Encapsulation of Pineapple Peel Extracts by Ionotropic Gelation Using Corn Starch, Weissella confusa Exopolysaccharide, and Sodium Alginate as Wall Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pineapple Peels and Obtaining the Extract
2.2.1. Total Soluble Solids, pH, Color and Moisture Content
2.2.2. Microencapsulation Process
2.2.3. Process Yield
2.3. Microparticle Analysis
2.3.1. Particle Morphology and Size Distribution
2.3.2. Moisture Content
2.3.3. Wettability and Solubility
2.3.4. Bulk Density and Tapped Density
2.3.5. Flowability and Cohesiveness
2.4. Evaluation of Phenolic Content and Antioxidant Activity of the Encapsulated Compounds
2.4.1. Preparation of the Extracts from the Microcapsules
Total Phenolic Content (TPC)
Antioxidant Activity by DPPH (2,2-Diphenyl-1-picrylhydrazyl)
Antioxidant Activity by ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
2.5. Identification of Bioactive Compounds by High Performance Liquid Chromatography (UHPLC)
2.6. Fourier Transform Infrared Spectroscopy (F-TIR)
2.7. Microbiologic Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Total Soluble Solids, pH, Color, Moisture Content, and Antioxidant Activity
3.2. Process Yield
3.3. Microencapsulates
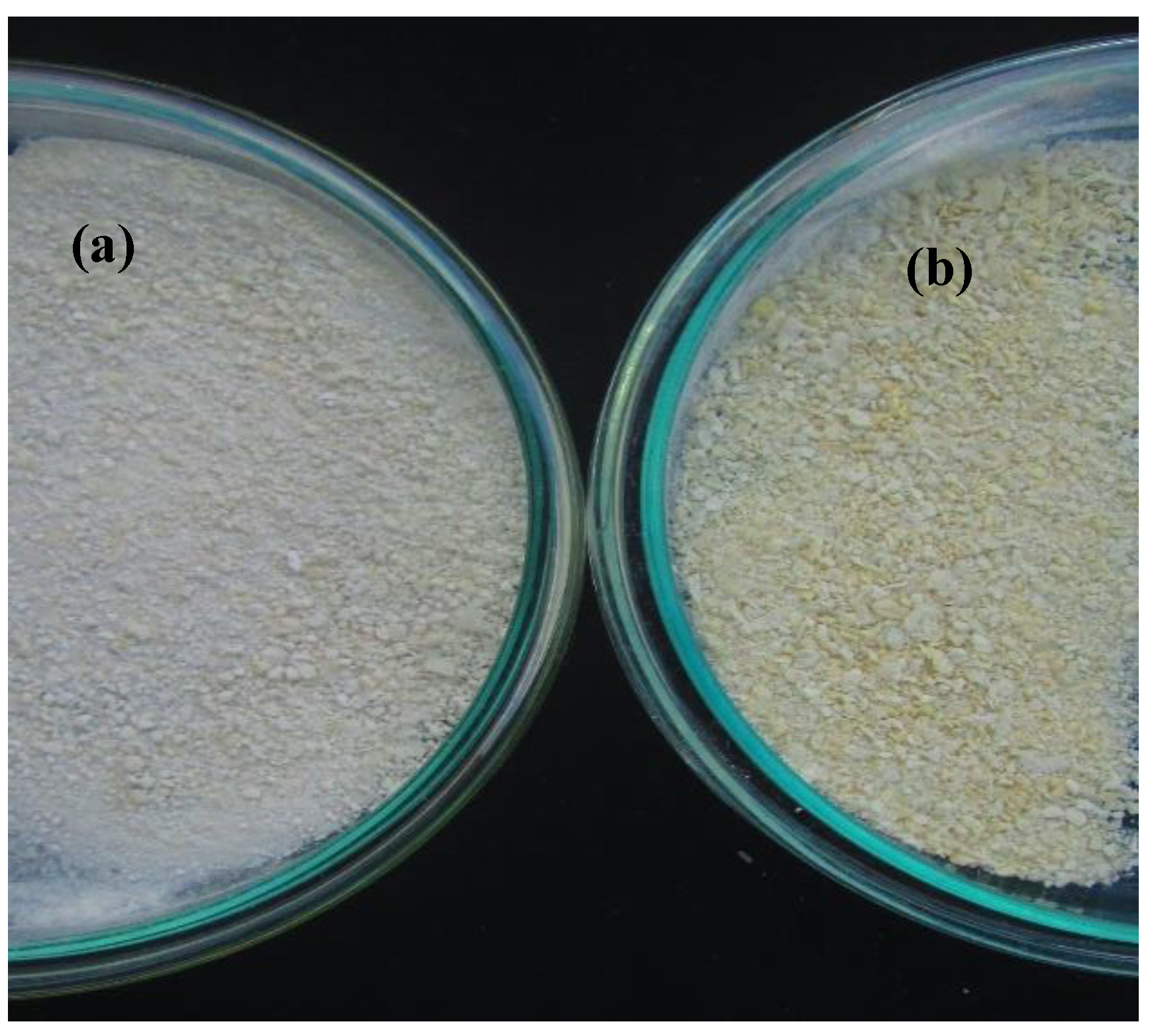
3.3.1. Particle Morphology and Size Distribution
3.3.2. Bulk Density, Tapped Density, Flowability, and Cohesiveness
3.3.3. Moisture Content and Water Activity
3.3.4. Wettability and Solubility
3.3.5. Total Phenolic Content and Antioxidant Activity by DPPH and ABTS
3.3.6. Encapsulation Efficiency
3.3.7. Compound Identification by UHPLC
3.3.8. Fourier Transform Infrared Spectroscopy (F-TIR)
3.3.9. Microbiological Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hikal, W.M.; Mahmoud, A.A.; Ahl, H.A.H.S.-A.; Bratovcic, A.; Tkachenko, K.G.; Kačániová, M.; Rodriguez, R.M. Pineapple (Ananas comosus L. Merr.), Waste Streams, Characterisation and Valorisation: An Overview. Open J. Ecol. 2021, 11, 610–634. [Google Scholar] [CrossRef]
- Polanía, M.; Londoño, L.; Ramírez, C.; Bolivar, G.; Aguilar, C.N. Valorization of pineapple waste as novel source of nutraceuticals and biofunctional compounds. Biomass Convers. Biorefin. 2022, 13, 3593–3618. [Google Scholar] [CrossRef]
- Difonzo, G.; Vollmer, K.; Caponio, F.; Pasqualone, A.; Carle, R.; Steingass, C.B. Characterisation and classification of pineapple (Ananas comosus [L.] Merr.) juice from pulp and peel. Food Control 2019, 96, 260–270. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of Pineapple Peel Extract by Spray Drying Using Maltodextrin, Inulin, and Arabic Gum as Wall Matrices. Foods 2020, 9, 718. [Google Scholar] [CrossRef]
- Dai, H.; Ou, S.; Liu, Z.; Huang, H. Pineapple peel carboxymethyl cellulose/polyvinyl alcohol/mesoporous silica SBA-15 hydrogel composites for papain immobilization. Carbohydr. Polym. 2017, 169, 504–514. [Google Scholar] [CrossRef]
- Lu, X.-H.; Sun, D.-Q.; Wu, Q.-S.; Liu, S.-H.; Sun, G.-M. Physico-Chemical Properties, Antioxidant Activity and Mineral Contents of Pineapple Genotypes Grown in China. Molecules 2014, 19, 8518–8532. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. A comparative study between natural and synthetic antioxidants: Evaluation of their performance after incorporation into biscuits. Food Chem. 2017, 216, 342–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Song, X.; Sui, X.; Qi, B.; Wang, Z.; Li, Y.; Jiang, L. Rosemary extract can be used as a synthetic antioxidant to improve vegetable oil oxidative stability. Ind. Crops Prod. 2016, 80, 141–147. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Campos, D.A.; Gómez-García, R.; Pintado, M.; Oliveira, M.C.; Santos, D.I.; Corrêa-Filho, L.C.; Moldão-Martins, M.; Alves, V.D. Optimization of Natural Antioxidants Extraction from Pineapple Peel and Their Stabilization by Spray Drying. Foods 2021, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Franco, P.; de Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.; Costa, R.; Rocha, F.; Estevinho, B.N.; Santos, L. Design of microparticles containing natural antioxidants: Preparation, characterization and controlled release studies. Powder Technol. 2017, 313, 287–292. [Google Scholar] [CrossRef]
- Bamidele, P.; Amiri-Rigi, A.; Emmambux, M.N. Encapsulation of ascorbyl palmitate in corn starch matrix by extrusion cooking: Release behavior and antioxidant activity. Food Chem. 2023, 399, 133981. [Google Scholar] [CrossRef] [PubMed]
- Haładyn, K.; Tkacz, K.; Wojdyło, A.; Nowicka, P. The Types of Polysaccharide Coatings and Their Mixtures as a Factor Affecting the Stability of Bioactive Compounds and Health-Promoting Properties Expressed as the Ability to Inhibit the α-Amylase and α-Glucosidase of Chokeberry Extracts in the Microencapsulation Process. Foods 2021, 10, 1994. [Google Scholar] [CrossRef] [PubMed]
- Sobieralska, M.; Kurek, M.A. Beta-Glucan as Wall Material in Encapsulation of Elderberry (Sambucus nigra) Extract. Plant Foods Hum. Nutr. 2019, 74, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Fangmeier, M.; Lehn, D.N.; Maciel, M.J.; de Souza, C.F.V. Encapsulation of Bioactive Ingredients by Extrusion with Vibrating Technology: Advantages and Challenges. Food Bioprocess Technol. 2019, 12, 1472–1486. [Google Scholar] [CrossRef]
- Suganya, V.; Anuradha, V. Microencapsulation and Nanoencapsulation: A Review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Emamifar, A.; Bavaisi, S. Nanocomposite coating based on sodium alginate and nano-ZnO for extending the storage life of fresh strawberries (Fragaria × ananassa Duch.). J. Food Meas. Charact. 2020, 14, 1012–1024. [Google Scholar] [CrossRef]
- Zheng, J.; Zeng, R.; Zhang, F.; Kan, J. Effects of sodium carboxymethyl cellulose on rheological properties and gelation behaviors of sodium alginate induced by calcium ions. LWT 2019, 103, 131–138. [Google Scholar] [CrossRef]
- McKinney, J.M.; Pucha, K.A.; Doan, T.N.; Wang, L.; Weinstock, L.D.; Tignor, B.T.; Fowle, K.L.; Levit, R.D.; Wood, L.B.; Willett, N.J. Sodium alginate microencapsulation of human mesenchymal stromal cells modulates paracrine signaling response and enhances efficacy for treatment of established osteoarthritis. Acta Biomater. 2021, 141, 315–332. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, B.; Tian, Y. Highly branched corn starch: Preparation, encapsulation, and release of ascorbic acid. Food Chem. 2021, 343, 128485. [Google Scholar] [CrossRef]
- Cassano, R.; Trombino, S. Drug Delivery Systems: Smart Polymeric Materials. In Advanced Polymers in Medicine; Springer International Publishing: Cham, Switzerland, 2015; pp. 341–370. [Google Scholar] [CrossRef]
- Shao, W.; Pan, X.; Liu, X.; Teng, F.; Yuan, S. Microencapsulation of Docosahexaenoic Acid Algal Oil Using Spray Drying Technology. Food Technol. Biotechnol. 2018, 56, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tian, Z.; Chen, L. A novel process for microencapsulation of fish oil with barley protein. Food Res. Int. 2011, 44, 2735–2741. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Torres, C.A.; Nunes, D.; Duarte, P.; Freitas, F.; Reis, M.A.; Fortunato, E.; Moldão-Martins, M.; da Costa, L.B.; Alves, V.D. Using a bacterial fucose-rich polysaccharide as encapsulation material of bioactive compounds. Int. J. Biol. Macromol. 2017, 104, 1099–1106. [Google Scholar] [CrossRef]
- Öner, E.T. Microbial Production of Extracellular Polysaccharides from Biomass. In Pretreatment Techniques for Biofuels and Biorefineries; Springer: Berlin/Heidelberg, Germany, 2013; pp. 35–56. [Google Scholar] [CrossRef]
- Abid, Y.; Gharsallaoui, A.; Dumas, E.; Ghnimi, S.; Azabou, S. Spray-drying microencapsulation of nisin by complexation with exopolysaccharides produced by probiotic Bacillus tequilensis-GM and Leuconostoc citreum-BMS. Colloids Surf. B Biointerfaces 2019, 181, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.K.; Michaud, P.; Singhania, R.R.; Soccol, C.R.; Pandey, A. Polysaccharides from Probiotics: New Developments as Food Additives. Food Technol. Biotechnol. 2010, 48, 451–463. [Google Scholar]
- Briceño, D. Efecto de las Bacterias Lácticas Microencapsuladas en la Calidad Microbiológica y en las Propiedades Fisicoquímicas de Salchichas Cocidas. Master’s Thesis, Universidad del Valle, Cali, Colombia, 2020. [Google Scholar]
- Yang, Y.; Peng, Q.; Guo, Y.; Han, Y.; Xiao, H.; Zhou, Z. Isolation and characterization of dextran produced by Leuconostoc citreum NM105 from manchurian sauerkraut. Carbohydr. Polym. 2015, 133, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Polanía, M.; Londoño, L.; Ramírez, C.; Bolívar, G. Influence of Ultrasound Application in Fermented Pineapple Peel on Total Phenolic Content and Antioxidant Activity. Fermentation 2022, 8, 314. [Google Scholar] [CrossRef]
- Alejandra. González Obtención y Evaluación de Polisacáridos de una Bacteria ácido Láctica para uso Como Aditivo Alimentario. Master’s Thesis, Universidad del Valle, Cali, Colombia, 2017. [Google Scholar]
- Nadzirah, K.Z.; Zainal, S.; Noriham, A.; Normah, I.; Siti Roha, A.M.; Nadya, H. Physico- chemical properties of pineapple variety N36 harvested and stored at different maturity stages. Int. Food Res. J. 2013, 20, 225–231. [Google Scholar]
- Turhan, E.Ü.; Erginkaya, Z.; Polat, S.; Özer, E.A. Design of probiotic dry fermented sausage (sucuk) production with microencapsulated and free cells of Lactobacillus rhamnosus. Turk. J. Vet. Anim. Sci. 2017, 41, 598–603. [Google Scholar] [CrossRef]
- Shaharuddin, S.; Muhamad, I.I. Microencapsulation of alginate-immobilized bagasse with Lactobacillus rhamnosus NRRL 442: Enhancement of survivability and thermotolerance. Carbohydr. Polym. 2015, 119, 173–181. [Google Scholar] [CrossRef]
- Braga, V.; Guidi, L.R.; de Santana, R.C.; Zotarelli, M.F. Production and characterization of pineapple-mint juice by spray drying. Powder Technol. 2020, 375, 409–419. [Google Scholar] [CrossRef]
- Albán, Á.B.; Ramos, M.; Alvarez, M. Estudio de la Vida Útilde Fresas (Fragaria vesca) Mediante Tratamiento con RadiaciónUltravioleta de Onda Corta (UV-C). Rev. Tecnol. Espolrte 2010, 23, 17–24. Available online: http://www.rte.espol.edu.ec/index.php/tecnologica/article/view/51 (accessed on 15 March 2023).
- Sun, K.A.; Thumthanaruk, B.; Lekhavat, S.; Jumnongpon, R. Effect of spray drying conditions on physical characteristics of coconut sugar powder. Int. Food Res. J. 2016, 23, 1315–1319. [Google Scholar]
- Ee, S.C.; Jamilah, B.; Muhammad, K.; Hashim, D.M.; Adzahan, N. Physico-chemical properties of spray-dried red pitaya (Hylocereus polyrhizus) peel powder during storage. Int. Food Res. J. 2014, 21, 155–160. [Google Scholar]
- Fernandes, R.V.D.B.; Borges, S.V.; Botrel, D.A. Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Rocha, G.A.; Fávaro-Trindade, C.S.; Grosso, C.R.F. Microencapsulation of lycopene by spray drying: Characterization, stability and application of microcapsules. Food Bioprod. Process. 2012, 90, 37–42. [Google Scholar] [CrossRef]
- Gutiérrez Avella, D.M.; García, C.A.O.; Cisneros, M. Medición de Fenoles y Actividad Antioxidante en Malezas Usadas para Alimentación Animal; Universidad Autónoma de Querétaro: Santiago de Querétaro, Mexico, 2008; Available online: https://www.cenam.mx/simposio2008/sm_2008/memorias/M2/SM2008-M220-1108.pdf (accessed on 17 April 2023).
- Escobar-Avello, D.; Avendaño-Godoy, J.; Santos, J.; Lozano-Castellón, J.; Mardones, C.; von Baer, D.; Luengo, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Gómez-Gaete, C. Encapsulation of Phenolic Compounds from a Grape Cane Pilot-Plant Extract in Hydroxypropyl Beta-Cyclodextrin and Maltodextrin by Spray Drying. Antioxidants 2021, 10, 1130. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Mesa-Vanegas, A.M.; Zapata-Uribe, S.; Arana, L.M.; Zapata, I.C.; Monsalve, Z.; Rojano, B. Actividad antioxidante de extractos de diferente polaridad de Ageratum conyzoides L. Bol. Latinoam. Y Caribe Plantas Med. Y Aromat. 2015, 14, 1–10. [Google Scholar]
- Selani, M.; Guidolin, S.; Tadeu, C.; Ratnayake, W.S.; Flores, R.A.; Bianchini, A. Characterisation and potential application of pineapple pomace in an extruded product for fibre enhancement. Food Chem. 2014, 163, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assawarachan, R.; Noomhorm, A. Changes in color and rheological behavior of pineapple concentrate through various evaporation methods. Int. J. Agric. Biol. Eng. 2010, 3, 74–84. [Google Scholar]
- Ding, P.; Syazwani, S. Physicochemical quality, antioxidant compounds and activity of MD-2 pineapple fruit at five ripening stages. Int. Food Res. J. 2016, 23, 549–555. [Google Scholar]
- Rashima, R.S.; Maizura, M.; Hafzan, W.M.W.N.; Hazzeman, H. Physicochemical properties and sensory acceptability of pineapples of different varieties and stages of maturity. Food Res. 2019, 3, 491–500. [Google Scholar] [CrossRef]
- Samarakoon, S.M.G.K.; Rajapakse, C.S.K. Peel of pineapple (Ananas comosus) as a potential source of antioxidants and photo-protective agents for sun protection cosmetics. Med. Plants Int. J. Phytomed. Relat. Ind. 2022, 14, 95–104. [Google Scholar] [CrossRef]
- Valdés García, A.; Martínez, M.I.D.; Landete, M.P.; Moya, M.S.P.; Beltrán, A. Sanahuja Potential of Industrial Pineapple (Ananas comosus (L.) Merrill) By-Products as Aromatic and Antioxidant Sources. Antioxidants 2021, 10, 1767. [Google Scholar] [CrossRef]
- Dey, T.B.; Kuhad, R.C. Upgrading the antioxidant potential of cereals by their fungal fermentation under solid-state cultivation conditions. Lett. Appl. Microbiol. 2014, 59, 493–499. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef]
- Muzaffar, K.; Kumar, P. Parameter optimization for spray drying of tamarind pulp using response surface methodology. Powder Technol. 2015, 279, 179–184. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Wang, L.; Charles, V.; Rooke, J.C.; Su, B.-L. Robust and Biocompatible Hybrid Matrix with Controllable Permeability for Microalgae Encapsulation. ACS Appl. Mater. Interfaces 2016, 8, 8939–8946. [Google Scholar] [CrossRef]
- Kalajahi, S.E.M.; Ghandiha, S. Optimization of spray drying parameters for encapsulation of Nettle (Urtica dioica L.) extract. LWT 2022, 158, 113149. [Google Scholar] [CrossRef]
- Ipar, V.S.; Singhal, R.S.; Devarajan, P.V. An innovative approach using microencapsulated turmeric oleoresin to develop ready-to-use turmeric milk powder with enhanced oral bioavailability. Food Chem. 2022, 373, 131400. [Google Scholar] [CrossRef] [PubMed]
- Pongjanyakul, T.; Rongthong, T. Enhanced entrapment efficiency and modulated drug release of alginate beads loaded with drug–clay intercalated complexes as microreservoirs. Carbohydr. Polym. 2010, 81, 409–419. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, L.; Zhang, J.; Ou, G.; Xiang, Y. Determination of Contents of the Main Nutritional Components, Functional Components of Akebia trifoliata Pericarp and the Antioxidant Activity of Its Extracts. Sci. Technol. Food Ind. 2022, 43, 388–394. [Google Scholar]
- Remanan, M.K.; Zhu, F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chem. 2021, 353, 128534. [Google Scholar] [CrossRef]
- Lin, L.; Cai, C.; Gilbert, R.G.; Li, E.; Wang, J.; Wei, C. Relationships between amylopectin molecular structures and functional properties of different-sized fractions of normal and high-amylose maize starches. Food Hydrocoll. 2016, 52, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Alves, I.; Rodrigues, M.Z.; Pinto, M.R.M.R.; Vanzela, E.S.L.; Stringheta, P.C.; Perrone, T.; Ramos, A.M. Morphological characterization of pequi extract microencapsulated through spray drying. Int. J. Food Prop. 2017, 20 (Suppl. S2), 1298–1305. [Google Scholar] [CrossRef] [Green Version]
- Villacrez, J.L.; Carriazo, J.G.; Osorio, C. Microencapsulation of Andes Berry (Rubus glaucus Benth.) Aqueous Extract by Spray Drying. Food Bioprocess Technol. 2014, 7, 1445–1456. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Effect of Spray Drying of Four Fruit Juices on Physicochemical, Phytochemical and Antioxidant Properties. J. Food Process. Preserv. 2015, 39, 1656–1664. [Google Scholar] [CrossRef]
- Feltre, G.; Almeida, F.S.; Sato, A.C.K.; Dacanal, G.C.; Hubinger, M.D. Alginate and corn starch mixed gels: Effect of gelatinization and amylose content on the properties and in vitro digestibility. Food Res. Int. 2020, 132, 109069. [Google Scholar] [CrossRef]
- Ribeiro, L.C.; da Costa, J.M.C.; Afonso, M.R.A. Flow behavior of cocoa pulp powder containing maltodextrin. Braz. J. Food Technol. 2020, 23. [Google Scholar] [CrossRef]
- Leturia, M.; Benali, M.; Lagarde, S.; Ronga, I.; Saleh, K. Characterization of flow properties of cohesive powders: A comparative study of traditional and new testing methods. Powder Technol. 2014, 253, 406–423. [Google Scholar] [CrossRef]
- Niazi, M.B.K. Production of Plasticized Thermoplastic Starch by Spray Drying; University of Groningen: Groningen, The Netherlands, 2013. [Google Scholar]
- Šeregelj, V.; Ćetković, G.; Čanadanović-Brunet, J.; Šaponjac, V.T.; Vulić, J.; Stajčić, S. Encapsulation and Degradation Kinetics of Bioactive Compounds from Sweet Potato Peel During Storage. Food Technol. Biotechnol. 2020, 58, 314–324. [Google Scholar] [CrossRef]
- Zhang, Z.; Law, D.; Lian, G. Characterization Methods of Encapsulates. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: New York, NY, USA, 2010; pp. 101–125. [Google Scholar] [CrossRef]
- Beldarrain-Iznaga, T.; Villalobos-Carvajal, R.; Leiva-Vega, J.; Sevillano, E. Armesto Influence of multilayer microencapsulation on the viability of Lactobacillus casei using a combined double emulsion and ionic gelation approach. Food Bioprod. Process. 2020, 124, 57–71. [Google Scholar] [CrossRef]
- Jittanit, W.; Niti-Att, S.; Techanuntachaikul, O. Study of spray drying of pineapple juice using maltodextrin as an adjunct. Chiang Mai J. Sci. 2010, 37, 498–506. [Google Scholar]
- Moreira, G.É.G.; Costa, M.G.M.; de Souza, A.C.R.; de Brito, E.S.; Medeiros, M.D.F.D.D.; de Azeredo, H.M.C. Physical properties of spray dried acerola pomace extract as affected by temperature and drying aids. LWT Food Sci. Technol. 2009, 42, 641–645. [Google Scholar] [CrossRef] [Green Version]
- Martinić, A.; Kalušević, A.; Lević, S.; Nedović, V.; Cebin, A.V.; Karlović, S.; Špoljarić, I.; Mršić, G.; Žižek, K.; Komes, D. Microencapsulation of Dandelion (Taraxacum officinale L.) Leaf Extract by Spray Drying. Food Technol. Biotechnol. 2022, 60, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Norcino, L.B.; Mendes, J.F.; Figueiredo, J.D.A.; Oliveira, N.L.; Botrel, D.A.; Mattoso, L.H.C. Development of alginate/pectin microcapsules by a dual process combining emulsification and ultrasonic gelation for encapsulation and controlled release of anthocyanins from grapes (Vitis labrusca L.). Food Chem. 2022, 391, 133256. [Google Scholar] [CrossRef]
- Ricci, J.; Mejia, A.A.; Versari, A.; Chiarello, E.; Bordoni, A.; Parpinello, G.P. Microencapsulation of polyphenolic compounds recovered from red wine lees: Process optimization and nutraceutical study. Food Bioprod. Process. 2022, 132, 1–12. [Google Scholar] [CrossRef]
- Bustamante, A.; Hinojosa, A.; Robert, P.; Escalona, V. Extraction and microencapsulation of bioactive compounds from pomegranate (Punica granatum var. Wonderful) residues. Int. J. Food Sci. Technol. 2017, 52, 1452–1462. [Google Scholar] [CrossRef]
- Tan, S.; Kha, T.; Parks, S.; Stathopoulos, C.; Roach, P. Optimising the Encapsulation of an Aqueous Bitter Melon Extract by Spray-Drying. Foods 2015, 4, 400–419. [Google Scholar] [CrossRef] [Green Version]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The physicochemical properties of spray-dried watermelon powders. Chem. Eng. Process. Process Intensif. 2007, 46, 386–392. [Google Scholar] [CrossRef]
- Daza, L.D.; Fujita, A.; Fávaro-Trindade, C.S.; Rodrigues-Ract, J.N.; Granato, D.; Genovese, M.I. Effect of spray drying conditions on the physical properties of Cagaita (Eugenia dysenterica DC.) fruit extracts. Food Bioprod. Process. 2016, 97, 20–29. [Google Scholar] [CrossRef]
- Hoyos-Leyva, J.D.; Bello-Pérez, L.A.; Alvarez-Ramirez, J.; Garcia, H.S. Microencapsulation using starch as wall material: A review. Food Rev. Int. 2018, 34, 148–161. [Google Scholar] [CrossRef]
- No, J.; Shin, M. Preparation and characteristics of octenyl succinic anhydride-modified partial waxy rice starches and encapsulated paprika pigment powder. Food Chem. 2019, 295, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Kobo, G.K.; Kaseke, T.; Fawole, O.A. Micro-Encapsulation of Phytochemicals in Passion Fruit Peel Waste Generated on an Organic Farm: Effect of Carriers on the Quality of Encapsulated Powders and Potential for Value-Addition. Antioxidants 2022, 11, 1579. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Emam-Djomeh, Z.; Mousavi, M.; Kobarfard, F.; Zbicinski, I. Developing spray-dried powders containing anthocyanins of black raspberry juice encapsulated based on fenugreek gum. Adv. Powder Technol. 2015, 26, 462–469. [Google Scholar] [CrossRef]
- Nthimole, T.; Kaseke, T.; Fawole, O.A. Micro-Encapsulation and Characterization of Anthocyanin-Rich Raspberry Juice Powder for Potential Applications in the Food Industry. Processes 2022, 10, 1038. [Google Scholar] [CrossRef]
- Essifi, K.; Brahmi, M.; Berraaouan, D.; Ed-Daoui, A.; El Bachiri, A.; Fauconnier, M.-L.; Tahani, A. Influence of Sodium Alginate Concentration on Microcapsules Properties Foreseeing the Protection and Controlled Release of Bioactive Substances. J. Chem. 2021, 2021, 5531479. [Google Scholar] [CrossRef]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of Probiotic Cells for Food Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef]
- Shi, L.-E.; Li, Z.-H.; Zhang, Z.-L.; Zhang, T.-T.; Yu, W.-M.; Zhou, M.-L.; Tang, Z.-X. Encapsulation of Lactobacillus bulgaricus in carrageenan-locust bean gum coated milk microspheres with double layer structure. LWT Food Sci. Technol. 2013, 54, 147–151. [Google Scholar] [CrossRef]
- Yousefi, S.; Emam-Djomeh, Z.; Mousavi, S.M. Effect of carrier type and spray drying on the physicochemical properties of powdered and reconstituted pomegranate juice (Punica granatum L.). J. Food Sci. Technol. 2011, 48, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quoc, L.P.T. Effect of Different Carrier Agents on Physicochemical Properties of Spray-dried Pineapple (Ananas comosus Merr.) Powder. J. Korean Chem. Soc. 2020, 64, 259–266. [Google Scholar]
- Šárka, E.; Dvořáček, V. Waxy starch as a perspective raw material (a review). Food Hydrocoll. 2017, 69, 402–409. [Google Scholar] [CrossRef]
- Phisut, N. Spray drying technique of fruit juice powder: Some factors influencing the properties of product. Int. Food Res. J. 2012, 19, 1297–1306. Available online: http://www.agris.upm.edu.my:8080/dspace/handle/0/11737 (accessed on 24 September 2022).
- Anuar, N.H.S.; Kormin, F.; Isa, N.L.M.; Iwansyah, A.C.; Abu Bakar, M.F.; Fuzi, S.F.Z.M.; Sabran, F. Spray Drying Microencapsulation of Kantan Extract (Etlingera Elatior) with Various Wall Materials. IOP Conf. Ser. Earth Environ. Sci. 2021, 736, 012007. [Google Scholar] [CrossRef]
- Olga, G.; Styliani, C.; Ioannis, R.G. Coencapsulation of Ferulic and Gallic acid in hp-b-cyclodextrin. Food Chem. 2015, 185, 33–40. [Google Scholar] [CrossRef]
- Wongverawattanakul, C.; Suklaew, P.; Chusak, C.; Adisakwattana, S.; Thilavech, T. Encapsulation of Mesona chinensis Benth Extract in Alginate Beads Enhances the Stability and Antioxidant Activity of Polyphenols under Simulated Gastrointestinal Digestion. Foods 2022, 11, 2378. [Google Scholar] [CrossRef]
- Lavelli, V.; Sri, P.S.C. Harsha Microencapsulation of grape skin phenolics for pH controlled release of antiglycation agents. Food Res. Int. 2019, 119, 822–828. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Extraction of bromelain from pineapple peels. Food Sci. Technol. Int. 2011, 17, 395–402. [Google Scholar] [CrossRef]
- Li, T.; Shen, P.; Liu, W.; Liu, C.; Liang, R.; Yan, N.; Chen, J. Major Polyphenolics in Pineapple Peels and their Antioxidant Interactions. Int. J. Food Prop. 2014, 17, 1805–1817. [Google Scholar] [CrossRef]
- Rasheed, A.; Cobham, E.I.; Zeighmami, M.; Ong, S.P. Extraction of Phenolic Compounds from Pineapple Fruit. In Proceedings of the 2nd International Symposium on Processing & Drying of Foods, Fruits & Vegetables, Kuala Lumpur, Malaysia, 18–19 June 2012; Volume 2, pp. 8–19. [Google Scholar]
- Roda, A.; Lucini, L.; Torchio, F.; Dordoni, R.; de Faveri, D.M.; Lambri, M. Metabolite profiling and volatiles of pineapple wine and vinegar obtained from pineapple waste. Food Chem. 2017, 229, 734–742. [Google Scholar] [CrossRef]
- Lubaina, S.; Renjith, P.R.; Roshni, A.S. Identification and Quantification of Polyphenols from Pineapple Peel by High Performance Liquid Chromatography Analysis. Adv. Zool. Bot. 2020, 8, 431–438. [Google Scholar] [CrossRef]
- Veras, K.S.; Fachel, F.N.S.; Teixeira, H.F.; Koester, L.S. Technological strategies applied for rosmarinic acid delivery through different routes—A review. J. Drug Deliv. Sci. Technol. 2022, 68, 103054. [Google Scholar] [CrossRef]
- Rahbardar, M.G.; Hosseinzadeh, H. Effects of rosmarinic acid on nervous system disorders: An updated review. Naunyn Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1779–1795. [Google Scholar] [CrossRef]
- Sik; Kapcsándi, V.; Székelyhidi, R.; Hanczné, E.L.; Ajtony, Z. Recent Advances in the Analysis of Rosmarinic Acid From Herbs in the Lamiaceae Family. Nat. Prod. Commun. 2019, 14, 1934578X1986421. [Google Scholar] [CrossRef] [Green Version]
- Esquivel-González, E.; Medina-Torres, L.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M.; Rocha-Guzmán, N.E.; Calderas, F.; Varela—Santos, E.D.C. Microencapsulation of betanins by spray drying with mixtures of sweet potato starch and maltodextrin as wall materials to prepare natural pigments delivery systems. J. Food Process. Preserv. 2022, 46, e16431. [Google Scholar] [CrossRef]
- Fang, J.; Fowler, P.A.; Tomkinson, J.; Hill, C.A.S. The preparation and characterisation of a series of chemically modified potato starches. Carbohydr. Polym. 2002, 47, 245–252. [Google Scholar] [CrossRef]
- Dai, H.; Ou, S.; Huang, Y.; Huang, H. Utilization of pineapple peel for production of nanocellulose and film application. Cellulose 2018, 25, 1743–1756. [Google Scholar] [CrossRef]
- Lubaina, S.; Renjith, P.R.; Dinesh, K.V. Babu Phytochemical Analysis and FT-IR Fingerprinting of Pineapple Peel-A Natural Resource of Bioactive Compounds. Int. J. Pharm. Biol. Sci. 2019, 9, 1229–1237. [Google Scholar]
- Mendez-Flores, A.; Hérnandez-Almanza, A.; Sáenz-Galindo, A.; Morlett-Chávez, J.; Aguilar, C.N.; Ascacio-Valdés, J. Ultrasound-assisted extraction of antioxidant polyphenolic compounds from Nephelium lappaceum L. (Mexican variety) husk. Asian Pac. J. Trop. Med. 2018, 11, 676–681. [Google Scholar]
- Pongjanyakul, T.; Puttipipatkhachorn, S. Xanthan–alginate composite gel beads: Molecular interaction and in vitro characterization. Int. J. Pharm. 2007, 331, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.S.; de Lima, P.M.; de Sá, S.H.G.; Chen, D.; Campanella, O.H.; Rodrigues, C.E.d.C.; Favaro-Trindade, C.S. Encapsulation of Rich-Carotenoids Extract from Guaraná (Paullinia cupana) Byproduct by a Combination of Spray Drying and Spray Chilling. Foods 2022, 11, 2557. [Google Scholar] [CrossRef]
- Ivanov, M.; Kostić, M.; Stojković, D.; Soković, M. Rosmarinic acid–Modes of antimicrobial and antibiofilm activities of a common plant polyphenol. S. Afr. J. Bot. 2022, 146, 521–527. [Google Scholar] [CrossRef]





| Parameter | Results 1 | |
|---|---|---|
| Moisture content (w.b%) | 95.90 ± 0.05 | |
| pH | 3.85 ± 0.02 | |
| Color | L * | 24.65 ± 0.13 |
| a * | 1.75 ± 0.03 | |
| b * | 29.06 ± 0.14 | |
| Soluble solids | °Brix | 10.13 ± 0.06 |
| Total phenolic content | Folin–Ciocalteau (mg GAE/g d.m) | 882.71 ± 2.07 |
| Antioxidant activity | DPPH (% inhibition) | 63.53 ± 1.10 |
| ABTS (% inhibition) | 80.06 ± 2.18 | |
| Compound | tr (min) | MQL (mg/kg) | Concentration (mg/kg) |
|---|---|---|---|
| Theobromine | 2.6 | 0.1 | <0.1 * |
| Theophylline | 2.8 | 0.1 | <0.1 * |
| Epigallocatechin (EGC) | 2.9 | 0.1 | <0.1 * |
| Catechin © | 3.0 | 0.1 | <0.1 * |
| Epicatechin (EC) | 3.2 | 0.1 | <0.1 * |
| p-Hydroxybenzoic acid | 3.2 | 0.1 | <0.1 * |
| Caffeine | 3.1 | 0.1 | <0.1 * |
| Caffeic acid | 3.2 | 0.1 | 5.1 ± 0.1 |
| Vanillic acid | 3.2 | 0.1 | 5.7 ± 0.2 |
| Rutin | 3.2 | 0.1 | 0.1 ± 0.01 |
| p-coumaric acid | 3.6 | 0.1 | 2.6 ± 0.1 |
| Epicatechin gallate (ECG) | 3.6 | 0.1 | <0.1 * |
| Ferulic acid | 3.7 | 0.1 | 3.9 ± 0.2 |
| Quercetin | 4.5 | 0.1 | 0.5 ± 0.01 |
| Rosmarinic acid | 4.0 | 2.0 | 10.0 ± 0.2 |
| Cyanidin | 3.8 | 0.1 | <0.1 * |
| Luteolin | 4.4 | 0.1 | <0.1 * |
| Kaempferol | 4.9 | 0.1 | <0.1 * |
| Trans-cinnamic acid | 3.7 | 0.4 | <0.1 * |
| Naringenin | 4.8 | 0.1 | <0.1 * |
| Pelargonidin | 3.6 | 0.1 | <0.1 * |
| Apigenin | 4.8 | 0.1 | <0.1 * |
| Pinocembrin | 5.8 | 0.1 | <0.1 * |
| Carnosic acid | 7.3 | 0.4 | <0.1 * |
| Ursolic acid | 8.6 | 0.1 | <0.1 * |
| Cyanidin 3-rutinoside | 3.0 | 0.1 | <0.1 * |
| Pelargonidin 3-glucoside | 3.1 | 0.1 | <0.1 * |
| Quercetin 3-glucoside | 3.6 | 0.1 | <0.1 * |
| Kaempferol 3-glucoside | 3.8 | 0.1 | <0.1 * |
| Rutin | 3.5 | 0.1 | <0.1 * |
| Gallic acid | 1.8 | 0.1 | 1.7 ± 0.1 |
| Microorganism | Permissible Range | Results |
|---|---|---|
| Mesophilic aerobic bacterial count, CFU/g | 10,000–50,000 | Absence |
| Escherichia coli count, in CFU/g | <10 | Absence |
| Fungi and yeast count, CFU/g | 50–500 | Absence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polanía, A.M.; Ramírez, C.; Londoño, L.; Bolívar, G.; Aguilar, C.N. Encapsulation of Pineapple Peel Extracts by Ionotropic Gelation Using Corn Starch, Weissella confusa Exopolysaccharide, and Sodium Alginate as Wall Materials. Foods 2023, 12, 2943. https://doi.org/10.3390/foods12152943
Polanía AM, Ramírez C, Londoño L, Bolívar G, Aguilar CN. Encapsulation of Pineapple Peel Extracts by Ionotropic Gelation Using Corn Starch, Weissella confusa Exopolysaccharide, and Sodium Alginate as Wall Materials. Foods. 2023; 12(15):2943. https://doi.org/10.3390/foods12152943
Chicago/Turabian StylePolanía, Anna María, Cristina Ramírez, Liliana Londoño, German Bolívar, and Cristobal Noe Aguilar. 2023. "Encapsulation of Pineapple Peel Extracts by Ionotropic Gelation Using Corn Starch, Weissella confusa Exopolysaccharide, and Sodium Alginate as Wall Materials" Foods 12, no. 15: 2943. https://doi.org/10.3390/foods12152943









