microbiological and Chemical Quality of a Traditional Salted-Fermented Fish (Hout-Kasef) Product of Jazan Region, Saudi Arabia. Saudi J. Biol. Sci. 2019, 26, 137–140. [Google Scholar] [CrossRef]
- Anggo, A.; Ma’ruf, W.; Swastawati, F.; Rianingsih, L. Changes of Amino and Fatty Acids in Anchovy (Stolephorus sp.) Fermented Fish Paste with Different Fermentation Periods. Procedia Environ. Sci. 2015, 23, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; He, Y.; Yan, Y.; Xie, N.; Song, Y.; Yan, X.; Ding, Z. Textural Properties of Stinky Mandarin Fish (Siniperca Chuatsi) during Fermentation: Effects of the State of Moisture. Int. J. Food Prop. 2017, 20, 1530–1538. [Google Scholar] [CrossRef] [Green Version]
- Ji, C.; Hu, X.; Ji, H.; Chen, S.; Li, L. Effects of Porphyra Yezoensis Polysaccharides on Gel Characteristics and Antioxidant Activity of Hypophthalmichthys Molitrix Surimi. Food Ferment. Ind. 2022, 48, 144–152. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S. Improvement of Gel Properties of Sardine (Sardinella Albella) Surimi Using Coconut Husk Extracts. Food Hydrocoll. 2015, 51, 146–155. [Google Scholar] [CrossRef]
- Wang, L.; Yang, K.; Wang, X.; Wu, D.; You, X.; Ma, J.; Zhang, Y.; Xiong, G.; Wang, L.; Sun, W. Gel Properties and Thermal Gelling Mechanism in Myofibrillar Protein of Grass Carp (Ctenopharyngodon Idellus) under the Synergistic Effects of Radio Frequency Combined with Magnetic Field. J. Food Sci. 2022, 87, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- ZHANG, L.; Zeng, J.; Gao, H.; ZHANG, K.; WANG, M. Effects of Different Frozen Storage Conditions on the Functional Properties of Wheat Gluten Protein in Nonfermented Dough. Food Sci. Technol. 2022, 42, e97821. [Google Scholar] [CrossRef]
- Dingjin, L.I.; Zhenhua, D.; Yan, L.I.U.; Qiuxia, D.; Shoulin, Y.E.; Xianghao, Z.H.U.; Yuxia, Y. Variation in Water Content during Vacuum Microwave Drying of Flavored Yam Chips Process Analyzed by Low-Field Nuclear Magnetic Resonance Imaging. Food Sci. 2019, 40, 116. [Google Scholar] [CrossRef]
- Soottawat, B.; Thomas, A.S.; Michael, T.M.; Haejung, A. Physicochemical Changes in Pacific Whiting Muscle Proteins during Iced Storage. Food Sci. 1997, 62, 729–733. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Riebroy, S.; Ishizaki, S.; Tanaka, M. Gel-forming Properties of Surimi Produced from Bigeye Snapper, Priacanthus Tayenus and P Macracanthus, Stored in Ice. J. Sci. Food Agric. 2002, 82, 1442–1451. [Google Scholar] [CrossRef]
- Riebroy, S.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effect of Iced Storage of Bigeye Snapper (Priacanthus Tayenus) on the Chemical Composition, Properties and Acceptability of Som-Fug, a Fermented Thai Fish Mince. Food Chem. 2007, 102, 270–280. [Google Scholar] [CrossRef]
- Chanphai, P.; Tajmir-Riahi, H.A. Tea Polyphenols Bind Serum Albumins:A Potential Application for Polyphenol Delivery. Food Hydrocoll. 2019, 89, 461–467. [Google Scholar] [CrossRef]
- Qiu, L.; Xing, Q.; Deng, Z.; Zheng, L. The Interaction of Plant Polyphenols with Silver Carp Myosin and Its Effects on the Structure and Gel Formation of Myofibrillar Protein. J. Chin. Inst. Food Sci. Technol. 2021, 21, 48–56. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat Flavor Precursors and Factors Influencing Flavor Precursors—A Systematic Review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Wu, N.; Wang, X.-C. Identification of Important Odorants Derived from Phosphatidylethanolamine Species in Steamed Male Eriocheir Sinensis Hepatopancreas in Model Systems. Food Chem. 2019, 286, 491–499. [Google Scholar] [CrossRef]
- Zeng, X.; Xia, W.; Jiang, Q.; Xu, Y.; Fan, J. Contribution of Mixed Starter Cultures to Flavor Profile of Suanyu—A Traditional Chinese Low-Salt Fermented Whole Fish. J. Food Process. Preserv. 2017, 41, e13131. [Google Scholar] [CrossRef]
- Zhou, X.; Chong, Y.; Ding, Y.; Gu, S.; Liu, L. Determination of the Effects of Different Washing Processes on Aroma Characteristics in Silver Carp Mince by MMSE–GC–MS, e-Nose and Sensory Evaluation. Food Chem. 2016, 207, 205–213. [Google Scholar] [CrossRef]
- Tian, D.; Jiao, H.; Tao, W.; Bi, L.; Zhou, Y. Analysis of Volatile Flavor Components of Five Kinds of Marine Fish. Food Ferment. Ind. 2015, 41, 155–159. [Google Scholar] [CrossRef]
- Hu, M.; Wang, S.; Liu, Q.; Cao, R.; Xue, Y. Flavor Profile of Dried Shrimp at Different Processing Stages. LWT 2021, 146, 111403. [Google Scholar] [CrossRef]
- Wu, T.; Zhan, P.; Wang, P. Inhibitory Effect of Perilla Frutescens Leaves on Fishy Smell Based on GC-O-MS and Chemometrics. Food Res. Dev. 2022, 43, 9–18. [Google Scholar]
- Alasalvar, C.; Taylor, K.D.A.; Shahidi, F. Comparison of Volatiles of Cultured and Wild Sea Bream (Sparus Aurata) during Storage in Ice by Dynamic Headspace Analysis/Gas Chromatography−Mass Spectrometry. J. Agric. Food Chem. 2005, 53, 2616–2622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lee, T. Gas Chromatography—Mass Spectrometry Analysis of Volatile Flavor Compounds in Mackerel for Assessment of Fish Quality. In Proceedings of the Flavor and Lipid Chemistry of Seafoods, Orlando, FL, USA, 25 August 1996. [Google Scholar]
- Yi, W.; YanQun, X.; Kun, X.; ZiSheng, L. The variation of fishy odor at the different processing stage of soft-shelled turtle by HS-SPME-GC-MS. Food Ferment. Ind. 2016, 42, 189–193. [Google Scholar]
- Luo, J.; Li, M.; Zhang, Y.; Liu, Y.; Guan, Z. Effect of different drying methods on volatile components of tilapia fillets analyzed by electronic nose combined with GC-MS. South China Fish. Sci. 2022, 18, 135–143. [Google Scholar]
- Benkerroum, N. Biogenic Amines in Dairy Products: Origin, Incidence, and Control Means. Compr. Rev. Food Ence Food Saf. 2016, 15, 801–826. [Google Scholar] [CrossRef] [PubMed]


| Number | Compounds | CAS | Relative Content/% | ||||
|---|---|---|---|---|---|---|---|
| Blank | 0 h | 1 h | 3 h | 5 h | |||
| 1 | Ethanol | 000064-17-5 | 2.64 | 2.04 | 3.48 | 6.57 | 12.06 |
| 2 | 1,3-Butanediol, (S)- | 024621-61-2 | nd | 0.39 | nd | nd | nd |
| 3 | 1-Penten-3-ol | 000616-25-1 | 2.2 | 2 | 2.71 | 3.26 | 4.13 |
| 4 | Piperidine-4-ol, 1,2,5-trimethyl-4-(2,4,5-trimethylphenyl)- | 1000259-69-6 | nd | 0.59 | nd | nd | nd |
| 5 | 1-Hexanol | 000111-27-3 | 1.52 | 1.38 | nd | 3 | 4.2 |
| 6 | 1H-Inden-5-ol, 2,3-dihydro- | 001470-94-6 | nd | 0.59 | nd | nd | nd |
| 7 | Ethanol, 2-(vinyloxy)- | 000764-48-7 | nd | 0.76 | nd | nd | nd |
| 8 | 2-[2-[2-[2-[2-(tert-Butyldimethylsilyloxy)ethoxy]ethoxy]ethoxy]ethoxy]ethanol | 1000352-10-3 | nd | 0.39 | nd | nd | 0.68 |
| 9 | 1-Pentanol | 000071-41-0 | 0.74 | nd | 0.97 | 0.94 | 1.33 |
| 10 | 2-Penten-1-ol, (Z)- | 001576-95-0 | 0.64 | nd | 0.55 | 0.53 | 0.89 |
| 11 | Ethanol, 2-[2-(2-butoxyethoxy)ethoxy]- | 000143-22-6 | nd | nd | 0.54 | nd | nd |
| 12 | (3-Methyl-oxiran-2-yl)-methanol | 1000194-22-9 | nd | nd | nd | 0.75 | nd |
| 13 | Silanol, trimethyl- | 001066-40-6 | nd | nd | nd | nd | 1.07 |
| 14 | 1-Butanol, 3-methyl- | 000123-51-3 | 1.18 | nd | nd | nd | 8.79 |
| 15 | 2-Octanol | 000123-96-6 | nd | nd | nd | nd | 1.01 |
| 16 | 1-Octen-3-ol | 003391-86-4 | 3.81 | nd | nd | nd | 2.89 |
| 17 | Phenylethyl Alcohol | 000060-12-8 | 1.49 | nd | nd | nd | 1.67 |
| 18 | 2-Octanol | 000123-96-6 | 2.27 | nd | nd | nd | nd |
| 19 | 2-Octen-1-ol, (E)- | 018409-17-1 | 0.7 | nd | nd | nd | nd |
| 20 | Cyclobutaneethanol, .beta.-methylene- | 116203-80-6 | 1.18 | nd | nd | nd | nd |
| 21 | Ethyl Acetate | 000141-78-6 | 2.33 | 0.63 | 1.63 | 2.53 | 3.15 |
| 22 | 2-Ethyl-1-hexanol, pentafluoropropionate | 1000365-51-1 | nd | 1.44 | nd | nd | nd |
| 23 | 2-Ethyl-1-hexanol, trifluoroacetate | 1000365-19-6 | 0.77 | nd | nd | nd | 3.05 |
| 24 | Formic acid, hex-2-yl ester | 1000368-21-9 | nd | nd | 1.07 | nd | nd |
| 25 | Arsenous acid, tris(trimethylsilyl) ester | 055429-29-3 | nd | nd | 2.06 | nd | nd |
| 26 | Hexyl chloroformate | 006092-54-2 | nd | nd | 2.01 | nd | nd |
| 27 | 2-Ethyl-1-hexanol, pentafluoropropionate | 1000365-51-1 | nd | nd | 2.58 | nd | nd |
| 28 | 3-Hydroxymandelic acid, ethyl ester, di-TMS | 1000071-88-9 | nd | nd | 1.27 | nd | nd |
| 29 | Thiocyanic acid carbazol-3,6-diyl ester | 040736-18-3 | 1.39 | nd | 0.98 | 0.98 | nd |
| 30 | Silanol, trimethyl-, propanoate | 016844-98-7 | nd | nd | 0.75 | 0.74 | nd |
| 31 | Propanoic acid, pentyl ester | 000624-54-4 | nd | nd | nd | 2.83 | nd |
| 32 | 1-Octyl trifluoroacetate | 002561-21-9 | nd | nd | nd | 0.69 | nd |
| 33 | Arsenous acid, tris(trimethylsilyl) ester | 055429-29-3 | 5.95 | nd | nd | 1.2 | nd |
| 34 | Dicyclopropylmethanol, chlorodifluoroacetate | 1000376-25-1 | 0.84 | nd | nd | nd | nd |
| 35 | Formic acid, heptyl ester | 000112-23-2 | 1.41 | nd | nd | nd | nd |
| 36 | .gamma.-Guanidinobutyric acid | 000463-00-3 | nd | 0.54 | nd | nd | nd |
| 37 | Pentanoic acid, 3-methyl- | 000105-43-1 | nd | 2.84 | nd | nd | nd |
| 38 | Octanoic acid | 000124-07-2 | nd | 1.67 | nd | nd | nd |
| 39 | n-Hexadecanoic acid | 000057-10-3 | nd | 18.4 | 8.35 | 5.54 | nd |
| 40 | 19,19-Dimethyl-eicosa-8,11-dienoic acid | 1000297-01-9 | nd | nd | 0.38 | nd | nd |
| 41 | cis-5,8,11,14,17-Eicosapentaenoic acid | 010417-94-4 | 0.54 | nd | nd | nd | nd |
| 42 | o-Xylene | 000095-47-6 | nd | 0.34 | nd | nd | nd |
| 43 | p-Xylene | 000106-42-3 | 14.14 | nd | 2.28 | 0.51 | 4.28 |
| 44 | Benzene, 1,3-dimethyl- | 000108-38-3 | nd | 1.49 | nd | 2.46 | nd |
| 45 | Benzene, 1,1′-(oxydi-2,1-ethanediyl)bis[3-ethyl- | 055044-09-2 | nd | 2.44 | nd | nd | nd |
| 46 | Benzene, 4-ethyl-1,2-dimethyl- | 000934-80-5 | nd | 4.51 | 1.24 | 2.74 | nd |
| 47 | Benzene, 1-ethyl-2,3-dimethyl- | 000933-98-2 | nd | 1.47 | 2.56 | nd | 5.44 |
| 48 | Benzene, 1-ethyl-3,5-dimethyl- | 000934-74-7 | nd | 1.21 | 2.03 | 1.89 | nd |
| 49 | Benzene, 2-ethyl-1,3-dimethyl- | 002870-04-4 | nd | nd | 2.59 | nd | nd |
| 50 | Benzene, 2-ethyl-1,4-dimethyl- | 001758-88-9 | nd | nd | nd | nd | 0.76 |
| 51 | Benzene, 2,4-diethyl-1-methyl- | 001758-85-6 | nd | nd | 1.27 | nd | nd |
| 52 | Benzene, 1,2,4,5-tetramethyl- | 000095-93-2 | nd | 9.63 | 11.48 | 17.22 | 10.62 |
| 53 | Benzene, 1,2,3,5-tetramethyl- | 000527-53-7 | nd | 3.86 | nd | 6.41 | nd |
| 54 | Benzene, 1,2,3,4-tetramethyl- | 000488-23-3 | nd | nd | 8.84 | nd | nd |
| 55 | Toluene | 000108-88-3 | nd | nd | 0.87 | nd | nd |
| 56 | Benzene, 1,3-diethyl-5-methyl- | 002050-24-0 | nd | nd | nd | 1.6 | 1.86 |
| 57 | Benzene, 1-methyl-4-(2-propenyl)- | 003333-13-9 | nd | nd | nd | 1.24 | 1.09 |
| 58 | Butanal, 3-methyl- | 000590-86-3 | nd | 0.53 | 2 | 1.87 | 2.14 |
| 59 | Hexanal | 000066-25-1 | 9.25 | 3.97 | 3.79 | 4.58 | 5.66 |
| 60 | Benzaldehyde, 2,4-dimethyl- | 015764-16-6 | nd | 0.69 | nd | nd | nd |
| 61 | Benzaldehyde | 000100-52-7 | 0.64 | 0.48 | 1.5 | 0.98 | 1.33 |
| 62 | Nonanal | 000124-19-6 | 1.66 | nd | nd | nd | 0.71 |
| 63 | Pentanal | 000110-62-3 | 1.05 | nd | nd | nd | nd |
| 64 | Heptanal | 000111-71-7 | 4.42 | nd | nd | nd | nd |
| 65 | 1,3-Cyclopentadiene, 1,2,3,4-tetramethyl-5-methylene- | 076089-59-3 | nd | 3.09 | 2.11 | 5.17 | nd |
| 66 | Squalene | 000111-02-4 | nd | 12.09 | nd | nd | nd |
| 67 | 2-Pentene | 000109-68-2 | nd | nd | 4.42 | nd | nd |
| 68 | 1,3,6-Octatriene, (E,E)- | 022038-69-3 | nd | nd | 0.49 | nd | nd |
| 69 | 1-Methylcycloheptene | 055308-20-8 | nd | nd | nd | 0.5 | nd |
| 70 | 1,7-Octadiene, 3,6-dimethylene- | 003382-59-0 | nd | nd | nd | nd | 0.94 |
| 71 | 1,4-Octadiene | 005675-25-2 | nd | nd | nd | nd | 0.57 |
| 72 | 2,4-Octadiene | 013643-08-8 | 3.86 | nd | nd | nd | nd |
| 73 | 3,5-Octadiene, (Z,Z)- | 007348-80-3 | 1.47 | nd | nd | nd | nd |
| 74 | Cyclooctene, 3-ethenyl- | 002213-60-7 | 0.85 | nd | nd | nd | nd |
| 75 | 1,4-Cyclooctadiene | 001073-07-0 | 0.52 | nd | nd | nd | nd |
| 76 | 2-Hexene, 3,5,5-trimethyl- | 026456-76-8 | 3.85 | nd | nd | nd | nd |
| 77 | 4-Aminophenol, N,O-bis(pentafluoropropionyl)- | 1000364-80-0 | nd | 0.12 | nd | nd | nd |
| 78 | 3-Aminophenol, N,O-bis(pentafluoropropionyl)- | 1000364-81-6 | nd | 0.81 | nd | nd | nd |
| 79 | Phenol | 000108-95-2 | 2.42 | 0.93 | 1.25 | 1.19 | 1.51 |
| 80 | p-Cresol | 000106-44-5 | 0.48 | nd | nd | nd | nd |
| 81 | Silane, [[4-[1,2-bis[(trimethylsilyl)oxy]ethyl]-1,2-phenylene]bis(oxy)]bis[trimethyl- | 056114-62-6 | nd | 0.48 | 0.41 | nd | 0.49 |
| 82 | Heptane, 2,6-dimethyl- | 001072-05-5 | nd | 0.75 | nd | nd | nd |
| 83 | 3,8-Dioxatricyclo[5.1.0.0(2,4)]octane, 4-ethenyl- | 053966-43-1 | nd | 0.46 | nd | nd | nd |
| 84 | Tris(tert-butyldimethylsilyloxy)arsane | 1000366-57-5 | nd | 1.14 | 1.52 | nd | nd |
| 85 | Methyltris(trimethylsiloxy)silane | 017928-28-8 | nd | nd | 1.77 | 1.76 | nd |
| 86 | Pentane, 1-chloro- | 000543-59-9 | nd | nd | nd | 5.6 | nd |
| 87 | Silane, chloroethylmethyl- | 006374-21-6 | nd | nd | nd | 0.79 | nd |
| 88 | 6,6-Diethylhoctadecane | 1000360-41-8 | 2.39 | nd | nd | nd | nd |
| 89 | 1-Methyl-2-methylenecyclohexane | 002808-75-5 | 1.42 | nd | nd | nd | nd |
| 90 | Heptadecane, 2,6-dimethyl- | 054105-67-8 | 2.36 | nd | nd | nd | nd |
| 91 | 7-Oxabicyclo[4.1.0]heptane, 3-oxiranyl- | 000106-87-6 | 1.52 | nd | nd | nd | nd |
| 92 | Heptadecane | 000629-78-7 | 0.86 | nd | nd | nd | nd |
| 93 | 3-Hexanone | 000589-38-8 | nd | nd | 0.88 | nd | 1.22 |
| 94 | 2-Hexanone | 000591-78-6 | nd | nd | nd | 0.84 | nd |
| 95 | 2H-1,4-Benzodiazepin-2-one, 7-chloro-1,3-dihydro-5-phenyl-1-(trimethylsilyl)- | 055299-24-6 | nd | nd | 0.82 | nd | nd |
| 96 | 1(3H)-Isobenzofuranone, 6-(dimethylamino)-3,3-bis[4-(dimethylamino)phenyl]- | 001552-42-7 | nd | nd | 0.24 | nd | nd |
| 97 | 2-Butanone | 000078-93-3 | nd | nd | nd | nd | 1.4 |
| 98 | 2-Nonanone | 000821-55-6 | 0.71 | nd | nd | nd | 0.51 |
| 99 | 3-Octanone, 2-methyl- | 000923-28-4 | 0.48 | nd | nd | nd | nd |
| 100 | 2-Undecanone | 000112-12-9 | 0.84 | nd | nd | nd | nd |
| 101 | 16-Methyl-heptadecane-1,2-diol, trimethylsilyl ether | 1000336-70-6 | nd | 0.28 | nd | nd | nd |
| 102 | Octaethylene glycol monododecyl ether | 003055-98-9 | nd | 1.44 | nd | nd | nd |
| 103 | 2,5-Dihydroxyacetophenone, bis(trimethylsilyl) ether | 1000352-83-7 | 1.22 | nd | nd | 2.1 | nd |
| 104 | 4-(2-Acetylamino-1-(trimethylsilyloxy)ethyl)phenol | 1000373-43-5 | 1.26 | nd | nd | nd | nd |
| 105 | Benzamide, N-[7-(1-hydroxypropyl)-2,3-dihydrobenzo[1,4]dioxin-6-yl]-4-methyl- | 1000316-96-2 | nd | 0.39 | nd | nd | nd |
| 106 | Hydrazinecarbothioamide, N-ethyl- | 013431-34-0 | nd | 0.61 | nd | nd | nd |
| 107 | Acetamide, 2-(2-hydroxyethoxy)- | 000123-85-3 | nd | 0.72 | nd | 1 | nd |
| 108 | Acetamide, N-(4-imidazo[1,2-a]pyrimidin-2-ylphenyl)-2-methoxy- | 1000338-04-4 | nd | 0.69 | nd | nd | nd |
| 109 | n-Hexylmethylamine | 035161-70-7 | nd | nd | nd | nd | 1.16 |
| 110 | N,N′,N″-Methylidynetrisformamide | 004774-33-8 | nd | nd | nd | nd | 1.06 |
| 111 | 4-Amino-5-imidazole carboxamide,N,N,O- tris(trimethylsilyl)- | 1000079-30-4 | nd | nd | nd | nd | 0.38 |
| 112 | Benzenamine, 4-bromo-3-chloro-N-(4-methylthiobenzylydene)- | 314283-74-4 | 1.4 | nd | nd | nd | nd |
| 113 | o-Cymene | 000527-84-4 | nd | 0.57 | 5.17 | nd | nd |
| 114 | Furan, 2-ethyl- | 003208-16-0 | 1.31 | nd | 0.58 | 0.66 | |
| 115 | Furo[2′,3′:4,5]thiazolo[3,2-g]purine-8-methanol, 4-amino-6.alpha.,7,8,9a-tetrahydro-7-hydroxy-, [6aS-(6a.alpha.,7.alpha.,8.beta.,9a.alpha.)]- | 016667-76-8 | nd | nd | 1.13 | nd | nd |
| 116 | Benzofuran, 2,3-dihydro-2-methyl- | 001746-11-8 | 0.54 | nd | nd | nd | nd |
| 117 | 5-[Cyano-(3,4-dimethyl-5-oxo-1,5-dihydro-pyrrol-2-ylidene)-methyl]-2,3,3-trimethyl-3,4-dihydro-2H-pyrrole-2-carbonitrile | 1000186-14-3 | nd | 1.31 | nd | nd | nd |
| 118 | 7H-Dibenzo(a,g)carbazole | 000207-84-1 | nd | 0.37 | nd | nd | nd |
| 119 | 1-(6-Methyl-benzothiazol-2-yl)-3-(4-methyl-benzoyl)-thiourea | 131120-14-4 | nd | 1.17 | nd | 0.34 | 0.53 |
| 120 | m-Tolualdehyde, thiosemicarbazone | 005706-82-1 | 1.93 | nd | nd | nd | nd |
| 121 | methoxy-phenyl-Oxime | 1000222-86-6 | 3.42 | 2.13 | 2.95 | 2.49 | 3.25 |
| 122 | Naphthalene | 000091-20-3 | 1.73 | 1.53 | 3.19 | 3.4 | 3.01 |
| 123 | Naphthalene, 2-methyl- | 000091-57-6 | nd | 0.66 | nd | nd | nd |
| 124 | 1H-Indene, 1-methylene | 002471-84-3 | nd | 1.85 | nd | nd | nd |
| 125 | 1H-Indene, 2,3-dihydro-4-methyl- | 000824-22-6 | nd | nd | 0.99 | nd | nd |
| 126 | Oxazole, 2,5-dihydro-5-(4-methylphenyl)-4-phenyl- | 036879-73-9 | nd | 0.78 | nd | nd | nd |
| 127 | 2-Mercapto-4-phenylthiazole | 002103-88-0 | nd | nd | 0.83 | nd | 1.02 |
| 128 | 13H-Dibenzo[a,i]carbazole | 000239-64-5 | nd | nd | nd | 1.57 | nd |
| 129 | .beta.-D-Xylo-Hexopyranosid-4-ulose, methyl 2,3,6-tri-O-methyl-, (2,4-dinitrophenyl)hydrazine | 041545-25-9 | nd | 0.45 | nd | nd | nd |
| 130 | Pyrazolo[1,5-a]pyrimidine, 2,7-dimethyl-5-phenyl- | 1000267-29-6 | nd | 0.9 | nd | nd | nd |
| 131 | Azulene | 000275-51-4 | nd | nd | 0.86 | nd | nd |
| 132 | Octanoic acid, silver(1+) | 024927-67-1 | nd | nd | 0.61 | nd | nd |
| 133 | 3-Phenylindole | 001504-16-1 | nd | nd | nd | 0.92 | nd |
| 134 | 2-Methyl-7-phenylindole | 001140-08-5 | nd | nd | nd | nd | 1.5 |
| 135 | Methyl d-glycero-.beta.-d-gulo-heptoside | 1000130-15-2 | nd | nd | nd | 0.61 | nd |
| 136 | 2-Hydrazino-4,6-dimethylpyrimidine ditms peak 2 | 1000332-01-7 | nd | nd | nd | nd | 1.56 |
| 137 | 4H-3,1-Benzoxazine, 6,7-dimethoxy-2-(4-methoxyphenyl)-4-propyl- | 1000327-27-7 | nd | nd | nd | nd | 1.09 |
| 138 | N-(Trifluoroacetyl)-O,O′,O″-tris(trimethylsilyl)norepinephrine | 1000072-26-3 | 0.39 | nd | nd | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; You, S.; Cheng, L.; Zeng, H.; Zheng, B.; Zhang, Y. Physiochemical Quality, Microbial Diversity, and Volatile Components of Monascus-Fermented Hairtail Surimi. Foods 2023, 12, 2891. https://doi.org/10.3390/foods12152891
Li Y, You S, Cheng L, Zeng H, Zheng B, Zhang Y. Physiochemical Quality, Microbial Diversity, and Volatile Components of Monascus-Fermented Hairtail Surimi. Foods. 2023; 12(15):2891. https://doi.org/10.3390/foods12152891
Chicago/Turabian StyleLi, Yanpo, Shuyi You, Lujie Cheng, Hongliang Zeng, Baodong Zheng, and Yi Zhang. 2023. "Physiochemical Quality, Microbial Diversity, and Volatile Components of Monascus-Fermented Hairtail Surimi" Foods 12, no. 15: 2891. https://doi.org/10.3390/foods12152891






