Cuttlefish Species Authentication: Advancing Label Control through Near-Infrared Spectroscopy as Rapid, Eco-Friendly, and Robust Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cuttlefish Sampling and Species Identification
2.2. NIR Data Collection
2.3. Identification Species Model
3. Results and Discussion
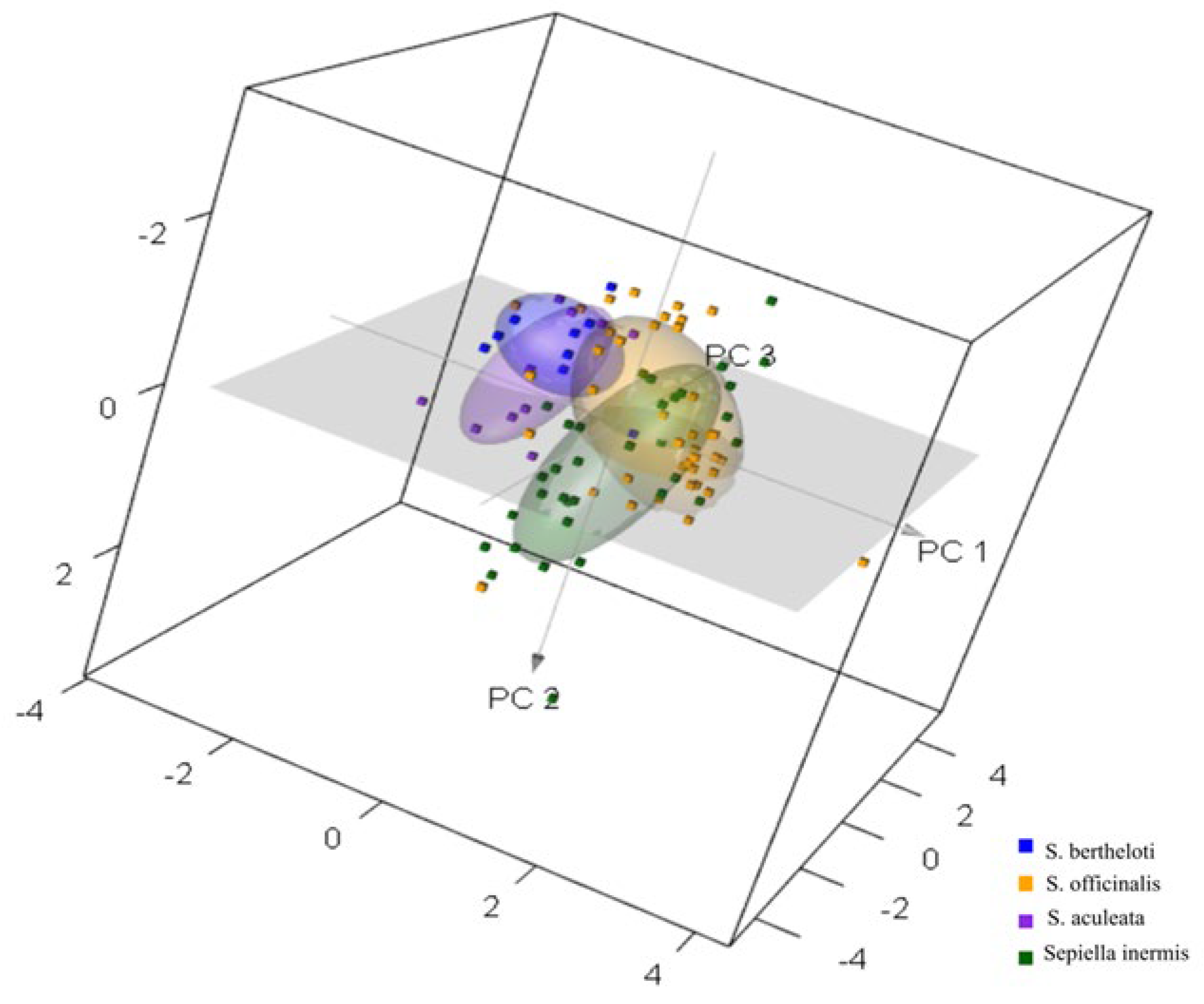
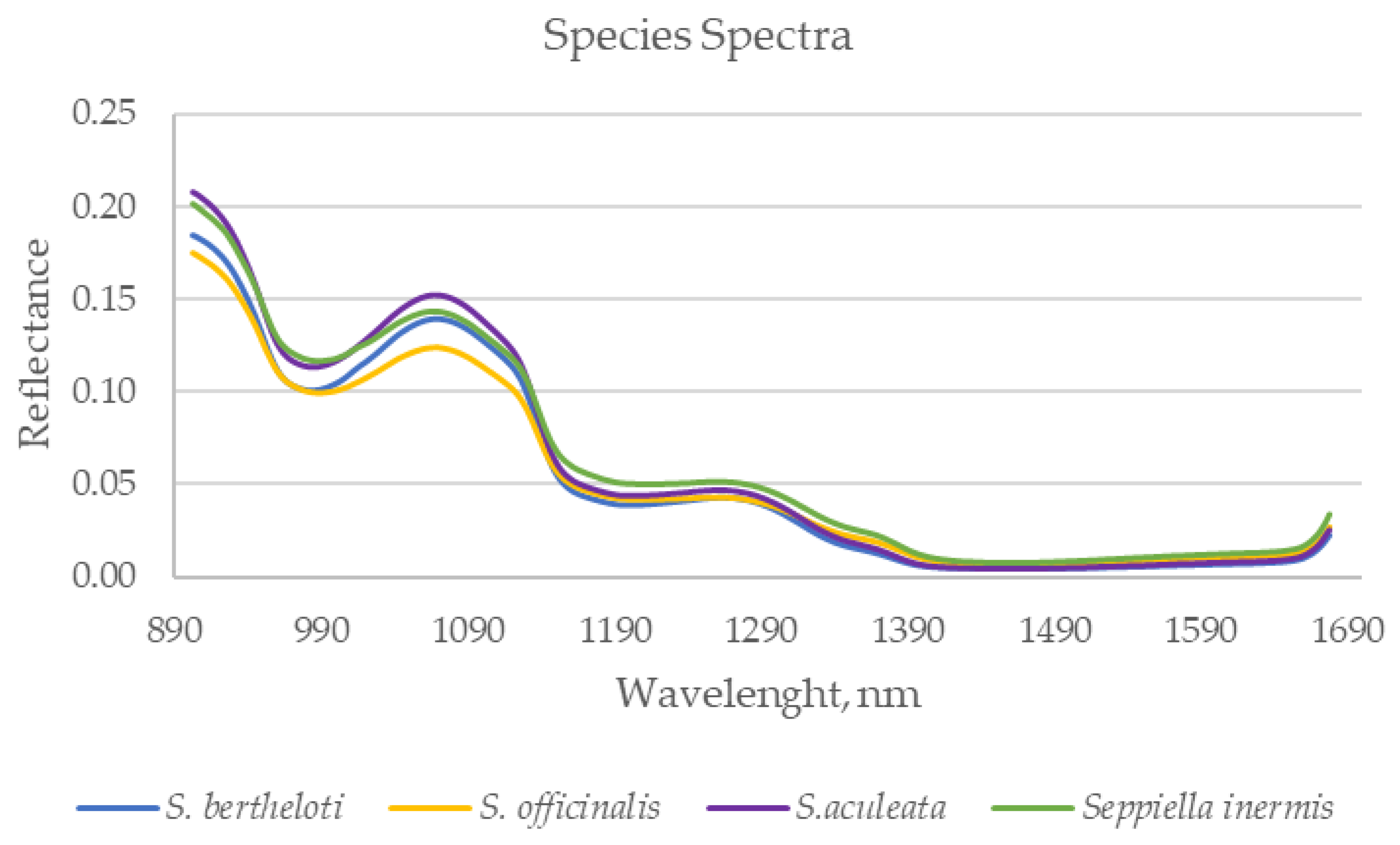
3.1. Exploratory Results
3.2. Classification by Species
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vargheese, S.; Basheer, V.S. Resolving the taxonomic ambiguity of Sepia ramani using integrative taxonomy. Molluscan Res. 2022, 42, 91–98. [Google Scholar] [CrossRef]
- MIPAAF; FEAMP 2014–2020. Seppia, Analisi Economica e Prospettive di Consumo. Available online: https://ittico.bmti.it/CaricaPdfRegolamenti.do?doc=20 (accessed on 6 July 2023).
- Hassoun, A.E.R.; Ujević, I.; Mahfouz, C.; Fakhri, M.; Roje-Busatto, R.; Jemaa, S.; Nazlić, N. Occurrence of domoic acid and cyclic imines in marine biota from Lebanon-Eastern Mediterranean Sea. Sci. Total Environ. 2021, 755, 142542. [Google Scholar] [CrossRef] [PubMed]
- Drábková, M.; Jachníková, N.; Tyml, T.; Sehadová, H.; Ditrich, O.; Myšková, E.; Hypša, V.; Štefka, J. Population co-divergence in common cuttlefish (Sepia officinalis) and its dicyemid parasite in the Mediterranean Sea. Sci. Rep. 2019, 9, 14300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jereb, P.; Roper, C.F. Cephalopods of the World—An Annotated and Illustrated Catalogue of Cephalopod Species Know to Date, 2nd ed.; FAO: Rome, Italy, 2010; Volume 2, ISBN 978-92-5-106720-8. [Google Scholar]
- Neige, P.; Neige, P.; Life, M. Morphometrics of hard structures in cuttlefish. Vie Milieu/Life Environ. 2006, 56, 121–127. [Google Scholar]
- Cermakova, E.; Lencova, S.; Mukherjee, S.; Horka, P.; Vobruba, S.; Demnerova, K.; Zdenkova, K. Identification of Fish Species and Targeted Genetic Modifications Based on DNA Analysis: State of the Art. Foods 2023, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Currò, S.; Fasolato, L.; Serva, L.; Boffo, L.; Carlo, J.; Novelli, E.; Balzan, S. Use of a portable near-infrared tool for rapid on-site inspection of freezing and hydrogen peroxide treatment of cuttlefish (Sepia officinalis). Food Control 2022, 132, 108524. [Google Scholar] [CrossRef]
- O’Brien, N.; Hulse, C.A.; Pfeifer, F.; Siesler, H.W. Near infrared spectroscopic authentication of seafood. J. Near Infrared Spectrosc. 2013, 21, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Cavallini, N.; Pennisi, F.; Giraudo, A.; Pezzolato, M.; Esposito, G.; Gavoci, G.; Magnani, L.; Pianezzola, A.; Geobaldo, F.; Savorani, F.; et al. Chemometric Differentiation of Sole and Plaice Fish Fillets Using Three Near-Infrared Instruments. Foods 2022, 11, 1643. [Google Scholar] [CrossRef] [PubMed]
- O’ Brien, N.A.; Hulse, C.A.; Siesler, H.W.; Hsiung, C. Spectroscopic Characterization of Seafood U.S. Patent No. US 9316628 B2, 19 April 2016.
- European Commission. Commission Implementing Regulation (EU) 2022/2503 Amending and correcting Implementing Regulation (EU) 2019/627 as regards practical arrangements for the performance of official controls in live bivalve molluscs, fishery products, or related to UV-radiation. Off. J. Eur. Union. 2022, L325, 58–61. [Google Scholar]
- Chapela, M.J.; Sotelo, C.G.; Calo-Mata, P.; Pérez-Martín, R.I.; Rehbein, H.; Hold, G.L.; Quinteiro, J.; Rey-Méndez, M.; Rosa, C.; Santos, A.T. Identification of Cephalopod Species (Ommastrephidae and Loliginidae) in Seafood Products by Forensically Informative Nucleotide Sequencing (FINS). J. Food Sci. 2002, 67, 1672–1676. [Google Scholar] [CrossRef]
- Currò, S.; Balzan, S.; Serva, L.; Boffo, L.; Ferlito, J.C.; Novelli, E.; Fasolato, L. Fast and green method to control frauds of geographical origin in traded cuttlefish using a portable infrared reflective instrument. Foods 2021, 10, 1678. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.; Mitchell, M.; Dean, M.; Elliott, C.; Campbell, K. The seafood supply chain from a fraudulent perspective. Food Secur. 2018, 10, 939–963. [Google Scholar] [CrossRef] [Green Version]
- Ghidini, S.; Varrà, M.O.; Zanardi, E. Approaching authenticity issues in fish and seafood products by qualitative spectroscopy and chemometrics. Molecules 2019, 24, 1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottavian, M.; Fasolato, L.; Facco, P.; Barolo, M. Foodstuff authentication from spectral data: Toward a species-independent discrimination between fresh and frozen-thawed fish samples. J. Food Eng. 2013, 119, 765–775. [Google Scholar] [CrossRef]
- Lv, H.; Xu, W.; You, J.; Xiong, S. Classification of freshwater fish species by linear discriminant analysis based on near infrared reflectance spectroscopy. J. Near Infrared Spectrosc. 2017, 25, 54–62. [Google Scholar] [CrossRef]
- Nieto-Ortega, S.; Lara, R.; Foti, G.; Melado-Herreros, Á.; Olabarrieta, I. Applications of near-infrared spectroscopy (NIRS) in fish value chain. In Infrared Spectroscopy; El-Azazy, M., Al-Saad, K., El-Shafie, A.S., Eds.; IntechOpen: Rijeka, Croatia, 2022; Chapter 5. [Google Scholar]
- Alamprese, C.; Casiraghi, E. Application of FT-NIR and FT-IR spectroscopy to fish fillet authentication. LWT Food Sci. Technol. 2015, 63, 720–725. [Google Scholar] [CrossRef]
- Cozzolino, D.; Chree, A.; Scaife, J.R.; Murray, I. Usefulness of Near-infrared reflectance (NIR) spectroscopy and chemometrics to discriminate fishmeal batches made with different fish species. J. Agric. Food Chem. 2005, 53, 4459–4463. [Google Scholar] [CrossRef] [PubMed]
- Varrà, M.O.; Ghidini, S.; Fabrile, M.P.; Ianieri, A.; Zanardi, E. Country of origin label monitoring of musky and common octopuses (Eledone spp. and Octopus vulgaris) by means of a portable near-infrared spectroscopic device. Food Control 2022, 138, 109052. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, J.I.; De Carvalho, C.J.B.; Pasquini, C.; De Lima, K.M.G.; Moura, M.O.; Arízaga, G.G.C. Barcoding without DNA? Species identification using near infrared spectroscopy. Zootaxa 2011, 2933, 46–54. [Google Scholar] [CrossRef] [Green Version]
- De Azevedo, R.A.; de Morais, J.W.; Lang, C.; de Sales Dambros, C. Discrimination of termite species using Near-Infrared Spectroscopy (NIRS). Eur. J. Soil Biol. 2019, 93, 103084. [Google Scholar] [CrossRef]
| n. of Samples | n. Per Species | FAO Fishing Area | |
|---|---|---|---|
| Training | 66 | S. bertheloti, n. 7 | 34 |
| S. officinalis, n. 28 | 27.7 (n. 22); 34 (n. 6) | ||
| S. aculeata, n. 7 | 51 | ||
| Sepiella inermis, n. 24 | 57 (n. 13), 71 (n. 11) | ||
| Testing | 27 | S. bertheloti, n. 2 | 34 |
| S. officinalis, n. 12 | 27.7 (n. 9); 34 (n. 3) | ||
| S. aculeata, n. 3 | 51 | ||
| Sepiella inermis, n. 10 | 57 (n. 6); 71 (n. 4) |
| Reference Species | ||||
|---|---|---|---|---|
| Predicted Species | S. bertheloti | S. officinalis | S. aculeata | Seppiella inermis |
| S. bertheloti | 2 | 0 | 0 | 0 |
| S. officinalis | 0 | 12 | 2 | 0 |
| S. aculeata | 0 | 0 | 1 | 0 |
| Seppiella inermis | 0 | 0 | 0 | 10 |
| Overall Accuracy (%) | 93 | |||
| Balanced Accuracy (%) | 100 | 93 | 66 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Currò, S.; Balzan, S.; Novelli, E.; Fasolato, L. Cuttlefish Species Authentication: Advancing Label Control through Near-Infrared Spectroscopy as Rapid, Eco-Friendly, and Robust Approach. Foods 2023, 12, 2973. https://doi.org/10.3390/foods12152973
Currò S, Balzan S, Novelli E, Fasolato L. Cuttlefish Species Authentication: Advancing Label Control through Near-Infrared Spectroscopy as Rapid, Eco-Friendly, and Robust Approach. Foods. 2023; 12(15):2973. https://doi.org/10.3390/foods12152973
Chicago/Turabian StyleCurrò, Sarah, Stefania Balzan, Enrico Novelli, and Luca Fasolato. 2023. "Cuttlefish Species Authentication: Advancing Label Control through Near-Infrared Spectroscopy as Rapid, Eco-Friendly, and Robust Approach" Foods 12, no. 15: 2973. https://doi.org/10.3390/foods12152973






