Bioactive Peptides from Edible Mushrooms—The Preparation, Mechanisms, Structure—Activity Relationships and Prospects
Abstract
1. Introduction
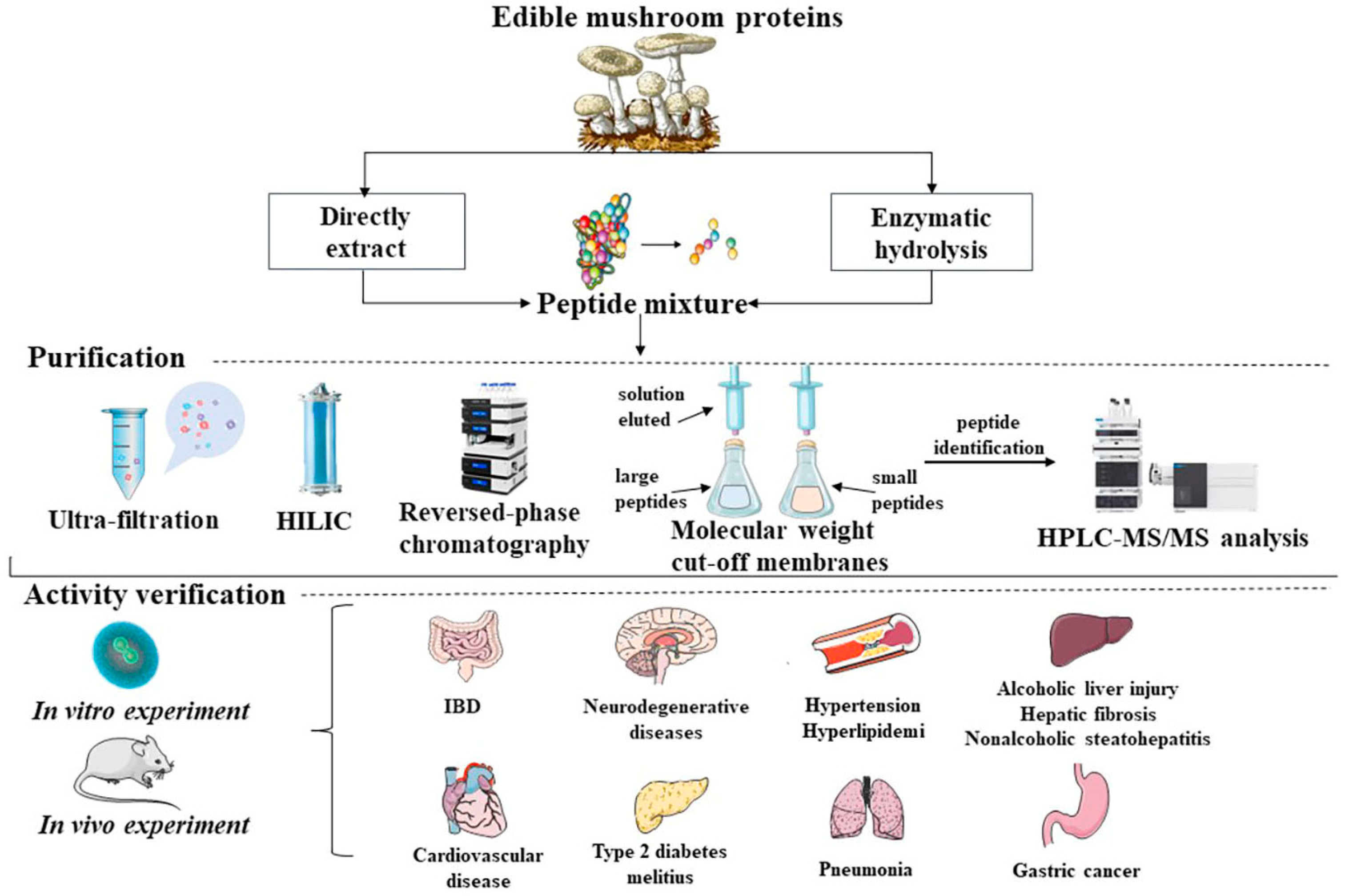
2. Preparation of MBPs
3. Bioactivities of MBPs
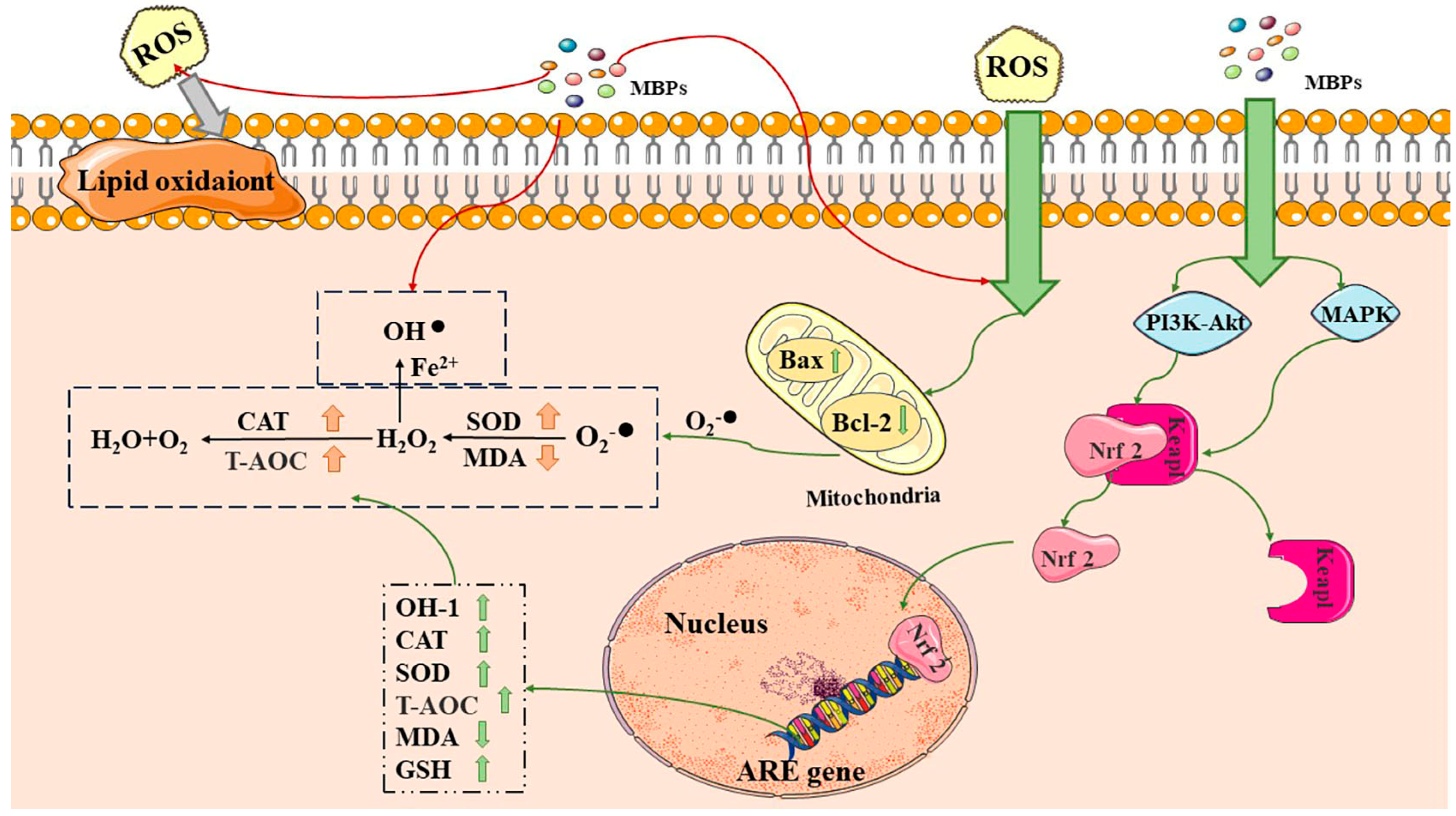
3.1. Antioxidant Activity
Anti-Aging Activity
| Edible Mushroom Category | Molecular Weight and Amino Acid Sequence | Mechanisms of Operation and Value | Reference |
|---|---|---|---|
| Agaricus blazei | Peptide mixtures | Alleviated D-gal-induced senescence response in NIH/3T3 cells. Decreased MDA and ROS contents Increased SOD, CAT and T-AOC activities | Feng et al. (2020) [46] |
| Ganoderma lucidum | Peptide mixtures | Inhibited expression of NOX4, TGF-β1 and 8-hydroxy-2′-deoxyguanosine (8-OHdG) Decreased MDA and ROS contents Increased SOD activities | Meng et al. (2022) [50] |
| Agaricus bisporus | MW of 1~3 kDa | DPPH radical scavenging activity with IC50 of 0.13 mg/mL Reduction in lactate dehydrogenase (LDH) leakage Decreased MDA and ROS contents Increased CAT and GSH activities | Kimatu et al. (2020) [51] |
| Tricholoma matsutake | Amino acid sequence: WALKGYK, WFNNAGP | Alleviated oxidative damage in DSS-induced mice DPPH radicals scavenging activity is 50% at 10 mg/mL Increased SOD contents Decreased MDA contents | Geng et al. (2016) [52,53] |
| Tricholoma matsutake | MW of 1~3 kDa, Amino acid sequence: EHEEHEEHEEPEDDPNSSEESYW | DPPH radical scavenging activity is 70% With EC 50 of 0.468 mg/mL Decreased MDA and ROS contents Increased CAT and T-AOC activities | Feng et al. (2020) [48] |
3.2. Antimicrobial Activity
| Edible Mushroom Category | Molecular Weight and Amino Acid Sequence | Inhibited Bacterial Species and Inhibition Values | Reference |
|---|---|---|---|
| Polyporus alveolaris | MW of 28 kDa | F. oxysporum, Physalospora piricola, M. arachidicola, Botrytis cinereal, with inhibitory concentration of 8 mg/mL for all | Wang et al. (2004) [62] |
| Pseudoplectania nigrella | MW of 4398.80 Da | Streptococcus pneumoniae in mice, with inhibitory concentration of 10 mg/kg | Mygind et al. (2005) [63] |
| Agaricus bisporus | Peptide mixtures | Pseudomonas aeruginosa, reduced to 26.64% at 0.25 mg/mL | Farzaneh et al. (2018) [64] |
| Terfezia claveryi | Peptide mixtures | Bacillus cereus, reduced to 27.44% at 0.25 mg/mL | Farzaneh et al. (2018) [64] |
| Ganoderma lucidum | Peptide mixtures | Salmonella typhi with minimal inhibitory concentration (MIC) of 52 μg Escherichia coli with MIC of 60 μg | Mishra et al. (2018) [18] |
| Pleurotus ostreatus | MW of 7 kDa N-terminal: VRPYLVAF | F. oxysporum, reduced to 20% at dosage of 15.6 μM M. arachidicola, reduced to 45% at dosage of 15.6 μM P. piricola, reduced to 63% at dosage of 15.6 μM | Chu et al. (2005) [65] |
| Agrocybe cylindracea | MW of reduced 9 kDa N-terminal: ANDPQCLYGNVAAKF | Human immunodeficiency virus type 1 with IC50 of 60 μM F. oxysporum with IC50 of 125 μM M. arachidicola with IC50 of 60 μM | Ngai et al. (2005) [58] |
| Cordyceps militaris | MW of 10.9 kDa N-terminal: AMAPPYGYRTPDAAQ | Bipolaris maydis with IC50 of 50 μM Mycosphaerella arachidicola with IC50 of 10 μM Candida albicans with IC50 of 0.75 mM Rhizoctonia solani with IC50 of 80 μM Human immunodeficiency virus type 1 with IC50 of 55 μM | Wong et al. (2011) [66] |
| Lentinus squarrosulus | MW of 17 kDa | Trichophyton mentagrophytes with inhibition zone diameter of 25.7 mm T. rubrum with inhibition zone diameter of 22.8 mm Aspergillus niger with inhibition zone diameter of 12.64 mm Candida tropicalis with inhibition zone diameter of 20.54 mm C. albicans with inhibition zone diameter of 20.62 mm | Poompouang and Suksomtip (2016) [67] |
| Russula paludosa | MW of 4.5 kDa N-terminal: KREHGQHCEF | Human immunodeficiency virus type 1 with IC50 of 11 μM | Wang et al. (2007) [68] |
3.3. Anti-Inflammatory Activity
3.4. Memory and Cognitive Improvement Activity
3.5. Anti-Hypertensive Activity
3.6. Antitumour Activity
3.7. Other Activities
4. Structure—Activity Relationships of MBPs
4.1. Molecular Weight
4.2. Electric Charge
4.3. Aromatic Amino Acids
4.4. Hydrophobic Amino Acids
4.5. Amino Acid Sequence
5. MBPs in Functional Foods
6. Challenges and Prospects
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A Review on Bioactive Peptides: Physiological Functions, Bioavailability and Safety. Int. J. Pept. Res. Ther. 2019, 26, 139–150. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Peñas, E.; Frias, J. Bioactive Peptides in Fermented Foods. In Fermented Foods in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–47. [Google Scholar] [CrossRef]
- Liu, Y.F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Bioactive peptides derived from egg proteins: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2508–2530. [Google Scholar] [CrossRef]
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014, 98, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Maestri, E.; Pavlicevic, M.; Montorsi, M.; Marmiroli, N. Meta-Analysis for Correlating Structure of Bioactive Peptides in Foods of Animal Origin with Regard to Effect and Stability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Maestri, E.; Marmiroli, M.; Marmiroli, N. Bioactive peptides in plant-derived foodstuffs. J. Proteom. 2016, 147, 140–155. [Google Scholar] [CrossRef]
- Daroit, D.J.; Brandelli, A. In vivo bioactivities of food protein-derived peptides—A current review. Curr. Opin. Food Sci. 2021, 39, 120–129. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, M.; Wu, S.; Liao, X.; Wang, J.; Wu, Q.; Zhuang, M.; Ding, Y. A review on mushroom-derived bioactive peptides: Preparation and biological activities. Food Res. Int. 2020, 134, 109230. [Google Scholar] [CrossRef]
- Erjavec, J.; Kos, J.; Ravnikar, M.; Dreo, T.; Sabotic, J. Proteins of higher fungi--from forest to application. Trends Biotechnol. 2012, 30, 259–273. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Dhanasekaran, D.; Krishnaraj, V.; Anbukumaran, A.; Ramasamy, T.; Manickam, M. Edible Mushrooms: A Promising Bioresource for Prebiotics. In Advances in Probiotics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 81–97. [Google Scholar] [CrossRef]
- Barros, A.B.; Ferrao, J.; Fernandes, T. A safety assessment of Coriolus versicolor biomass as a food supplement. Food Nutr. Res. 2016, 60, 29953. [Google Scholar] [CrossRef]
- Bains, A.; Tripathi, A. Evaluation of Antioxidant and Anti-Inflammatory Properties of Aqueous Extract of Wild Mushrooms Collected from Himachal Pradesh. Asian J. Pharm. Clin. Res. 2017, 10, 467–472. [Google Scholar] [CrossRef]
- Bains, A.; Chawla, P.; Kaur, S.; Najda, A.; Fogarasi, M.; Fogarasi, S. Bioactives from Mushroom: Health Attributes and Food Industry Applications. Materials 2021, 14, 7640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.J.; Huo, C.Y.; Qian, Y.; Ren, D.F.; Lu, J. Ultra-high-pressure processing improves proteolysis and release of bioactive peptides with activation activities on alcohol metabolic enzymes in vitro from mushroom foot protein. Food Chem. 2017, 231, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, H.; Chen, J.; Zhang, X. Bioactive proteins from mushrooms. Biotechnol. Adv. 2011, 29, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Daliri, E.B.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Mishra, J.; Rajput, R.; Singh, K.; Puri, S.; Goyal, M.; Bansal, A.; Misra, K. Antibacterial Natural Peptide Fractions from Indian Ganoderma lucidum. Int. J. Pept. Res. Ther. 2017, 24, 543–554. [Google Scholar] [CrossRef]
- Chai, K.F.; Voo, A.Y.H.; Chen, W.N. Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3825–3885. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Tritean, N.; Bărbieru, O.-G.; Constantinescu-Aruxandei, D.; Oancea, F. Extraction and Plastein Reaction of Bioactive Peptides from Agaricus Bisporus Mushrooms. Proceedings 2019, 29, 106. [Google Scholar] [CrossRef]
- Ghorai, S.; Banik, S.P.; Verma, D.; Chowdhury, S.; Mukherjee, S.; Khowala, S. Fungal biotechnology in food and feed processing. Food Res. Int. 2009, 42, 577–587. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullon, P.; Sivaraman, G.K.; McClements, D.J.; Gullon, B.; Lorenzo, J.M. Edible Mushrooms as Functional Ingredients for Development of Healthier and More Sustainable Muscle Foods: A Flexitarian Approach. Molecules 2021, 26, 2463. [Google Scholar] [CrossRef]
- Sousa, A.S.; Araujo-Rodrigues, H.; Pintado, M.E. The Health-promoting Potential of Edible Mushroom Proteins. Curr. Pharm. Des. 2023, 29, 804–823. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.C.; Abdullah, N.; Shuib, A.S.; Aminudin, N. Novel angiotensin I-converting enzyme inhibitory peptides derived from edible mushroom Agaricus bisporus (J.E. Lange) Imbach identified by LC-MS/MS. Food Chem. 2014, 148, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Paisansak, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. Angiotensin-I converting enzyme inhibitory peptide derived from the shiitake mushroom (Lentinula edodes). J. Food Sci. Technol. 2021, 58, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Prandi, B.; Cigognini, I.M.; Faccini, A.; Zurlini, C.; Rodríguez, Ó.; Tedeschi, T. Comparative Study of Different Protein Extraction Technologies Applied on Mushrooms By-products. Food Bioprocess Technol. 2023, 16, 1570–1581. [Google Scholar] [CrossRef]
- Piovesana, S.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Zenezini Chiozzi, R.; Lagana, A. Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation. Anal. Bioanal. Chem. 2018, 410, 3425–3444. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Samperi, R.; Lagana, A. Recent trends in the analysis of bioactive peptides in milk and dairy products. Anal. Bioanal. Chem. 2016, 408, 2677–2685. [Google Scholar] [CrossRef]
- Islam, T.; Ganesan, K.; Xu, B. New insight into mycochemical profiles and antioxidant potential of edible and medicinal mushrooms: A review. Int. J. Med. Mushroom 2019, 21, 237–251. [Google Scholar] [CrossRef]
- Tonolo, F.; Folda, A.; Cesaro, L.; Scalcon, V.; Marin, O.; Ferro, S.; Bindoli, A.; Rigobello, M.P. Milk-derived bioactive peptides exhibit antioxidant activity through the Keap1-Nrf2 signaling pathway. J. Funct. Foods 2020, 64, 103696. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Li, W. Antioxidant, antitumor and immunostimulatory activities of the polypeptide from Pleurotus eryngii mycelium. Int. J. Biol. Macromol. 2017, 97, 323–330. [Google Scholar] [CrossRef]
- Sun, J.; He, H.; Xie, B.J. Novel Antioxidant Peptides from Fermented Mushroom Ganoderma lucidum. J. Agric. Food Chem. 2004, 52, 6646–6652. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, J.; He, H.; Guo, H.; Zhang, S. Hepatoprotective effects of Ganoderma lucidum peptides against D-galactosamine-induced liver injury in mice. J. Ethnopharmacol. 2008, 117, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Z.; Zhuang, J.; Zhang, J.; Shen, F.; Yu, P.; Zhong, H.; Feng, F. Antiaging function of Chinese pond turtle (Chinemys reevesii) peptide through activation of the Nrf2/Keap1 signaling pathway and its structure-activity relationship. Front. Nutr. 2022, 9, 961922. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhou, J.; Yang, C.; Zhang, X.; Shi, Y.; Liu, J.; Yan, X.; Liang, J.; Liu, X.; Luo, L.; et al. gamma-Glutamylcysteine ameliorates D-gal-induced senescence in PC12 cells and mice via activating AMPK and SIRT1. Food Funct. 2022, 13, 7560–7571. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Xu, F.; Zhang, Y.; Li, Z.; Ju, X.; Wang, L. Insoluble-bound polyphenols of adlay seed ameliorate H(2)O(2)-induced oxidative stress in HepG2 cells via Nrf2 signalling. Food Chem. 2020, 325, 126865. [Google Scholar] [CrossRef]
- Huang, P.; Luo, F.J.; Ma, Y.C.; Wang, S.X.; Huang, J.; Qin, D.D.; Xue, F.F.; Liu, B.Y.; Wu, Q.; Wang, X.L.; et al. Dual antioxidant activity and the related mechanisms of a novel pentapeptide GLP4 from the fermented mycelia of Ganoderma lingzhi. Food Funct. 2022, 13, 9032–9048. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Vudhya Gowrisankar, Y.; Chen, X.Z.; Yang, Y.C.; Yang, H.L. The Antiaging Activity of Ergothioneine in UVA-Irradiated Human Dermal Fibroblasts via the Inhibition of the AP-1 Pathway and the Activation of Nrf2-Mediated Antioxidant Genes. Oxid. Med. Cell. Longev. 2020, 2020, 2576823. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, J.; Shan, Y.; Qi, X.; Wang, H.; Jia, L. A combination of chicken embryo extract and a nutritional supplement protect a rat model of aging against d-galactose-induced dysfunction of mitochondria and autophagy. Food Funct. 2019, 10, 2774–2784. [Google Scholar] [CrossRef]
- Cheng, J.Q.; Xu, G.; Du, P.; An, L.; Lv, G.; Miao, L.; Guangxin, Y.; Moffitt, H.L.; Kim, I.; Chi, Z.T.; et al. Research progress of active polypeptides of Cordyceps militaris. BIO Web Conf. 2017, 8, 1002. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Cao, B.; Zhao, H.; Feng, J. Emerging Roles of Ganoderma lucidum in Anti-Aging. Aging Dis. 2017, 8, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Wu, J. Dietary peptides in aging: Evidence and prospects. Food Sci. Hum. Wellness 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Mehra, A.; Zaidi, K.U.; Mani, A.; Thawani, V. The health benefits of Cordyceps militaris—A review. Kavaka 2017, 48, 27–32. [Google Scholar]
- Feng, Q.; Lu, X.; Yuan, G.; Zhang, Q.; An, L. Effects of Agaricus blazei polypeptide on cell senescence by regulation of Keap1/Nrf2/ARE and TLR4/NF-κBp65 signaling pathways and its mechanism in D-gal-induced NIH/3T3 cells. J. Funct. Foods 2020, 72, 104037. [Google Scholar] [CrossRef]
- Erdmann, K.; Cheung, B.W.; Schroder, H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J. Nutr. Biochem. 2008, 19, 643–654. [Google Scholar] [CrossRef]
- Feng, Q.; Li, Y.; Lu, X.; Yu, Y.; Yuan, G.; Sun, J.; Tian, C.; Hu, L.; Xu, G.; An, L.; et al. Agaricus blazei polypeptide exerts a protective effect on D-galactose-induced aging mice via the Keap1/Nrf2/ARE and P53/Trim32 signaling pathways. J. Food Biochem. 2021, 45, e13555. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Meng, J.; Ma, A.; Zhang, S.; Lin, D.; Lin, S.; Li, M.; Zhou, H.; Yang, B. Ganoderma Lucidum Polysaccharide Peptide attenuates post myocardial infarction fibrosis via down-regulating TGF-β1/SMAD and relieving oxidative stress. Pharmacol. Res. Mod. Chin. Med. 2022, 4, 100152. [Google Scholar] [CrossRef]
- Kimatu, B.M.; Fang, D.; Zhao, L.; Hu, Q. Agaricus bisporus peptide fractions confer cytoprotective ability against hydrogen peroxide-induced oxidative stress in HepG2 and Caco-2 cells. J. Food Meas. Charact. 2020, 14, 2503–2519. [Google Scholar] [CrossRef]
- Geng, X.; Tian, G.; Zhang, W.; Zhao, Y.; Zhao, L.; Wang, H.; Ng, T.B. A Tricholoma matsutake Peptide with Angiotensin Converting Enzyme Inhibitory and Antioxidative Activities and Antihypertensive Effects in Spontaneously hypertensive Rats. Sci. Rep. 2016, 6, 24130. [Google Scholar] [CrossRef]
- Li, M.; Lv, R.; Wang, C.; Ge, Q.; Du, H.; Lin, S. Tricholoma matsutake-derived peptide WFNNAGP protects against DSS-induced colitis by ameliorating oxidative stress and intestinal barrier dysfunction. Food Funct. 2021, 12, 11883–11897. [Google Scholar] [CrossRef]
- Le, P.; Kunold, E.; Macsics, R.; Rox, K.; Jennings, M.C.; Ugur, I.; Reinecke, M.; Chaves-Moreno, D.; Hackl, M.W.; Fetzer, C.; et al. Repurposing human kinase inhibitors to create an antibiotic active against drug-resistant Staphylococcus aureus, persisters and biofilms. Nat. Chem. 2020, 12, 145–158. [Google Scholar] [CrossRef]
- Theron, G.; Limberis, J.; Venter, R.; Smith, L.; Pietersen, E.; Esmail, A.; Calligaro, G.; Te Riele, J.; de Kock, M.; van Helden, P.; et al. Bacterial and host determinants of cough aerosol culture positivity in patients with drug-resistant versus drug-susceptible tuberculosis. Nat. Med. 2020, 26, 1435–1443. [Google Scholar] [CrossRef]
- Krishnan, M.; Choi, J.; Jang, A.; Kim, Y. A Novel Peptide Antibiotic, Pro10-1D, Designed from Insect Defensin Shows Antibacterial and Anti-Inflammatory Activities in Sepsis Models. Int. J. Mol. Sci. 2020, 21, 6216. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Eryngin, a novel antifungal peptide from fruiting bodies of the edible mushroom Pleurotus eryngii. Peptides 2004, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ngai, P.H.; Zhao, Z.; Ng, T.B. Agrocybin, an antifungal peptide from the edible mushroom Agrocybe cylindracea. Peptides 2005, 26, 191–196. [Google Scholar] [CrossRef]
- Leite, M.L.; da Cunha, N.B.; Costa, F.F. Antimicrobial peptides, nanotechnology, and natural metabolites as novel approaches for cancer treatment. Pharmacol. Ther. 2018, 183, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shan, Y.; Gao, H.; Wang, B.; Liu, X.; Dong, Y.; Liu, X.; Yao, N.; Zhou, Y.; Li, X.; et al. Expression of a recombinant hybrid antimicrobial peptide magainin II-cecropin B in the mycelium of the medicinal fungus Cordyceps militaris and its validation in mice. Microb. Cell Fact. 2018, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Sa-Ard, P.; Sarnthima, R.; Khammuang, S.; Kanchanarach, W. Antioxidant, antibacterial and DNA protective activities of protein extracts from Ganoderma lucidum. J. Food Sci. Technol. 2015, 52, 2966–2973. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B.; Liu, Q. Alveolarin, a novel antifungal polypeptide from the wild mushroom Polyporus alveolaris. Peptides 2004, 25, 693–696. [Google Scholar] [CrossRef]
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sonksen, C.P.; Ludvigsen, S.; Raventos, D.; Buskov, S.; Christensen, B.; De Maria, L.; et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 2005, 437, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, P.; Khanahamadi, M.; Ehsani, M.R.; Sharifan, A. Bioactive properties of Agaricus bisporus and Terfezia claveryi proteins hydrolyzed by gastrointestinal proteases. LWT 2018, 91, 322–329. [Google Scholar] [CrossRef]
- Chu, K.T.; Xia, L.; Ng, T.B. Pleurostrin, an antifungal peptide from the oyster mushroom. Peptides 2005, 26, 2098–2103. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B.; Wang, H.; Sze, S.C.; Zhang, K.Y.; Li, Q.; Lu, X. Cordymin, an antifungal peptide from the medicinal fungus Cordyceps militaris. Phytomedicine 2011, 18, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Suksomtip, M.; Poompouang, S. Isolation and characterization of an antifungal peptide from fruiting bodies of edible mushroom Lentinus squarrosulus Mont. Malays. J. Microbiol. 2016, 12, 43–49. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.X.; Ng, T.B. A peptide with HIV-1 reverse transcriptase inhibitory activity from the medicinal mushroom Russula paludosa. Peptides 2007, 28, 560–565. [Google Scholar] [CrossRef]
- Song, X.; Xu, X.; Chen, W. Antioxidant and Immunostimulatory Activities of Fermented Sour Soybean Milk Added With Polypeptides From Pleurotus eryngii. Front. Microbiol. 2022, 13, 750039. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, Q.; Liu, J.; Su, A.; Xu, H.; Li, X.; Huang, Q.; Zhou, J.; Mariga, A.M.; Yang, W. Isolation, purification and identification of immunologically active peptides from Hericium erinaceus. Food Chem. Toxicol. 2021, 151, 112111. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, L.; Rakariyatham, K.; Han, Y.; Gao, Z.; Muinde Kimatu, B.; Hu, Q.; Xiao, H. Isolation of a novel bioactive protein from an edible mushroom Pleurotus eryngii and its anti-inflammatory potential. Food Funct. 2017, 8, 2175–2183. [Google Scholar] [CrossRef]
- Yen, C.Y.; Yu, C.H.; Tsai, J.J.; Tseng, H.K.; Liao, E.C. Effects of Local Nasal Immunotherapy with FIP-fve Peptide and Denatured Tyrophagus putrescentiae for Storage Mite-Induced Airway Inflammation. Arch Immunol. Ther. Exp. 2022, 70, 6. [Google Scholar] [CrossRef]
- Yao, M.; Ge, W.; Zhou, Q.; Zhou, X.; Luo, M.; Zhao, Y.; Wei, B.; Ji, S. Exogenous glutathione alleviates chilling injury in postharvest bell pepper by modulating the ascorbate-glutathione (AsA-GSH) cycle. Food Chem. 2021, 352, 129458. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, M.; Sun, C.; Brennan, M.; Li, H.; Wang, G.; Lai, F.; Wu, H. Enzymatic preparation of immunomodulatory hydrolysates from defatted wheat germ (Triticum vulgare) globulin. Int. J. Food Sci. Technol. 2016, 51, 2556–2566. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N. Antioxidant, immunomodulatory and antiproliferative effects of gelatin hydrolysate from unicorn leatherjacket skin. J. Sci. Food Agric. 2016, 96, 3220–3226. [Google Scholar] [CrossRef]
- Xu, G.; Yuan, G.; Lu, X.; An, L.; Sheng, Y.; Du, P. Study on the effect of regulation of Cordyceps militaris polypeptide on the immune function of mice based on a transcription factor regulatory network. Food Funct. 2020, 11, 6066–6077. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.F.; Liu, Q.T.; Zhou, B.; Qiu, Y.F.; Liu, X.D.; Ma, Z.Y.; Feng, X.L.; Cao, R.B.; Chen, P.Y. The potential molecular effects of bursal septpeptide II on immune induction and antitumor activity. J. Vet. Sci. 2015, 16, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; An, L.; Sun, Y.; Xu, G.; Du, P. Improvement of Learning and Memory Induced by Cordyceps Polypeptide Treatment and the Underlying Mechanism. Evid. Based Complement. Altern. Med. 2018, 2018, 9419264. [Google Scholar] [CrossRef]
- Mori, K.; Obara, Y.; Moriya, T.; Inatomi, S.; Nakahata, N. Effects of Hericium erinaceus on amyloid β(25-35) peptide-induced learning and memory deficits in mice. Biomed. Res. 2011, 32, 67–72. [Google Scholar] [CrossRef]
- Yu, J.; Mikiashvili, N.; Bonku, R.; Smith, I.N. Allergenicity, antioxidant activity and ACE-inhibitory activity of protease hydrolyzed peanut flour. Food Chem. 2021, 360, 129992. [Google Scholar] [CrossRef]
- Kaprasob, R.; Khongdetch, J.; Laohakunjit, N.; Selamassakul, O.; Kaisangsri, N. Isolation and characterization, antioxidant, and antihypertensive activity of novel bioactive peptides derived from hydrolysis of King boletus mushroom. LWT 2022, 160, 113287. [Google Scholar] [CrossRef]
- Kang, M.G.; Kim, Y.H.; Bolormaa, Z.; Kim, M.K.; Seo, G.S.; Lee, J.S. Characterization of an antihypertensive angiotensin I-converting enzyme inhibitory peptide from the edible mushroom Hypsizygus marmoreus. Biomed. Res. Int. 2013, 2013, 283964. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Y.; Peng, K.; Wang, X.L.; Ding, Z.; Liu, L.; Xu, P.; Liu, G.Q. Isolation and Characterization of Three Antihypertension Peptides from the Mycelia of Ganoderma Lucidum (Agaricomycetes). J. Agric. Food Chem. 2019, 67, 8149–8159. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.C.; Lee, D.H.; Kim, J.H.; Yu, H.E.; Park, J.S.; Lee, J.S. Production and characterization of antihypertensive angiotensin I-converting enzyme inhibitor from Pholiota adiposa. J. Microbiol. Biotechnol. 2006, 16, 757–763. [Google Scholar]
- Jang, J.H.; Jeong, S.C.; Kim, J.H.; Lee, Y.H.; Ju, Y.C.; Lee, J.S. Characterisation of a new antihypertensive angiotensin I-converting enzyme inhibitory peptide from Pleurotus cornucopiae. Food Chem. 2011, 127, 412–418. [Google Scholar] [CrossRef]
- Girjal, V.U.; Neelagund, S.; Krishnappa, M. Antioxidant Properties of the Peptides Isolated From Ganoderma lucidum Fruiting Body. Int. J. Pept. Res. Ther. 2012, 18, 319–325. [Google Scholar] [CrossRef]
- Singh, B.P.; Aluko, R.E.; Hati, S.; Solanki, D. Bioactive peptides in the management of lifestyle-related diseases: Current trends and future perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 4593–4606. [Google Scholar] [CrossRef]
- Xiong, C.; Li, P.; Luo, Q.; Phan, C.W.; Li, Q.; Jin, X.; Huang, W. Induction of Apoptosis in HeLa Cells by a Novel Peptide from Fruiting Bodies of Morchella importuna via the Mitochondrial Apoptotic Pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 5563367. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.M.; Cao, W.; Yao, K.W.; Liu, Z.Q.; Guo, J.Y. Anti-inflammation and antioxidant effect of Cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Metab. Brain Dis. 2012, 27, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Butler, A.E.; Barreto, G.E.; Sahebkar, A. Antioxidative potential of antidiabetic agents: A possible protective mechanism against vascular complications in diabetic patients. J. Cell. Physiol. 2019, 234, 2436–2446. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell. Physiol. 2019, 234, 1300–1312. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Y.; Xu, F.; Xie, J.; Gao, X.; Li, L.; Tian, Y.; Sheng, J. Characterization of the structure, stability, and activity of hypoglycemic peptides from Moringa oleifera seed protein hydrolysates. Food Funct. 2022, 13, 3481–3494. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, C.; Wang, T.; Sun, Y.; Li, T.; Fan, G. Improvement of Biological Activity of Morchella esculenta Protein Hydrolysate by Microwave-Assisted Selenization. J. Food Sci. 2019, 84, 73–79. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ramirez, A.; Morales, D.; Soler-Rivas, C. Molecular actions of hypocholesterolaemic compounds from edible mushrooms. Food Funct. 2018, 9, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Sosalagere, C.; Adesegun Kehinde, B.; Sharma, P. Isolation and functionalities of bioactive peptides from fruits and vegetables: A reviews. Food Chem. 2022, 366, 130494. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wu, Y.; Zhang, Z.; Song, S.; Zhuang, H.; Xu, Z.; Yao, L.; Sun, M. Purification, Identification, and Sensory Evaluation of Kokumi Peptides from Agaricus bisporus Mushroom. Foods 2019, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- de Castro, R.J.S.; Sato, H.H. A response surface approach on optimization of hydrolysis parameters for the production of egg white protein hydrolysates with antioxidant activities. Biocatal. Agric. Biotechnol. 2015, 4, 55–62. [Google Scholar] [CrossRef]
- Saadi, S.; Saari, N.; Anwar, F.; Abdul Hamid, A.; Ghazali, H.M. Recent advances in food biopeptides: Production, biological functionalities and therapeutic applications. Biotechnol. Adv. 2015, 33, 80–116. [Google Scholar] [CrossRef]
- Ching, L.; Abdullah, N.; Shuib, A.S.; Aminudin, N. Characterization of antihypertensive peptides from Pleurotus cystidiosus OK Miller (abalone mushroom). In Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products (ICMBMP7), Arcachon, France, 4–7 October 2011; pp. 319–328. [Google Scholar]
- Raghavan, S.; Kristinsson, H.G. ACE-inhibitory activity of tilapia protein hydrolysates. Food Chem. 2009, 117, 582–588. [Google Scholar] [CrossRef]
- Sangtitanu, T.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Karnchanatat, A. Peptides obtained from edible mushrooms: Hericium erinaceus offers the ability to scavenge free radicals and induce apoptosis in lung cancer cells in humans. Food Funct. 2020, 11, 4927–4939. [Google Scholar] [CrossRef]
- Kimatu, B.M.; Zhao, L.; Biao, Y.; Ma, G.; Yang, W.; Pei, F.; Hu, Q. Antioxidant potential of edible mushroom (Agaricus bisporus) protein hydrolysates and their ultrafiltration fractions. Food Chem. 2017, 230, 58–67. [Google Scholar] [CrossRef]
- Rivero-Pino, F. Bioactive food-derived peptides for functional nutrition: Effect of fortification, processing and storage on peptide stability and bioactivity within food matrices. Food Chem. 2023, 406, 135046. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Yang, Y.; Fang, S.; Li, Y.; Chen, J.; Meng, Y. Mechanism of the antimicrobial activity of whey protein-epsilon-polylysine complexes against Escherichia coli and its application in sauced duck products. Int. J. Food Microbiol. 2020, 328, 108663. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Luppi, L.; Babut, T.; Petit, E.; Rolland, M.; Quemener, D.; Soussan, L.; Moradi, M.A.; Semsarilar, M. Antimicrobial polylysine decorated nano-structures prepared through polymerization induced self-assembly (PISA). Polym. Chem. 2019, 10, 336–344. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Yang, Q.; Cai, X.; Yan, A.; Tian, Y.; Du, M.; Wang, S. A specific antioxidant peptide: Its properties in controlling oxidation and possible action mechanism. Food Chem. 2020, 327, 126984. [Google Scholar] [CrossRef]
- Wongaem, A.; Reamtong, O.; Srimongkol, P.; Sangtanoo, P.; Saisavoey, T.; Karnchanatat, A. Antioxidant properties of peptides obtained from the split gill mushroom (Schizophyllum commune). J. Food Sci. Technol. 2021, 58, 680–691. [Google Scholar] [CrossRef]
- Khongdetch, J.; Laohakunjit, N.; Kaprasob, R. King Boletus mushroom-derived bioactive protein hydrolysate: Characterisation, antioxidant, ACE inhibitory and cytotoxic activities. Int. J. Food Sci. Technol. 2021, 57, 1399–1410. [Google Scholar] [CrossRef]
- Pavlicevic, M.; Marmiroli, N.; Maestri, E. Immunomodulatory peptides—A promising source for novel functional food production and drug discovery. Peptides 2022, 148, 170696. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Y.; Zhang, Z.; Wang, X.; Niu, Y.; Zhang, S.; Xu, W.; Ren, C. Advances of peptides for antibacterial applications. Colloids Surf. B Biointerfaces 2021, 202, 111682. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C.; Silva, O.N.; Lu, T.K.; Franco, O.L. Antimicrobial peptides: Role in human disease and potential as immunotherapies. Pharmacol. Ther. 2017, 178, 132–140. [Google Scholar] [CrossRef]
- Mishra, J.; Rajput, R.; Singh, K.; Bansal, A.; Misra, K. Antioxidant-Rich Peptide Fractions Derived from High-Altitude Chinese Caterpillar Medicinal Mushroom Ophiocordyceps sinensis (Ascomycetes) Inhibit Bacterial Pathogens. Int. J. Med. Mushrooms 2019, 21, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Pak, V.V.; Koo, M.S.; Kasymova, T.D.; Kwon, D.Y. Isolation and identification of peptides from soy 11S-globulin with hypocholesterolemic activity. Chem. Nat. Compd. 2005, 41, 710–714. [Google Scholar] [CrossRef]
- Valdez-Solana, M.A.; Corral-Guerrero, I.A.; Tellez-Valencia, A.; Avitia-Dominguez, C.; Meza-Velazquez, J.A.; de Casa, A.G.; Sierra-Campos, E. Cordyceps militaris Inhibited Angiotensin-Converting Enzyme through Molecular Interaction between Cordycepin and ACE C-Domain. Life 2022, 12, 1450. [Google Scholar] [CrossRef]
- Vogt, E.; Sonderegger, L.; Chen, Y.-Y.; Segessemann, T.; Künzler, M. Structural and functional analysis of peptides derived from KEX2-processed repeat proteins in agaricomycetes using reverse genetics and peptidomics. Microbiol. Spectr. 2022, 10, e02021-22. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; Magalhães, M.C.; Sousa-Lobo, J.M.; Almeida, I.F. Trending Anti-Aging Peptides. Cosmetics 2020, 7, 91. [Google Scholar] [CrossRef]
- Aursuwanna, T.; Noitang, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Puthong, S.; Reamtong, O.; Karnchanatat, A. Investigating the cellular antioxidant and anti-inflammatory effects of the novel peptides in lingzhi mushrooms. Heliyon 2022, 8, e11067. [Google Scholar] [CrossRef]
- Ko, H.J.; Kim, J.H.; Lee, G.S.; Shin, T. Sulforaphane controls the release of paracrine factors by keratinocytes and thus mitigates particulate matter-induced premature skin aging by suppressing melanogenesis and maintaining collagen homeostasis. Phytomedicine 2020, 77, 153276. [Google Scholar] [CrossRef]
- Guo, H.; Guo, S.; Liu, H. Antioxidant activity and inhibition of ultraviolet radiation-induced skin damage of Selenium-rich peptide fraction from selenium-rich yeast protein hydrolysate. Bioorg. Chem. 2020, 105, 104431. [Google Scholar] [CrossRef]
- Praca, F.G.; Viegas, J.S.R.; Peh, H.Y.; Garbin, T.N.; Medina, W.S.G.; Bentley, M. Microemulsion co-delivering vitamin A and vitamin E as a new platform for topical treatment of acute skin inflammation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110639. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Wu, L.; Tong, A.; Zhao, L.; Liu, B.; Zhao, C. Physicochemical characterization of polysaccharides from Chlorella pyrenoidosa and its anti-ageing effects in Drosophila melanogaster. Carbohydr. Polym. 2018, 185, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.L.; Wu, P.F.; Wang, S.; Zhang, J.J.; Shen, Z.C.; Luo, H.; Chen, H.; Long, L.H.; Chen, J.G.; Wang, F. Dimethyl sulfide protects against oxidative stress and extends lifespan via a methionine sulfoxide reductase A-dependent catalytic mechanism. Aging Cell 2017, 16, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Michalek, I.M.; Lelen-Kaminska, K.; Caetano Dos Santos, F.L. Peptides stimulating synthesis of extracellular matrix used in anti-aging cosmetics: Are they clinically tested? A systematic review of the literature. Australas. J. Dermatol. 2019, 60, e267–e271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, H.; Zheng, Y.; Zhang, L.; Wang, X.; Luo, Z.; Tang, J.; Lin, L.; Du, Z.; Dong, C. The effects and mechanism of collagen peptide and elastin peptide on skin aging induced by D-galactose combined with ultraviolet radiation. J. Photochem. Photobiol. B 2020, 210, 111964. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-T. Overview of mushroom cultivation and utilization as functional foods. In Mushrooms as Functional Foods; John Wiley & Son: Hoboken, NJ, USA, 2008; pp. 1–33. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Chen, Z.; Chen, Y.; Lin, Q.; Liang, Y. Exogenous Bioactive Peptides Have a Potential Therapeutic Role in Delaying Aging in Rodent Models. Int. J. Mol. Sci. 2022, 23, 1421. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Liu, X.; Wang, W.; Wang, J.; Li, X.; Sun, S. Preparation and Identification of Peptides with alpha-Glucosidase Inhibitory Activity from Shiitake Mushroom (Lentinus edodes) Protein. Foods 2023, 12, 2534. [Google Scholar] [CrossRef]
- Karaś, M. Influence of physiological and chemical factors on the absorption of bioactive peptides. Int. J. Food Sci. Technol. 2018, 54, 1486–1496. [Google Scholar] [CrossRef]
- Aguilar-Toala, J.E.; Quintanar-Guerrero, D.; Liceaga, A.M.; Zambrano-Zaragoza, M.L. Encapsulation of bioactive peptides: A strategy to improve the stability, protect the nutraceutical bioactivity and support their food applications. RSC Adv. 2022, 12, 6449–6458. [Google Scholar] [CrossRef]
- Pei, J.; Gao, X.; Pan, D.; Hua, Y.; He, J.; Liu, Z.; Dang, Y. Advances in the stability challenges of bioactive peptides and improvement strategies. Curr. Res. Food Sci. 2022, 5, 2162–2170. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Kapoor, B.; Jain, S.K.; Gowthamarajan, K.; Zacconi, F.; Chellappan, D.K.; Gupta, G.; et al. Development of mushroom polysaccharide and probiotics based solid self-nanoemulsifying drug delivery system loaded with curcumin and quercetin to improve their dissolution rate and permeability: State of the art. Int. J. Biol. Macromol. 2021, 189, 744–757. [Google Scholar] [CrossRef]
- Ohsu, T.; Amino, Y.; Nagasaki, H.; Yamanaka, T.; Takeshita, S.; Hatanaka, T.; Maruyama, Y.; Miyamura, N.; Eto, Y. Involvement of the calcium-sensing receptor in human taste perception. J. Biol. Chem. 2010, 285, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Real Hernandez, L.M.; Gonzalez de Mejia, E. Enzymatic Production, Bioactivity, and Bitterness of Chickpea (Cicer arietinum) Peptides. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1913–1946. [Google Scholar] [CrossRef] [PubMed]

| Edible Mushroom Category | Molecular Weight and Amino Acid Sequence | Mechanisms of Operation and Value | Reference |
|---|---|---|---|
| Hypsizygus marmoreus | MW of 1~5 kDa Amino acid sequence: LSMGSASLSP | Hydroxyl radical scavenging activity with IC50 of 190 μg/mL | Kang et al. (2013) [82] |
| Ganoderma Lucidum | Peptide mixtures | Activation of angiotensin I-mediated phosphorylation of endothelial nitric oxide synthase in human umbilical vein endothelial cells, with IC50 of 127.9 μmol/L | Wu et al. (2019) [83] |
| Tricholoma matsutake | MW < 5 kDa Amino acid sequence: WALKGYK | With IC50 of 0.40 μM Inhibition of ACE activity is 63.9% | Geng et al. (2016) [52] |
| Pholiota adiposa | MW of 1~2 kDa Amino acid sequence: GEGGP | With IC50 of 44 μg/mL | Koo et al. (2006) [84] |
| Pleurotus cornucopiae | Peptide mixtures | With IC50 of 0.46 mg/mL | Jang et al. (2011) [85] |
| Ganoderma lucidum | MW of 3.35 kDa | Hydroxyl radical scavenging activity is 72.87% Superoxide anion radical scavenging activity is 72.16% DPPH radical scavenging activity is 74.21% | Girjal et al. (2012) [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Gao, J.; Zhao, F.; Liu, X.; Ma, B. Bioactive Peptides from Edible Mushrooms—The Preparation, Mechanisms, Structure—Activity Relationships and Prospects. Foods 2023, 12, 2935. https://doi.org/10.3390/foods12152935
Li H, Gao J, Zhao F, Liu X, Ma B. Bioactive Peptides from Edible Mushrooms—The Preparation, Mechanisms, Structure—Activity Relationships and Prospects. Foods. 2023; 12(15):2935. https://doi.org/10.3390/foods12152935
Chicago/Turabian StyleLi, Haiyan, Ji’an Gao, Fen Zhao, Xinqi Liu, and Biao Ma. 2023. "Bioactive Peptides from Edible Mushrooms—The Preparation, Mechanisms, Structure—Activity Relationships and Prospects" Foods 12, no. 15: 2935. https://doi.org/10.3390/foods12152935
APA StyleLi, H., Gao, J., Zhao, F., Liu, X., & Ma, B. (2023). Bioactive Peptides from Edible Mushrooms—The Preparation, Mechanisms, Structure—Activity Relationships and Prospects. Foods, 12(15), 2935. https://doi.org/10.3390/foods12152935





