Reducing Antigenicity and Improving Antioxidant Capacity of β-Lactoglobulin through Covalent Interaction with Six Flavonoids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
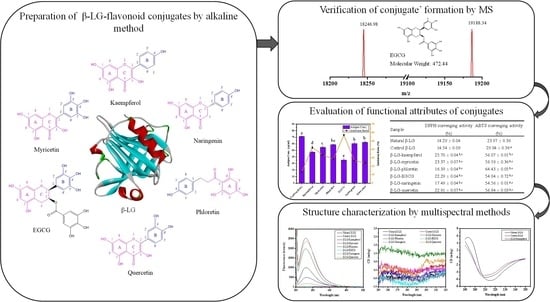
2.2. Preparation of β-LG-Flavonoid Conjugates
2.3. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS)
2.4. Enzyme-Linked Immunosorbent Assays (ELISA)
2.5. DPPH and ABTS Radical Scavenging Capacity of β-LG-Flavonoid Conjugates
2.6. Intrinsic Fluorescence Spectroscopy Measurements
2.7. Fourier Transform Infrared (FTIR) Spectroscopy Measurements
2.8. Circular Dichroism (CD) Spectroscopy Measurements
2.9. Scanning Electron Microscopy (SEM)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Generation of Covalent Bonds between β-LG-Flavonoids
3.2. The Antigenic Characteristics of β-LG-Flavonoid Conjugates
3.3. The Antioxidant Performance of β-LG-Flavonoid Conjugates
3.4. The Changes of the Microenvironment around the Tryptophan (Trp) Residue
3.5. The Covalent Interaction of Flavonoids-β-LG
3.6. The Structural Characteristics of β-LG Induced by Covalent Modification via Flavonoids
3.7. Morphological Observation of β-LG-Flavonoid Conjugates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giannetti, A.; Toschi Vespasiani, G.; Ricci, G.; Miniaci, A.; di Palmo, E.; Pession, A. Cow’s Milk Protein Allergy as a Model of Food Allergies. Nutrients 2021, 13, 1525. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G.; Zaripov, O.G. Beta–lactoglobulin–nutrition allergen and nanotransporter of different nature ligands therapy with therapeutic action. Res. Vet. Sci. 2020, 133, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Hu, J.; Han, L.; Cao, J.; Nishinari, K.; Yang, J.; Fang, Y.; Li, D. Conformational transition and gelation of κ-carrageenan in electrostatic complexation with β-lactoglobulin aggregates. Food Hydrocoll. 2021, 118, 106764. [Google Scholar] [CrossRef]
- Zhong, J.Z.; Liu, W.; Liu, C.M.; Wang, Q.H.; Li, T.; Tu, Z.C.; Luo, S.J.; Cai, X.F.; Xu, Y.J. Aggregation and conformational changes of bovine β-lactoglobulin subjected to dynamic high-pressure microfluidization in relation to antigenicity. J. Dairy Sci. 2012, 95, 4237–4245. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Li, K.; Wu, H.; Li, W.; Sun, Q. Multi-spectroscopic, conformational, and computational atomic-level insights into the interaction of β-lactoglobulin with apigenin at different pH levels. Food Hydrocoll. 2020, 105, 105810. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Zou, Y.; Shu, L.; Han, N.Y.; Yang, Y. Characterization of the molecular properties and allergenicity (IgE-binding capacity) of β-lactoglobulin by heat treatment using asymmetric-flow field-flow fractionation and ultra-performance liquid chromatography quadrupole time of flight mass chromatography. Food Chem. 2022, 374, 131748. [Google Scholar] [CrossRef]
- Li, H.; Pan, Y.; Yang, Z.; Rao, J.; Chen, B. Improving Antioxidant Activity of beta-Lactoglobulin by Nature-Inspired Conjugation with Gentisic Acid. J. Agric. Food Chem. 2019, 67, 11741–11751. [Google Scholar] [CrossRef]
- Abd El-Maksoud, A.A.; Abd El-Ghany, I.H.; El-Beltagi, H.S.; Anankanbil, S.; Banerjee, C.; Petersen, S.V.; Perez, B.; Guo, Z. Adding functionality to milk-based protein: Preparation, and physico-chemical characterization of beta-lactoglobulin-phenolic conjugates. Food Chem. 2018, 241, 281–289. [Google Scholar] [CrossRef]
- Geng, S.; Jiang, Z.; Ma, H.; Wang, Y.; Liu, B.; Liang, G. Interaction mechanism of flavonoids and bovine β-lactoglobulin: Experimental and molecular modelling studies. Food Chem. 2020, 312, 126066. [Google Scholar] [CrossRef]
- Li, T.; Hu, P.; Dai, T.; Li, P.; Ye, X.; Chen, J.; Liu, C. Comparing the binding interaction between β-lactoglobulin and flavonoids with different structure by multi-spectroscopy analysis and molecular docking. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 197–206. [Google Scholar] [CrossRef]
- Fusi, F.; Trezza, A.; Tramaglino, M.; Sgaragli, G.; Saponara, S.; Spiga, O. The beneficial health effects of flavonoids on the cardiovascular system: Focus on K+ channels. Pharmacol. Res. 2020, 152, 104625. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Song, J.; Jin, Y. A flavonoid monomer tricin in Gramineous plants: Metabolism, bio/chemosynthesis, biological properties, and toxicology. Food Chem. 2020, 320, 126617. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, J.; Chen, X. Bioactive flavonoids in Moringa oleifera and their health-promoting properties. J. Funct. Foods 2018, 47, 469–479. [Google Scholar] [CrossRef]
- Ramos, L.R.; Santos, J.S.; Daguer, H.; Valese, A.C.; Cruz, A.G.; Granato, D. Analytical optimization of a phenolic-rich herbal extract and supplementation in fermented milk containing sweet potato pulp. Food Chem. 2017, 221, 950–958. [Google Scholar] [CrossRef]
- Silva, B.; Biluca, F.C.; Gonzaga, L.V.; Fett, R.; Dalmarco, E.M.; Caon, T.; Costa, A.C.O. In vitro anti-inflammatory properties of honey flavonoids: A review. Food Res. Int. 2021, 141, 110086. [Google Scholar] [CrossRef]
- He, W.; Zhang, T.; Velickovic, T.C.; Li, S.; Lyu, Y.; Wang, L.; Yi, J.; Liu, Z.; He, Z.; Wu, X. Covalent conjugation with (−)-epigallo-catechin 3-gallate and chlorogenic acid changes allergenicity and functional properties of Ara h1 from peanut. Food Chem. 2020, 331, 127355. [Google Scholar] [CrossRef]
- You, J.; Luo, Y.; Wu, J. Conjugation of Ovotransferrin with Catechin Shows Improved Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 2581–2587. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Z.; Cheng, Y.; Xu, H.; Velickovic, T.C.; He, K.; Sun, F.; He, Z.; Liu, Z.; Wu, X. Changes in Allergenicity of Ovalbumin in vitro and in vivo on Conjugation with Quercetin. J. Agric. Food Chem. 2020, 68, 4027–4035. [Google Scholar] [CrossRef]
- Rohn, S. Possibilities and limitations in the analysis of covalent interactions between phenolic compounds and proteins. Food Res. Int. 2014, 65, 13–19. [Google Scholar] [CrossRef]
- Zhou, S.D.; Huang, L.; Meng, L.; Lin, Y.F.; Xu, X.; Dong, M.S. Soy protein isolate-(-)-epigallocatechin gallate conjugate: Covalent binding sites identification and IgE binding ability evaluation. Food Chem. 2020, 333, 127400. [Google Scholar] [CrossRef]
- Bordenave, N.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2013, 5, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Keppler, J.K.; Schwarz, K.; van der Goot, A.J. Covalent modification of food proteins by plant-based ingredients (polyphenols and organosulphur compounds): A commonplace reaction with novel utilization potential. Trends Food Sci. Technol. 2020, 101, 38–49. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; Gao, Y.; Mcclements, D.J. Food-grade covalent complexes and their application as nutraceutical delivery systems: A review. Compr. Rev. Food Sci. Food Saf. 2016, 16, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lu, Y.; Xu, H.; Lin, D.; He, Z.; Wu, H.; Liu, L.; Wang, Z. Reducing the allergenic capacity of beta-lactoglobulin by covalent conjugation with dietary polyphenols. Food Chem. 2018, 256, 427–434. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, W.; Fan, R.; Yuan, F.; Gao, Y. Evaluation of structural and functional properties of protein–EGCG complexes and their ability of stabilizing a model β-carotene emulsion. Food Hydrocoll. 2015, 45, 337–350. [Google Scholar] [CrossRef]
- Pu, P.; Zheng, X.; Jiao, L.; Chen, L.; Yang, H.; Zhang, Y.; Liang, G. Six flavonoids inhibit the antigenicity of β-lactoglobulin by noncovalent interactions: A spectroscopic and molecular docking study. Food Chem. 2021, 339, 128106. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; McClements, D.J.; Gao, Y. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocoll. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Gong, Y.; Hou, Z.; Gao, Y.; Xue, Y.; Liu, X.; Liu, G. Optimization of extraction parameters of bioactive components from defatted marigold (Tagetes erecta L.) residue using response surface methodology. Food Bioprod. Process. 2012, 90, 9–16. [Google Scholar] [CrossRef]
- Shao, Y.H.; Zhang, Y.; Liu, J.; Tu, Z.C. Influence of ultrasonic pretreatment on the structure, antioxidant and IgG/IgE binding activity of beta-lactoglobulin during digestion in vitro. Food Chem. 2020, 312, 126080. [Google Scholar] [CrossRef]
- Sui, X.; Sun, H.; Qi, B.; Zhang, M.; Li, Y.; Jiang, L. Functional and conformational changes to soy proteins accompanying anthocyanins: Focus on covalent and non-covalent interactions. Food Chem. 2018, 245, 871–878. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Hu, X.; Pan, J.; Gong, D.; Zhang, G. New insights into the binding mechanism between osthole and beta-lactoglobulin: Spectroscopic, chemometrics and docking studies. Food Res. Int. 2019, 120, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.J.; Ramalli, S.G.; Wallace, B.A. DichroWeb, a website for calculating protein secondary structure from circular dichroism spectroscopic data. Protein Sci. 2022, 31, 37–46. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Raghavan, V. Impact of microwave processing on the secondary structure, in-vitro protein digestibility and allergenicity of shrimp (Litopenaeus vannamei) proteins. Food Chem. 2021, 337, 127811. [Google Scholar] [CrossRef]
- El-Maksoud, A.A.A.; Cheng, W.; Petersen, S.V.; Pandiselvam, R.; Guo, Z. Covalent phenolic acid-grafted β-lactoglobulin conjugates: Synthesis, characterization, and evaluation of their multifunctional properties. LWT 2022, 172, 114215. [Google Scholar] [CrossRef]
- Liu, J.; Song, G.; Zhou, L.; Yuan, Y.; Wang, D.; Yuan, T.; Li, L.; He, G.; Xiao, G.; Chen, F.; et al. Sonochemical effects on fabrication, characterization and antioxidant activities of β-lactoglobulin-chlorogenic acid conjugates. Ultrason. Sonochem. 2023, 92, 106240. [Google Scholar] [CrossRef]
- Xue, Y.; Han, Y.-N.; Wang, Y.; Zhang, Y.; Yin, Y.-Q.; Liu, B.-H.; Zhang, H.; Zhao, X. Effect of ferulic acid covalent conjugation on the functional properties and antigenicity of β-lactoglobulin. Food Chem. 2023, 406, 135095. [Google Scholar] [CrossRef]
- Tao, F.; Xiao, C.; Chen, W.; Zhang, Y.; Pan, J.; Jia, Z. Covalent modification of β-lactoglobulin by (−)-epigallocatechin-3-gallate results in a novel antioxidant molecule. Int. J. Biol. Macromol. 2019, 126, 1186–1191. [Google Scholar] [CrossRef]
- Zhong, J.; Tu, Y.; Liu, W.; Luo, S.; Liu, C. Comparative study on the effects of nystose and fructofuranosyl nystose in the glycation reaction on the antigenicity and conformation of β-lactoglobulin. Food Chem. 2015, 188, 658–663. [Google Scholar] [CrossRef]
- Barry, R. Principles of fluorescence spectroscopy, 3rd Edition. J. Biomed. Cpt. USA 2008, 13, 029901. [Google Scholar] [CrossRef]
- Yang, W.; Liu, F.; Xu, C.; Yuan, F.; Gao, Y. Molecular interaction between (−)-epigallocatechin-3-gallate and bovine lactoferrin using multi-spectroscopic method and isothermal titration calorimetry. Food Res. Int. 2014, 64, 141–149. [Google Scholar] [CrossRef]
- Zhong, J.Z.; Xu, Y.J.; Liu, W.; Liu, C.M.; Luo, S.J.; Tu, Z.C. Antigenicity and functional properties of β-lactoglobulin conjugated with fructo-oligosaccharides in relation to conformational changes. J. Dairy Sci. 2013, 96, 2808–2815. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Sample | DPPH Scavenging Activity (%) | ABTS Scavenging Activity (%) |
|---|---|---|
| Natural β-LG | 14.29 ± 0.04 | 23.97 ± 0.36 |
| Control β-LG | 14.54 ± 0.09 | 29.04 ± 0.36 a |
| β-LG-kaempferol | 25.70 ± 0.04 b,c | 56.07 ± 0.01 b,c |
| β-LG-myricetin | 23.37 ± 0.07 b,c | 58.58 ± 0.36 b,c |
| β-LG-phloretin | 16.39 ± 0.04 b,c | 64.43 ± 0.05 b,c |
| β-LG-EGCG | 22.29 ± 0.04 b,c | 54.04 ± 0.72 b,c |
| β-LG-naringenin | 17.49 ± 0.04 b,c | 54.56 ± 0.01 b,c |
| β-LG-quercetin | 22.81 ± 0.07 b,c | 56.84 ± 0.03 b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, P.; Deng, Z.; Chen, L.; Yang, H.; Liang, G. Reducing Antigenicity and Improving Antioxidant Capacity of β-Lactoglobulin through Covalent Interaction with Six Flavonoids. Foods 2023, 12, 2913. https://doi.org/10.3390/foods12152913
Pu P, Deng Z, Chen L, Yang H, Liang G. Reducing Antigenicity and Improving Antioxidant Capacity of β-Lactoglobulin through Covalent Interaction with Six Flavonoids. Foods. 2023; 12(15):2913. https://doi.org/10.3390/foods12152913
Chicago/Turabian StylePu, Pei, Zhifen Deng, Lang Chen, Han Yang, and Guizhao Liang. 2023. "Reducing Antigenicity and Improving Antioxidant Capacity of β-Lactoglobulin through Covalent Interaction with Six Flavonoids" Foods 12, no. 15: 2913. https://doi.org/10.3390/foods12152913
APA StylePu, P., Deng, Z., Chen, L., Yang, H., & Liang, G. (2023). Reducing Antigenicity and Improving Antioxidant Capacity of β-Lactoglobulin through Covalent Interaction with Six Flavonoids. Foods, 12(15), 2913. https://doi.org/10.3390/foods12152913