The Effects of Poly-γ-Glutamic Acid on the Postharvest Physiology and Quality of Strawberry cv. Hongyan during Cold Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Assays of Weight Loss, Decay Rate, and Index of Fruit
2.3. Assays of Antioxidant Enzymes in Fruit
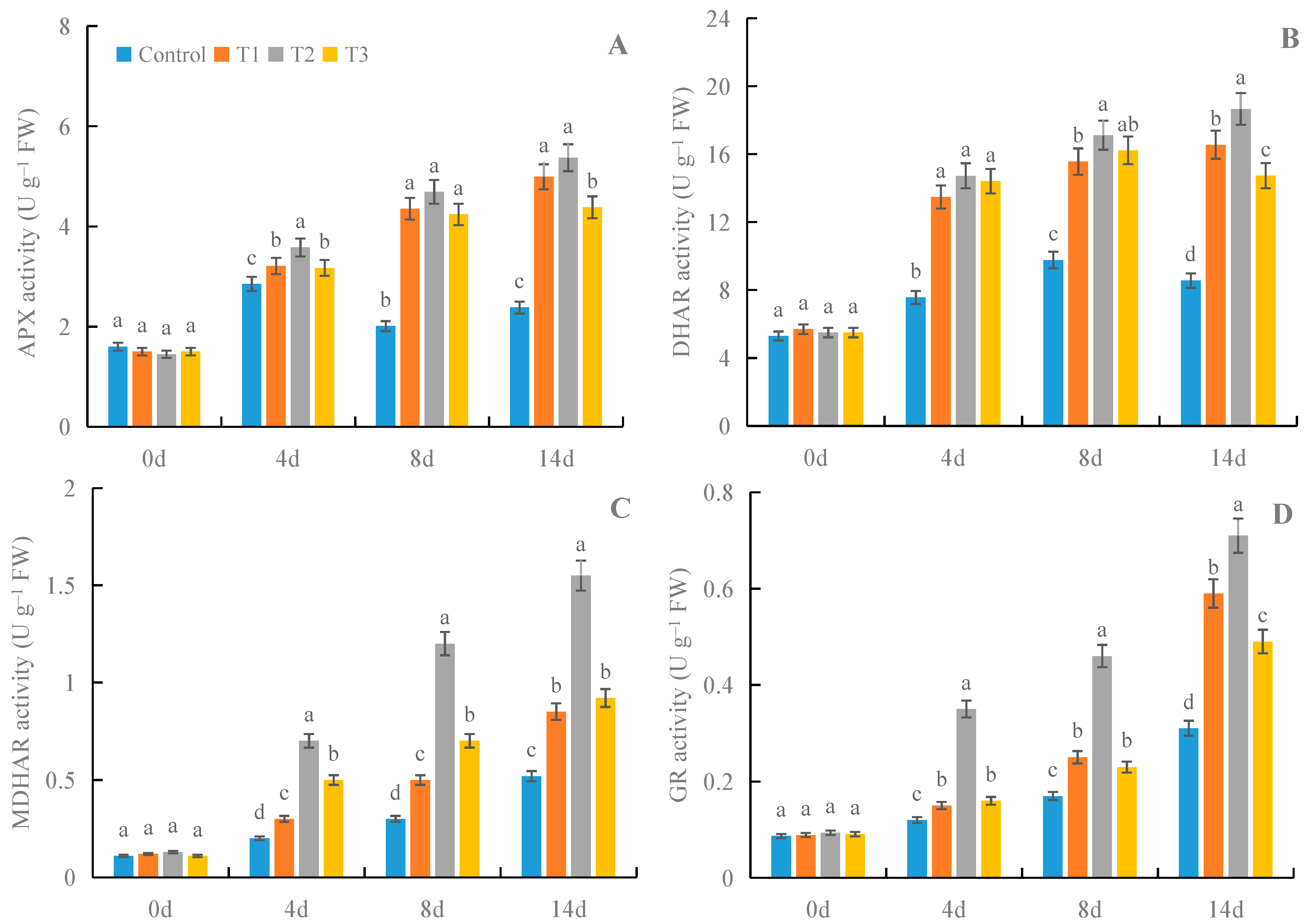
2.4. Assays of Enzymes in AsA-GSH Cycle in Fruit
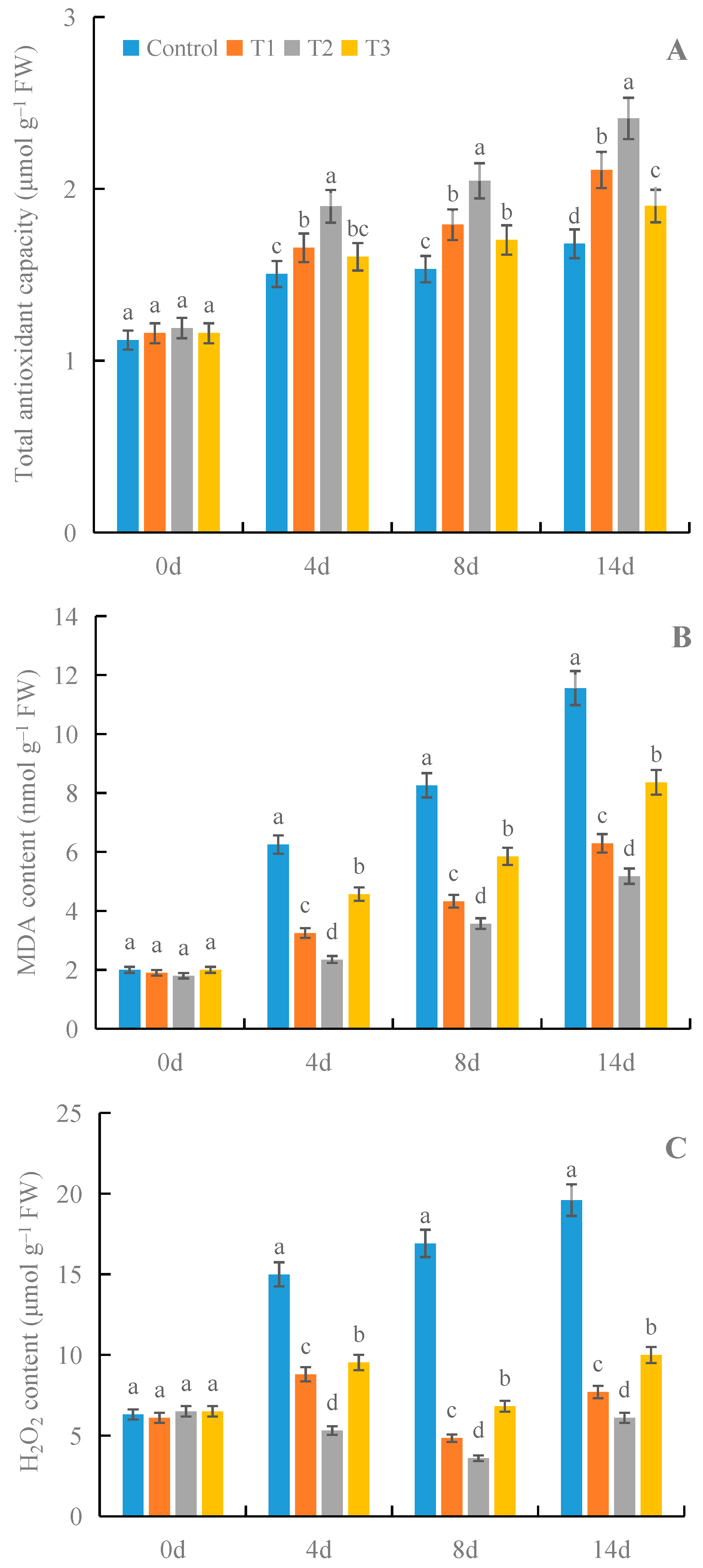
2.5. Assays of Total Antioxidant Capacity and the Contents of MDA and H2O2 in Fruit
2.6. Assay of Fruit Firmness
2.7. Assays of Enzymes Responsible for Cell Wall Degradation in Fruit
2.8. Measurement of Fruit Color Parameters
2.9. Determination of Fruit Nutritional Quality
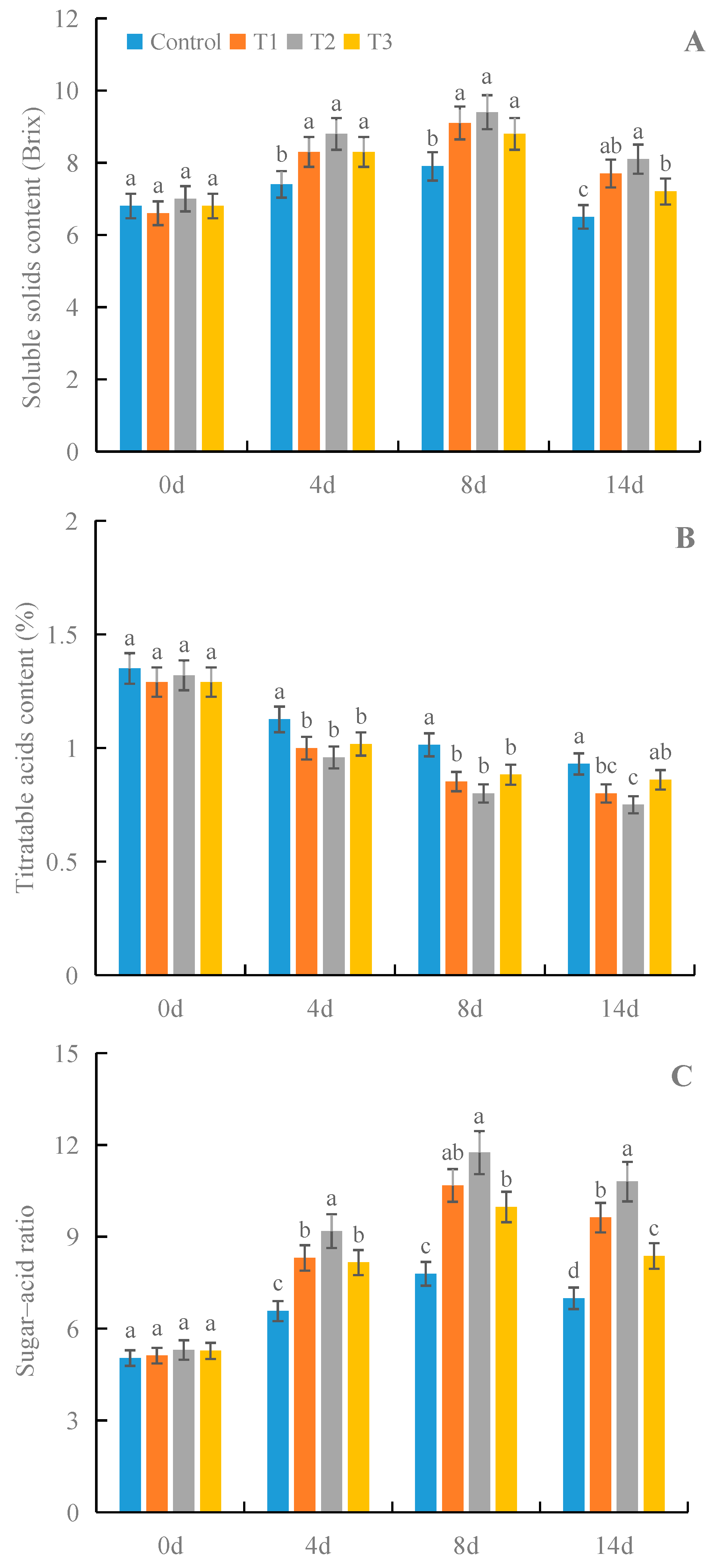
2.10. Determination of Fruit Sugar−Acid Ratio
2.11. Statistical Analysis
3. Results
3.1. Effects of γ-PGA on the Appearance and Freshness of Strawberry under Cold Storage
3.2. Effects of γ-PGA on the Activities of SOD, POD, and CAT in Fruit under Cold Storage
3.3. Effects of γ-PGA on the Activities of Enzymes in AsA-GSH Cycle in Fruit under Cold Storage
3.4. Effects of γ-PGA on Total Antioxidant Capacity and the Contents of MDA and H2O2 in Fruit under Cold Storage
3.5. Effects of γ-PGA on Fruit Firmness and the Activities of Enzymes Responsible for Cell Wall Degradation under Cold Storage
3.6. Effects of γ-PGA on Fruit Color Parameters of Strawberry under Cold Storage
3.7. Effects of γ-PGA on Fruit Nutritional Quality of Strawberry under Cold Storage
3.8. Effects of γ-PGA on the Contents of SSs and TAs and the SAR of Strawberry Fruit under Cold Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, Q.; Kurubas, M.S.; Ustun, H.; Balkhi, M.; Erkan, M. Evaluation of foliar organic fertilizer, biofertilizer and biological fungicide on the antioxidant compounds and postharvest quality attributes of strawberry fruit. Erwerbs-Obstbau 2022, 64, 365–376. [Google Scholar] [CrossRef]
- Gundogdu, M.; Berk, S.; Yıldız, K.; Kaki, B.; Tuna, S.; Canan, I.; Okatan, V.; Ercisli, S. Influence of foliar application with gibberellic acid on phenolic and bioactive compounds of strawberry fruits. Erwerbs-Obstbau 2021, 63, 15–23. [Google Scholar] [CrossRef]
- Wang, K.; Li, T.; Chen, S.; Li, Y.; Rashid, A. The biochemical and molecular mechanisms of softening inhibition by chitosan coating in strawberry fruit (Fragaria × ananassa) during cold storage. Sci. Hortic. 2020, 271, 109483. [Google Scholar] [CrossRef]
- Bahmani, R.; Razavi, F.; Mortazavi, S.N.; Gohari, G.; Juárez-Maldonado, A. Evaluation of proline-coated chitosan nanoparticles on decay control and quality preservation of strawberry fruit (cv. Camarosa) during cold storage. Horticulturae 2022, 8, 648. [Google Scholar] [CrossRef]
- Bal, E.; Ürün, B.A. Effects of chitosan coating with putrescine on bioactive compounds and quality of strawberry cv. San Andreas during cold storage. Erwerbs-Obstbau 2021, 63, 7–14. [Google Scholar] [CrossRef]
- Panou, A.A.; Akrida-Demertzi, K.; Demertzis, P.; Riganakos, K.A. Effect of gaseous ozone and heat treatment on quality and shelf life of fresh strawberries during cold storage. Int. J. Fruit Sci. 2021, 21, 218–231. [Google Scholar] [CrossRef]
- Shehata, S.A.; Emad, A.A.; Marwa, R.A.; Reda, M.M.; Rwotonen, I.B.; Karima, F.A. Effect of some citrus essential oils on post-harvest shelf life and physicochemical quality of strawberries during cold storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Alikhani-Koupaei, M. Exogenous phytosulfokine α (PSKα) applying delays senescence and relief decay in strawberry fruits during cold storage by sufficient intracellular ATP and NADPH availability. Food Chem. 2021, 336, 127685. [Google Scholar] [CrossRef]
- Donghee, A.; Inhwan, K.; Jeong-Ho, L.; Jeong, H.C.; Kee-Jai, P.; Jihyun, L. The effect of high CO2 treatment on targeted metabolites of ‘Seolhyang’ strawberry (Fragaria × ananassa) fruits during cold storage. LWT-Food Sci. Technol. 2021, 143, 111156. [Google Scholar]
- Balogun-Agbaje, O.A.; Odeniyi, O.A.; Odeniyi, M.A. Drug delivery applications of poly-γ-glutamic acid. Futur. J. Pharm. Sci. 2021, 7, 125. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Yu, B. Poly-γ-glutamic acid: Recent achievements, diverse applications and future perspectives. Trends Food Sci. Tech. 2022, 119, 1–12. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, C.; Xu, X.; Feng, X.; Xu, H. Effect of poly (γ-glutamic acid) on wheat productivity, nitrogen use efficiency and soil microbes. J. Soil Sci. Plant Nut. 2013, 13, 744–755. [Google Scholar] [CrossRef]
- Liang, J.; Shi, W.; He, Z.; Pang, L.; Zhang, Y. Effects of poly-γ-glutamic acid on water use efficiency, cotton yield, and fiber quality in the sandy soil of southern Xinjiang, China. Agr. Water Manage. 2019, 218, 48–59. [Google Scholar] [CrossRef]
- Bai, N.; Zhang, H.; He, Y.; Zhang, J.; Zheng, X.; Zhang, H.; Zhang, Y.; Lv, W.; Li, S. Effects of Bacillus subtilis A-5 and its fermented γ-polyglutamic acid on the rhizosphere bacterial community of Chinese cabbage. Front. Microbiol. 2022, 13, 954489. [Google Scholar] [CrossRef]
- Tao, L.; Long, H.; Zhang, J.; Qi, L.; Zhang, S.; Li, T.; Li, S. Preparation and coating application of γ-polyglutamic acid hydrogel to improve storage life and quality of shiitake mushrooms. Food Control 2021, 130, 108404. [Google Scholar] [CrossRef]
- Tao, L.; Tian, L.; Zhang, X.S.; Huang, X.; Long, H.Y.; Chang, F.Y.; Li, T.P.; Li, S.H. Effects of gamma-polyglutamic acid on the physicochemical properties and microstructure of grass carp (Ctenopharyngodon idellus) surimi during frozen storage. LWT-Food Sci. Technol. 2020, 134, 109960. [Google Scholar] [CrossRef]
- Wang, L.; Dou, G.; Guo, H.; Zhang, Q.; Qin, X.; Yu, W.; Jiang, C.; Xiao, H. Volatile organic compounds of Hanseniaspora uvarum increase strawberry fruit flavor and defense during cold storage. Food Sci. Nutr. 2019, 7, 2625–2635. [Google Scholar] [CrossRef] [Green Version]
- Martínez, K.; Ortiz, M.; Albis, A.; Gilma Gutiérrez Castañeda, C.; Valencia, M.E.; Grande Tovar, C.D. The Effect of edible chitosan coatings incorporated with Thymus capitatus essential oil on the shelf-life of strawberry (Fragaria × ananassa) during cold storage. Biomolecules 2018, 8, 155. [Google Scholar] [CrossRef] [Green Version]
- Lei, P.; Xu, Z.; Ding, Y.; Tang, B.; Zhang, Y.; Li, H.; Feng, X.; Xu, H. Effect of poly(γ-glutamic acid) on the physiological responses and calcium signaling of rape seedlings (Brassica napus L.) under cold stress. J. Agric. Food Chem. 2015, 63, 10399–10406. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, J.; Lei, P.; Wang, Q.; Feng, X.; Xu, H. Poly-γ-glutamic acid induces system tolerance to drought stress by promoting abscisic acid accumulation in Brassica napus L. Sci. Rep. 2020, 10, 252. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.R.; Rahmati-Joneidabad, M.; Noshad, M. Effect of chia seed mucilage/bacterial cellulose edible coating on bioactive compounds and antioxidant activity of strawberries during cold storage. Int. J. Biol. Macromol. 2021, 190, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Quintana, S.E.; Llalla, O.; Garcia-Risco, M.R.; Fornari, T. Comparison between essential oils and supercritical extracts into chitosan-based edible coatings on strawberry quality during cold storage. J. Supercrit. Fluid. 2021, 171, 105198. [Google Scholar] [CrossRef]
- Duan, Z.; Duan, W.; Li, F.; Li, Y.; Luo, P.; Liu, H. Effect of carboxymethylation on properties of fucoidan from Laminaria japonica: Antioxidant activity and preservative effect on strawberry during cold storage. Postharvest Biol. Technol. 2019, 151, 127–133. [Google Scholar] [CrossRef]
- Huang, H.; Gu, L.; Li, M.; Zheng, Y.; Jin, P. Effect of postharvest melatonin treatment on quality and reactive oxygen species metabolism in strawberry. Food Sci. 2021, 42, 187–193. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Scebba, F.; Sebastiani, L.; Vitagliano, C. Activities of antioxidant enzymes during senescence of Prunus armeniaca leaves. Biol. Plant. 2001, 44, 41–46. [Google Scholar] [CrossRef]
- Kato, M.; Shimizu, S. Chlorophyll metabolism in higher plants VII. Chlorophyll degradation in senescing tobacco leaves: Phenolic-dependent peroxidative degradation. Can. J. Bot. 1987, 65, 729–735. [Google Scholar] [CrossRef]
- Shan, C.; Liang, Z. Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress. Plant Sci. 2010, 178, 130–139. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Brennan, T.; Frenkel, C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q. The Experiment of Basic Biochemistry; Higher Education Press: Beijing, China, 2009; pp. 84–86. [Google Scholar]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines. Physiol. Plant. 1996, 98, 685–692. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituints of Prunus domestica. Quantitative analysis of phenolic constituints. J. Sci. Food Agr. 1959, 10, 63e68. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Li, X.X. Quality and Analytical Method of Fresh Fruits and Vegetables; China Agriculture Press: Beijing, China, 1994; pp. 208–209. [Google Scholar]
- Wu, Y.; Li, L.; Li, M.; Zhang, M.; Sun, H.; Sigrimis, N. Optimal fertigation for high yield and fruit quality of greenhouse strawberry. PLoS ONE 2020, 15, e0224588. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Yang, N.; Zhu, C.; Gan, L. Exogenously applied poly-γ-glutamic acid alleviates salt stress in wheat seedlings by modulating ion balance and the antioxidant system. Environ. Sci. Pollut. Res. 2017, 24, 6592–6598. [Google Scholar] [CrossRef]
- Ahmad Lone, W.; Majeed, N.; Yaqoob, U.; John, R. Exogenous brassinosteroid and jasmonic acid improve drought tolerance in Brassica rapa L. genotypes by modulating osmolytes, antioxidants and photosynthetic system. Plant Cell Rep. 2022, 41, 603–617. [Google Scholar] [CrossRef]
- Cisse, E.H.M.; Zhang, L.J.; Pu, Y.J.; Miao, L.F.; Li, D.D.; Zhang, J.; Yang, F. Exogenous Ca2+ associated with melatonin alleviates drought-induced damage in the eoody tree Dalbergia odorifera. J. Plant Growth Regul. 2022, 41, 2359–2374. [Google Scholar] [CrossRef]
- Momeni, M.M.; Kalantar, M.; Dehghani-Zahedani, M. H2O2 seed priming improves tolerance to salinity stress in durum wheat. Cereal Res. Commun. 2023, 51, 391–401. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Pang, X.; Li, H.; Feng, X.; Xu, H. Exogenous application of poly-γ-glutamic acid enhances stress defense in Brassica napus L. seedlings by inducing cross-talks between Ca2+, H2O2, brassinolide, and jasmonic acid in leaves. Plant Physiol. Biochem. 2017, 118, 460–470. [Google Scholar] [CrossRef]
- Mattus-Araya, E.; Stappung, Y.; Herrera, R.; Moya-león, M.A. Molecular actors involved in the softening of Fragaria chiloensis fruit accelerated by ABA treatment. J. Plant Growth Regul. 2023, 42, 433–448. [Google Scholar] [CrossRef]
- Lu, Y.; Li, D.; Li, L.; Belwal, T.; Xu, Y.; Lin, X.; Duan, Z.; Luo, Z. Effects of elevated CO2 on pigment metabolism of postharvest mandarin fruit for degreening. Food Chem. 2020, 318, 126462. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, Y.; Jin, Y.; Wang, B.; Shan, C. Selenomethionine regulates the sugar-acid ratio of strawberry fruit by modulating the activities of related enzymes. J. Hortic. Sci. Biotech. 2022, 97, 224–235. [Google Scholar] [CrossRef]




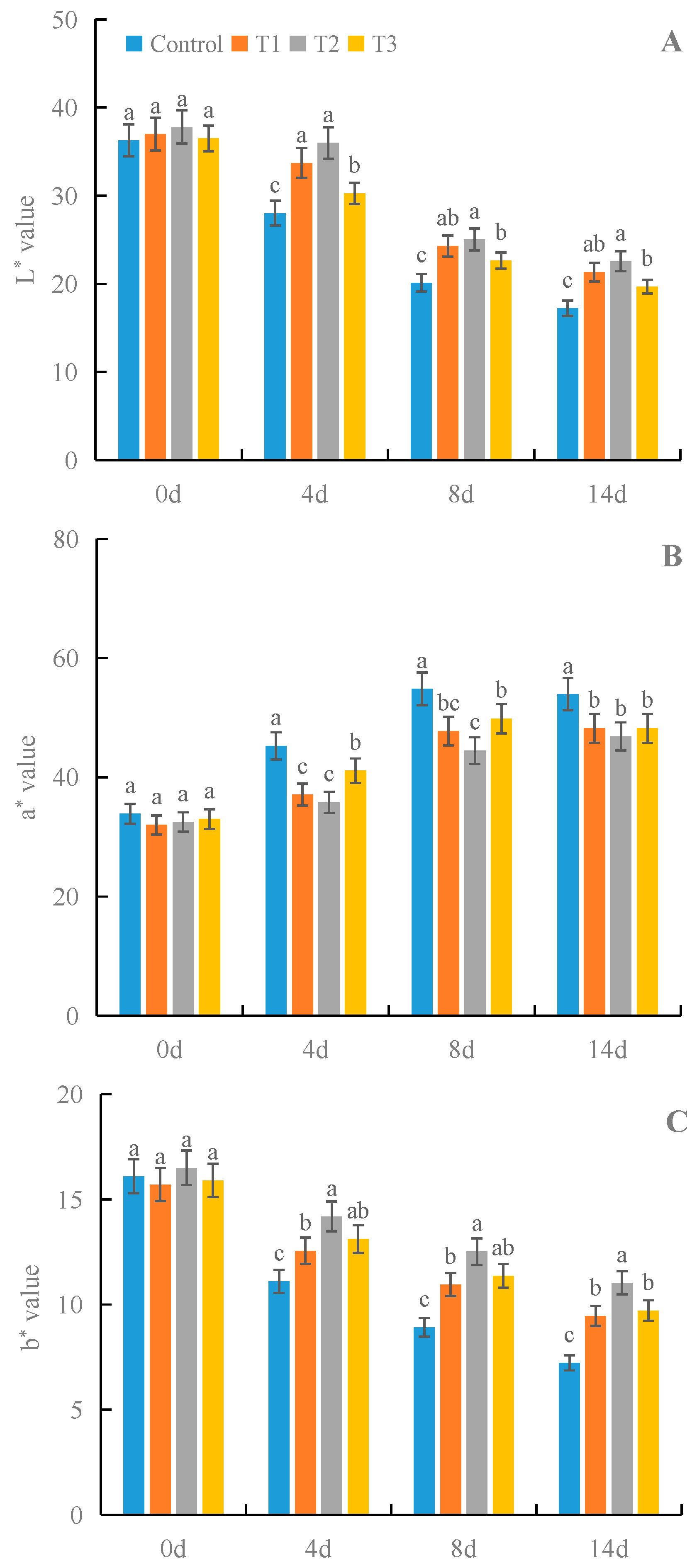

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, C.; Luo, Y.; Yang, C.; Gao, X. The Effects of Poly-γ-Glutamic Acid on the Postharvest Physiology and Quality of Strawberry cv. Hongyan during Cold Storage. Foods 2023, 12, 2944. https://doi.org/10.3390/foods12152944
Shan C, Luo Y, Yang C, Gao X. The Effects of Poly-γ-Glutamic Acid on the Postharvest Physiology and Quality of Strawberry cv. Hongyan during Cold Storage. Foods. 2023; 12(15):2944. https://doi.org/10.3390/foods12152944
Chicago/Turabian StyleShan, Changjuan, Yi Luo, Chen Yang, and Xinxia Gao. 2023. "The Effects of Poly-γ-Glutamic Acid on the Postharvest Physiology and Quality of Strawberry cv. Hongyan during Cold Storage" Foods 12, no. 15: 2944. https://doi.org/10.3390/foods12152944




