Proteomic Profiling of Thigh Meat at Different Ages of Chicken for Meat Quality and Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Samples Preparation
2.3. Meat Quality Characteristics
2.4. Protein Extraction, Digestion and TMT Labeling
2.5. LC-MS/MS Analysis and Protein Identification
2.6. LC-PRM/MS Analysis
2.7. Statistical Analysis and Bioinformation Analysis
3. Results
3.1. Characteristics of Thigh Meat Quality during Growth
3.2. Proteomic Expression Profiling of Thigh Muscle
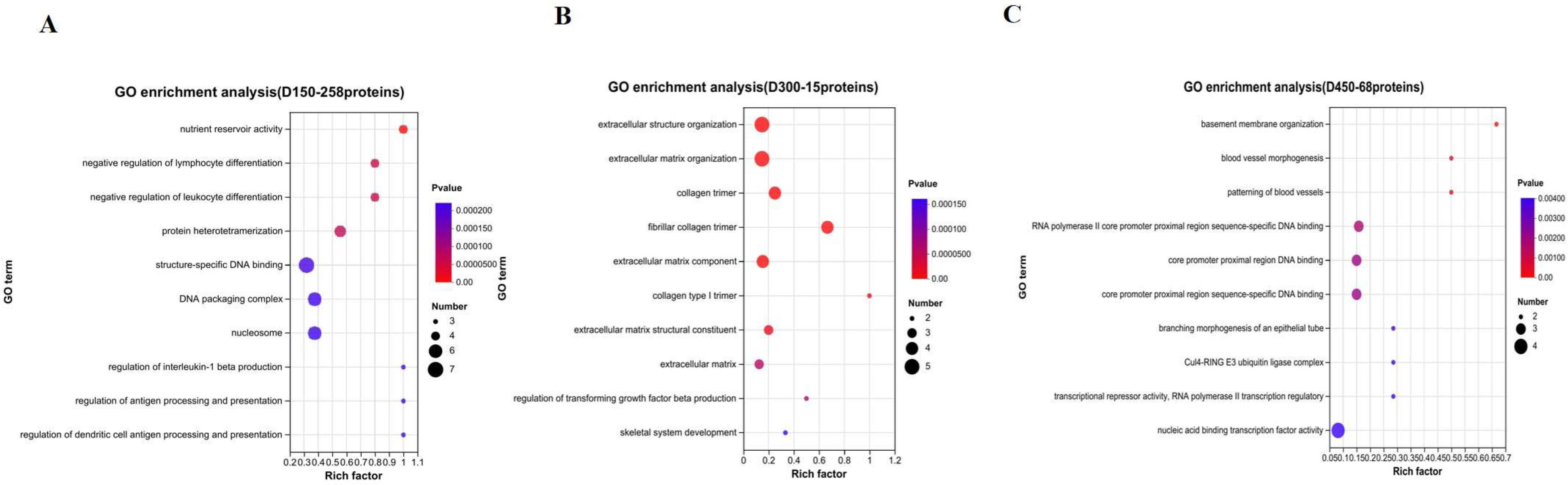
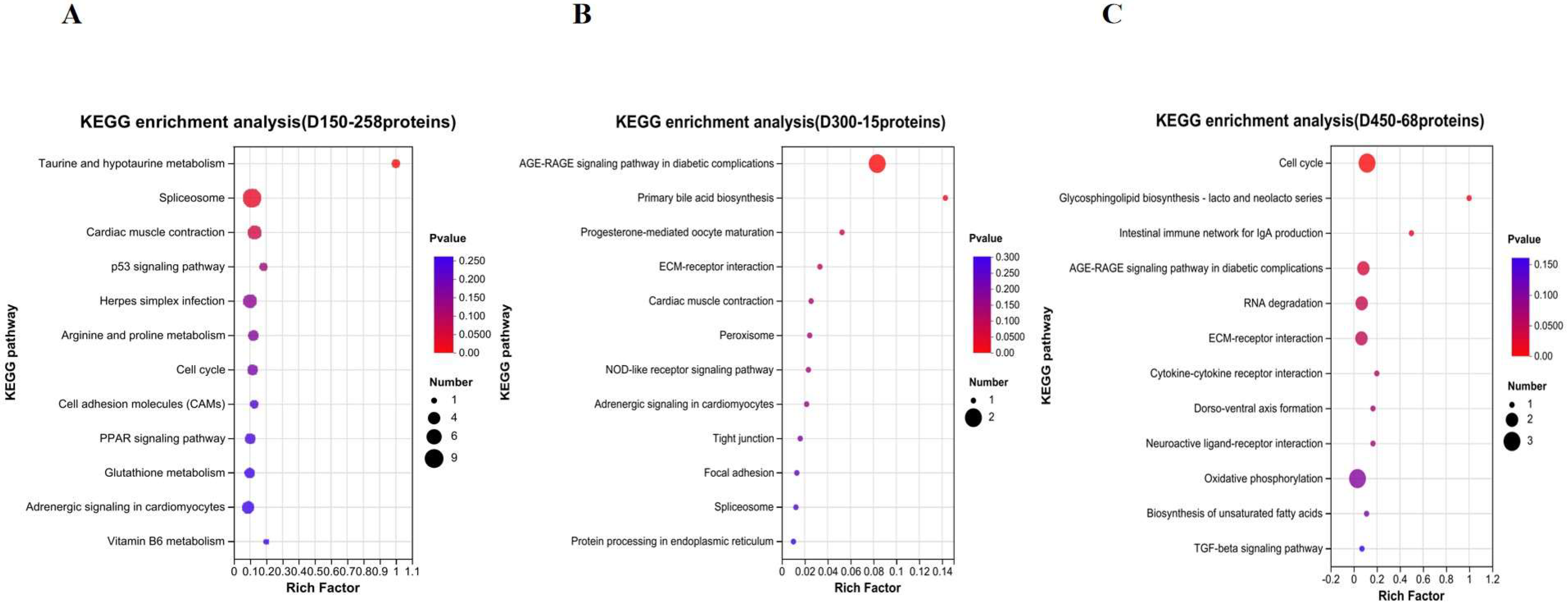
3.3. Functional Enrichment Analysis of DEPs
3.4. Subcluster Analysis of DEPs
3.5. Function Analysis of DEPs Based on Age Stages
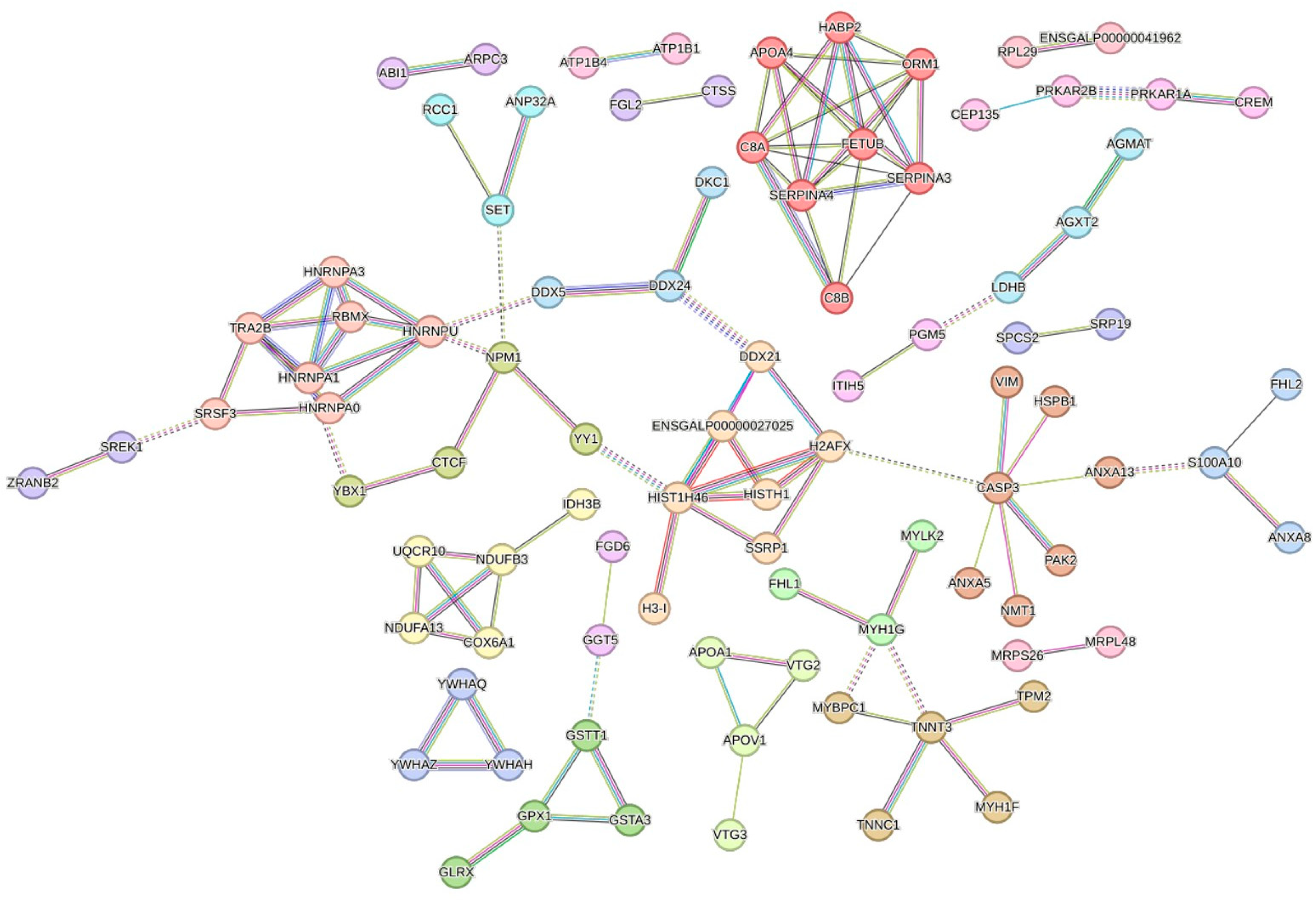
3.6. Interaction Networks of DEPs
3.7. PRM Validation of TMT-Based Results
4. Discussion
4.1. The Proteins and Pathways Related to FAAs and Peptides
4.2. The Proteins and Pathways Related to IMF
4.3. The Proteins and Pathways Related to Muscle Development and Contraction
4.4. The Proteins and Pathways Related to Follicle Development
4.5. The Proteins and Pathways Related to the Spliceosome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Yang, C.; Peng, H.; Yin, H.; Wang, Y.; Hu, Y.; Yu, C.; Jiang, X.; Du, H.; Li, Q.; et al. Effects of Slaughter Age on Muscle Characteristics and Meat Quality Traits of Da-Heng Meat Type Birds. Animals 2019, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Jiang, Y.; Wang, Z.; Chen, G.; Bai, H.; Chang, G. Indigenous, Yellow-Feathered Chickens Body Measurements, Carcass Traits, and Meat Quality Depending on Marketable Age. Animals 2022, 12, 2422. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Gai, K.; Li, Z.; Chen, Y.; Wang, L.; Qi, X.; Xing, K.; Wang, X.; Xiao, L.; Ni, H.; et al. HPLC-QTRAP-MS-based metabolomics approach investigates the formation mechanisms of meat quality and flavor of Beijing You chicken. Food Chem. X 2022, 17, 100550. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Ge, C.; Zhou, G.; Zhang, W.; Liao, G. 1H NMR-based metabolic characterization of Chinese Wuding chicken meat. Food Chem. 2019, 274, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhuang, H.; Cao, J.; Geng, A.; Wang, H.; Chu, Q.; Yan, Z.; Zhang, X.; Zhang, Y.; Liu, H. Breast Meat Fatty Acid Profiling and Proteomic Analysis of Beijing-You Chicken During the Laying Period. Front. Vet. Sci. 2022, 9, 908862. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, J.; Geng, A.; Zhang, X.; Wang, H.; Chu, Q.; Yan, Z.; Zhang, Y.; Liu, H.; Zhang, J. Integration of GC-MS and LC-MS for metabolite characteristics of thigh meat between fast- and slow-growing broilers at marketable age. Food Chem. 2023, 403, 134362. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zheng, M.; Zhao, G.; Liu, R.; Wen, J. Identification of differentially expressed genes and pathways for intramuscular fat metabolism between breast and thigh tissues of chickens. BMC Genom. 2018, 19, 55. [Google Scholar] [CrossRef]
- Ge, K.; Ye, P.; Yang, L.; Kuang, J.; Chen, X.; Geng, Z. Comparison of slaughter performance, meat traits, serum lipid parameters and fat tissue between Chaohu ducks with high- and low-intramuscular fat content. Anim. Biotechnol. 2020, 31, 245–255. [Google Scholar] [CrossRef]
- Farmer, L.J. Poultry meat flavor. In Poultry Meat Science; Richardson, R.I., Mead, G.C., Eds.; CABI Publishing: New York, NY, USA, 1999; pp. 127–158. [Google Scholar]
- Zotte, A.D.; Gleeson, E.; Franco, D.; Cullere, M.; Lorenzo, J.M. Proximate composition, amino acid profile, and oxidative stability of slow-growing indigenous chickens compared with commercial broiler chickens. Foods 2020, 9, 546. [Google Scholar] [CrossRef]
- Liu, J.; Fu, R.; Liu, R.; Zhao, G.; Zheng, M.; Cui, H.; Li, Q.; Song, J.; Wang, J.; Wen, J. Protein profiles for muscle development and intramuscular fat accumulation at different post-hatching ages in chickens. PLoS ONE 2016, 11, e0159722. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Geng, A.; Wang, H.; Chu, Q.; Yang, L.; Yan, Z.; Zhang, X.; Zhang, Y.; Dai, J.; et al. Comprehensive proteomic characterization of the pectoralis major at three chronological ages in Beijing-You chicken. Front. Physiol. 2021, 12, 658711. [Google Scholar] [CrossRef] [PubMed]
- Zerehdaran, S.; Vereijken, A.L.J.; van Arendonk, J.A.M.; van der Waaijt, E.H. Estimation of genetic parameters for fat deposition and carcass traits in broilers. Poult. Sci. 2004, 83, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K.; Li, K.; Zhang, G.; Jin, Z.; He, F.; et al. iProX: An integrated proteome resource. Nucleic Acids Res. 2019, 47, D1211–D1217. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Yin, L.; Li, Z.; Yu, C.; Du, H.; Jiang, X.; Yang, C.; Liu, Y. Integration analysis of metabolome and transcriptome profiles revealed the age-dependent dynamic change in chicken meat. Food Res. Int. 2022, 156, 111171. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. On the enzymology of amino acid transport. Science 1973, 180, 33–39. [Google Scholar] [CrossRef]
- Meister, A. The gamma-glutamyl cycle. Diseases associated with specific enzyme deficiencies. Ann. Intern. Med. 1974, 81, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Leveille, G.A. In vitro hepatic lipogenesis in the hen and chick. Comp. Biochem. Physiol. 1969, 28, 431–435. [Google Scholar] [CrossRef]
- Qiu, F.; Xie, L.; Ma, J.-E.; Luo, W.; Zhang, L.; Chao, Z.; Chen, S.; Nie, Q.; Lin, Z.; Zhang, X. Lower Expression of SLC27A1 Enhances Intramuscular Fat Deposition in Chicken via Down-Regulated Fatty Acid Oxidation Mediated by CPT1A. Front. Physiol. 2017, 8, 449. [Google Scholar] [CrossRef]
- Wang, C.S.; McConathy, W.J.; Kloer, H.U.; Alaupovic, P. Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein C-III. J. Clin. Investig. 1985, 75, 384–390. [Google Scholar] [CrossRef]
- Wu, X.; Liu, L.; Li, J.; Chi, F. Proteome analysis using iTRAQ reveals the differentiation between Tibetan and ordinary ovalbumin peptides. Int. J. Biol. Macromol. 2019, 132, 722–728. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, J.; Cheng, L.; Gao, S.; Li, S.; Qiu, N.; Li, H.; Peng, L.; Geng, F. A puzzle piece of protein N-glycosylation in chicken egg: N-glycoproteome of chicken egg vitelline membrane. Int. J. Biol. Macromol. 2020, 164, 3125–3132. [Google Scholar] [CrossRef]
- Cui, H.-X.; Liu, R.-R.; Zhao, G.-P.; Zheng, M.-Q.; Chen, J.-L.; Wen, J. Identification of differentially expressed genes and pathways for intramuscular fat deposition in pectoralis major tissues of fast- and slow-growing chickens. BMC Genom. 2012, 13, 213. [Google Scholar] [CrossRef]
- Ouyang, H.; Wang, Z.; Chen, X.; Yu, J.; Li, Z.; Nie, Q. Proteomic analysis of chicken skeletal muscle during Embryonic development. Front. Physiol. 2017, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- King, M.W.; Neff, A.W.; Mescher, A.L. Proteomics analysis of regenerating amphibian limbs: Changes during the onset of regeneration. Int. J. Dev. Biol. 2009, 53, 955–969. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xing, S.; Zhao, G.; Zheng, M.; Yang, X.; Sun, J.; Wen, J.; Liu, R. Identification of diverse cell populations in skeletal muscles and biomarkers for intramuscular fat of chicken by single-cell RNA sequencing. BMC Genom. 2020, 21, 752. [Google Scholar] [CrossRef]
- Wang, G.G.; Allis, C.D.; Chi, P. Chromatin remodeling and cancer, Part I: Covalent histone modifications. Trends Mol. Med. 2007, 13, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Fyodorov, D.V.; Zhou, B.-R.; Skoultchi, A.I.; Bai, Y. Emerging roles of linker histones in regulating chromatin structure and function. Nat. Rev. Mol. Cell Biol. 2018, 19, 192–206. [Google Scholar] [CrossRef]
- Woodhouse, S.; Pugazhendhi, D.; Brien, P.; Pell, J.M. Ezh2 maintains a key phase of muscle satellite cell expansion but does not regulate terminal differentiation. J. Cell Sci. 2013, 126, 565–579. [Google Scholar] [CrossRef]
- Kogelman, L.J.A.; Zhernakova, D.V.; Westra, H.-J.; Cirera, S.; Fredholm, M.; Franke, L.; Kadarmideen, H.N. An integrative systems genetics approach reveals potential causal genes and pathways related to obesity. Genome Med. 2015, 7, 105. [Google Scholar] [CrossRef]
- Hermeking, H.; Benzinger, A. 14-3-3 proteins in cell cycle regulation. Semin. Cancer Biol. 2006, 16, 183–192. [Google Scholar] [CrossRef]
- Zha, J.; Harada, H.; Yang, E.; Jockel, J.; Korsmeyer, S.J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 1996, 87, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Bai, X.; Luo, C.; Jiang, D.; Li, X.; Zhang, X.; Tian, Y.; Huang, Y. Quantitative proteomic analysis of chicken serum reveals key proteins affecting follicle development during reproductive phase transitions. Poult. Sci. 2021, 100, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Hermeking, H.; Lengauer, C.; Polyak, K.; He, T.C.; Zhang, L.; Thiagalingam, S.; Kinzler, K.W.; Vogelstein, B. 14-3-3sigma is a p53-Regulated inhibitor of G2/M Progression. Mol. Cell 1997, 1, 3–11. [Google Scholar] [CrossRef]
- De, S.; Marcinkiewicz, J.L.; Vijayaraghavan, S.; Kline, D. Expression of 14-3-3 protein isoforms in mouse oocytes, eggs and ovarian follicular development. BMC Res. Notes 2012, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Eisa, A.A.; De, S.; Detwiler, A.; Gilker, E.; Ignatious, A.C.; Vijayaraghavan, S.; Kline, D. YWHA (14-3-3) protein isoforms and their interactions with CDC25B phosphatase in mouse oogenesis and oocyte maturation. BMC Dev. Biol. 2019, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Paez, L.M.; Bordone, M.C.; Leote, A.C.; Saraiva-Agostinho, N.; Ascensão-Ferreira, M.; Barbosa-Morais, N.L. Alternative splicing: The pledge, the turn, and the prestige: The key role of alternative splicing in human biological systems. Hum. Genet. 2017, 136, 1015–1042. [Google Scholar] [CrossRef]
- Butcher, S.E.; Brow, D.A. Towards understanding the catalytic core structure of the spliceosome. Biochem. Soc. Trans. 2005, 33, 447–449. [Google Scholar] [CrossRef]
- Topp, J.D.; Jackson, J.; Melton, A.A.; Lynch, K.W. A cell-based screen for splicing regulators identifies hnRNP LL as a distinct signal-induced repressor of CD45 variable exon 4. RNA 2008, 14, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Mallory, M.J.; Jackson, J.; Weber, B.; Chi, A.; Heyd, F.; Lynch, K.W. Signal- and development-dependent alternative splicing of LEF1 in T cells is controlled by CELF2. Mol. Cell Biol. 2011, 31, 2184–2195. [Google Scholar] [CrossRef]

| Traits 2 | D150 | D300 | D450 |
|---|---|---|---|
| Live weight (g) | 1344 ± 63 c | 1951 ± 266 b | 2228 ± 294 a |
| Thigh muscle weight (g) | 55.7 ± 10.2 c | 99.5 ± 14.6 b | 134.2 ± 21.3 a |
| Percentage of thigh weight (%) | 8.3 ± 1.5 c | 10.3 ± 1.4 b | 12.0 ± 0.9 a |
| IMF (%) | 4.4 ± 0.8 c | 8.3 ± 1.2 b | 11.7 ± 2.3 a |
| Crude protein (%) | 79.18 ± 3.00 a | 72.28 ± 3.06 b | 61.83 ± 4.78 c |
| Dry matter (%) | 25.17 ± 1.15 c | 30.17 ± 1.28 b | 32.53 ± 1.80 a |
| Ans (mg/g) | 3.95 ± 0.43 a | 2.61 ± 0.32 b | 2.58 ± 0.36 b |
| Car (mg/g) | 0.39 ± 0.20 b | 1.95 ± 0.72 a | 2.17 ± 0.83 a |
| Ser (mg/g) | 0.09 ± 0.02 a | 0.05 ± 0.01 c | 0.07 ± 0.02 b |
| Tau (mg/g) | 2.17 ± 0.31 b | 2.18 ± 0.42 b | 2.55 ± 0.47 a |
| Asp (mg/g) | 0.07 ± 0.03 b | 0.08 ± 0.04 b | 0.11 ± 0.03 a |
| Thr (mg/g) | 0.07 ± 0.03 b | 0.11 ± 0.07 a | 0.10 ± 0.04 a |
| Ser (mg/g) | 0.27 ± 0.10 a | 0.20 ± 0.06 b | 0.23 ± 0.05 ab |
| Glu (mg/g) | 0.25 ± 0.07 b | 0.32 ± 0.10 a | 0.28 ± 0.04 ab |
| Gly (mg/g) | 0.13 ± 0.05 b | 0.19 ± 0.06 a | 0.22 ± 0.03 a |
| Val (mg/g) | 0.03 ± 0.01 b | 0.04 ± 0.01 a | 0.04 ± 0.01 a |
| Leu (mg/g) | 0.03 ± 0.01 b | 0.04 ± 0.01 a | 0.04 ± 0.01 a |
| Phe (mg/g) | 0.019 ± 0.002 b | 0.022 ± 0.005 a | 0.023 ± 0.002 a |
| Ala (mg/g) | 0.04 ± 0.02 b | 0.07 ± 0.02 a | 0.06 ± 0.02 a |
| His (mg/g) | 0.02 ± 0.00 c | 0.03 ± 0.01 b | 0.04 ± 0.01 a |
| Arg (mg/g) | 0.12 ± 0.03 a | 0.06 ± 0.02 c | 0.08 ± 0.02 b |
| Total amino acids (mg/g) | 3.71 ± 0.36 b | 3.81 ± 0.52 b | 4.26 ± 0.50 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Chen, X.; Cao, J.; Chang, C.; Geng, A.; Wang, H.; Chu, Q.; Yan, Z.; Zhang, Y.; Liu, H. Proteomic Profiling of Thigh Meat at Different Ages of Chicken for Meat Quality and Development. Foods 2023, 12, 2901. https://doi.org/10.3390/foods12152901
Zhang J, Chen X, Cao J, Chang C, Geng A, Wang H, Chu Q, Yan Z, Zhang Y, Liu H. Proteomic Profiling of Thigh Meat at Different Ages of Chicken for Meat Quality and Development. Foods. 2023; 12(15):2901. https://doi.org/10.3390/foods12152901
Chicago/Turabian StyleZhang, Jian, Xia Chen, Jing Cao, Cheng Chang, Ailian Geng, Haihong Wang, Qin Chu, Zhixun Yan, Yao Zhang, and Huagui Liu. 2023. "Proteomic Profiling of Thigh Meat at Different Ages of Chicken for Meat Quality and Development" Foods 12, no. 15: 2901. https://doi.org/10.3390/foods12152901
APA StyleZhang, J., Chen, X., Cao, J., Chang, C., Geng, A., Wang, H., Chu, Q., Yan, Z., Zhang, Y., & Liu, H. (2023). Proteomic Profiling of Thigh Meat at Different Ages of Chicken for Meat Quality and Development. Foods, 12(15), 2901. https://doi.org/10.3390/foods12152901








