Two-Step Upcycling Process of Lignocellulose into Edible Bacterial Nanocellulose with Black Raspberry Extract as an Active Ingredient
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
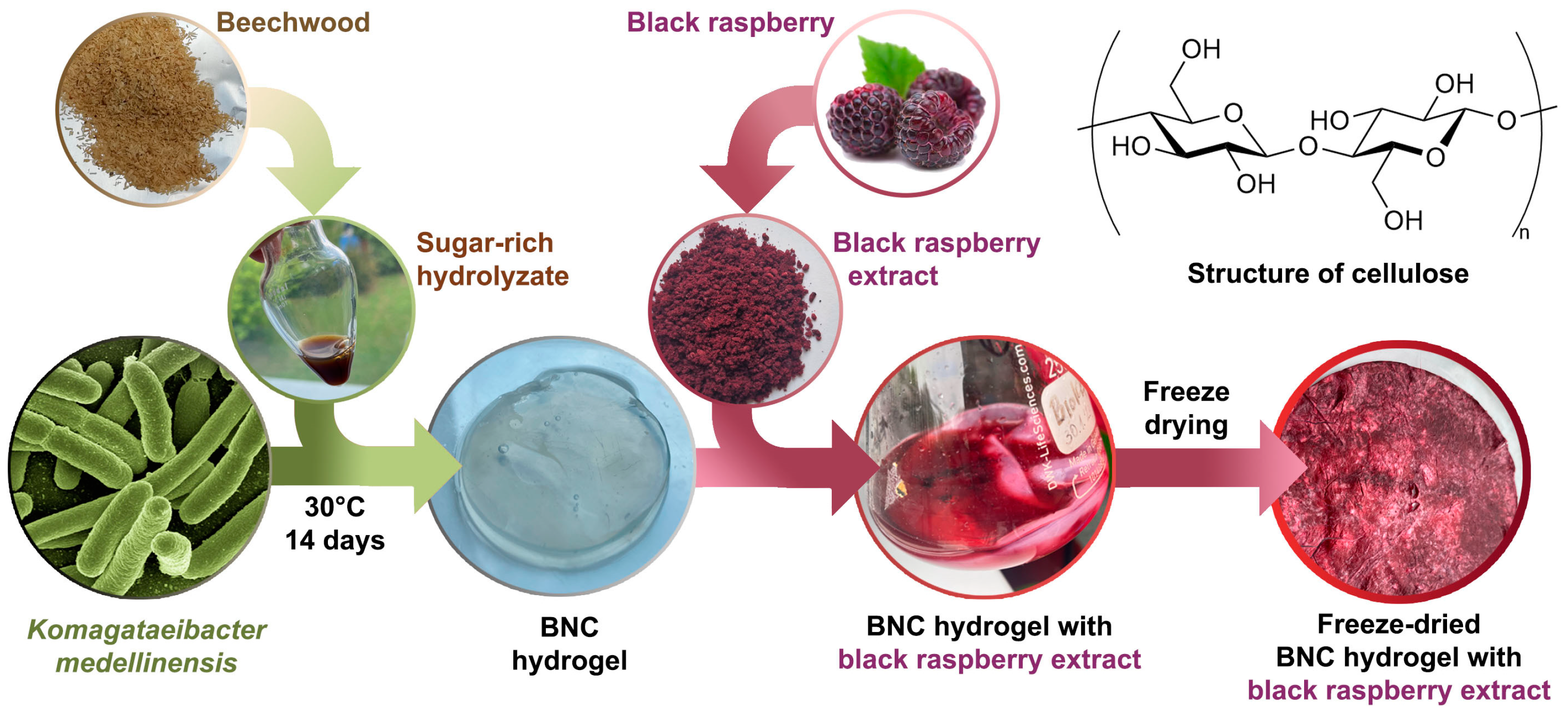
2.2. Black Raspberry Extraction
2.2.1. Black Raspberry Pomace
2.2.2. Black Raspberry Pomace Extraction
2.3. Preparation of a Sugar-Rich Hydrolysate from Pretreated Beechwood Biomass
2.4. Bacterial Nanocellulose Production
2.5. Bacterial Nanocellulose–Black Raspberry (BNC-BR) Film Preparation
Efficiency of Black Raspberry Extract Adsorption on BNC
2.6. Characterization of Obtained BNC-BR Film
2.6.1. Fourier-Transform Infrared Spectroscopy Analysis (ATR-FTIR)
2.6.2. Optical Microscopy (OM) and Scanning Electron Microscopy Analysis (SEM)
2.7. In Vitro Release Study
2.8. Kinetic Modeling of Cyanidin-3-O-Rutinoside Release
2.9. High-Performance Liquid Chromatography Analysis (HPLC)
2.10. Total Phenolic Content (TPC)
2.11. Total Anthocyanin Content (TAC)
2.12. DPPH Radical Scavenging Activity
2.13. Ferric-Reducing Antioxidant Power (FRAP) Assay
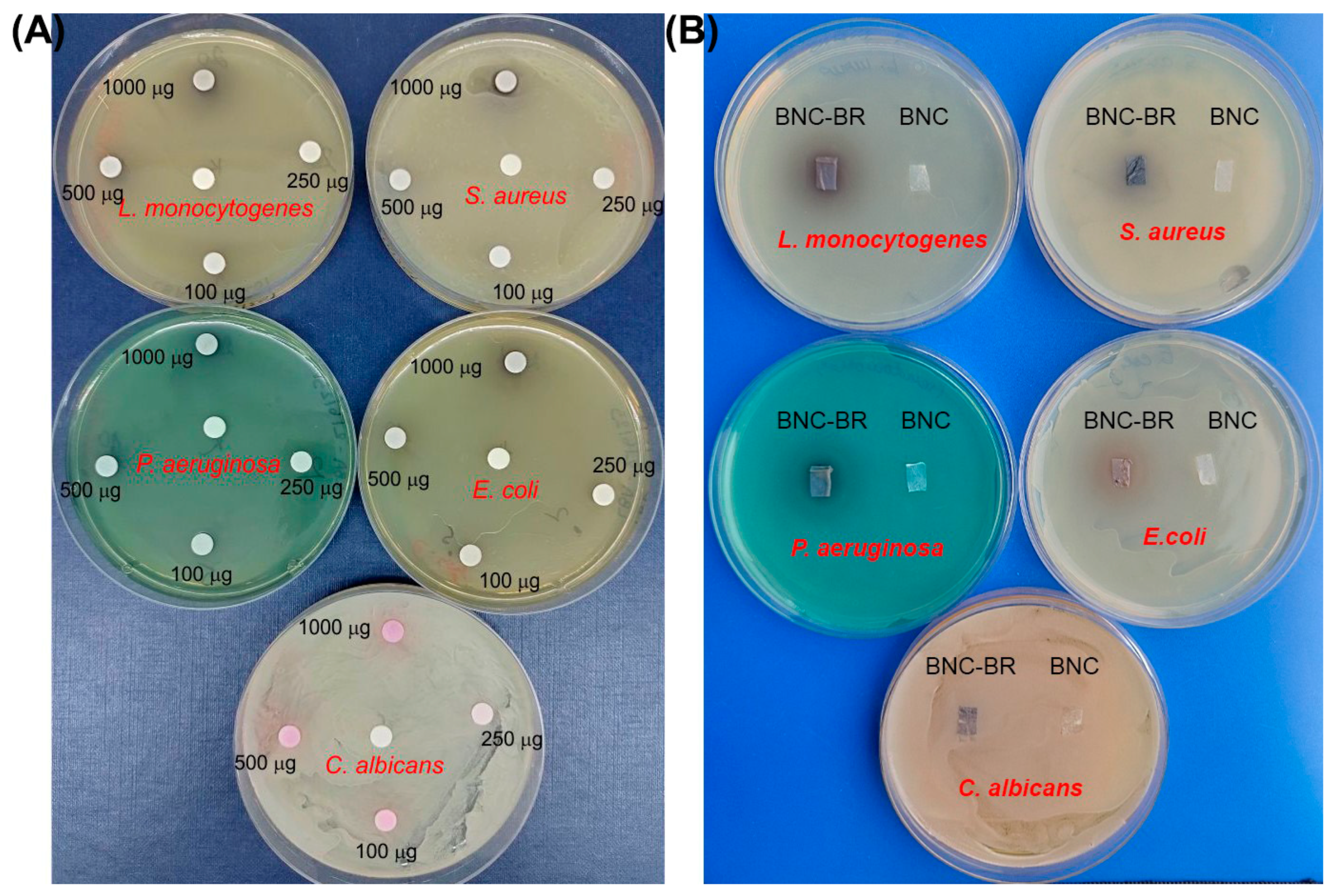
2.14. Antimicrobial Assay
2.15. Statistical Analysis
3. Results and Discussion
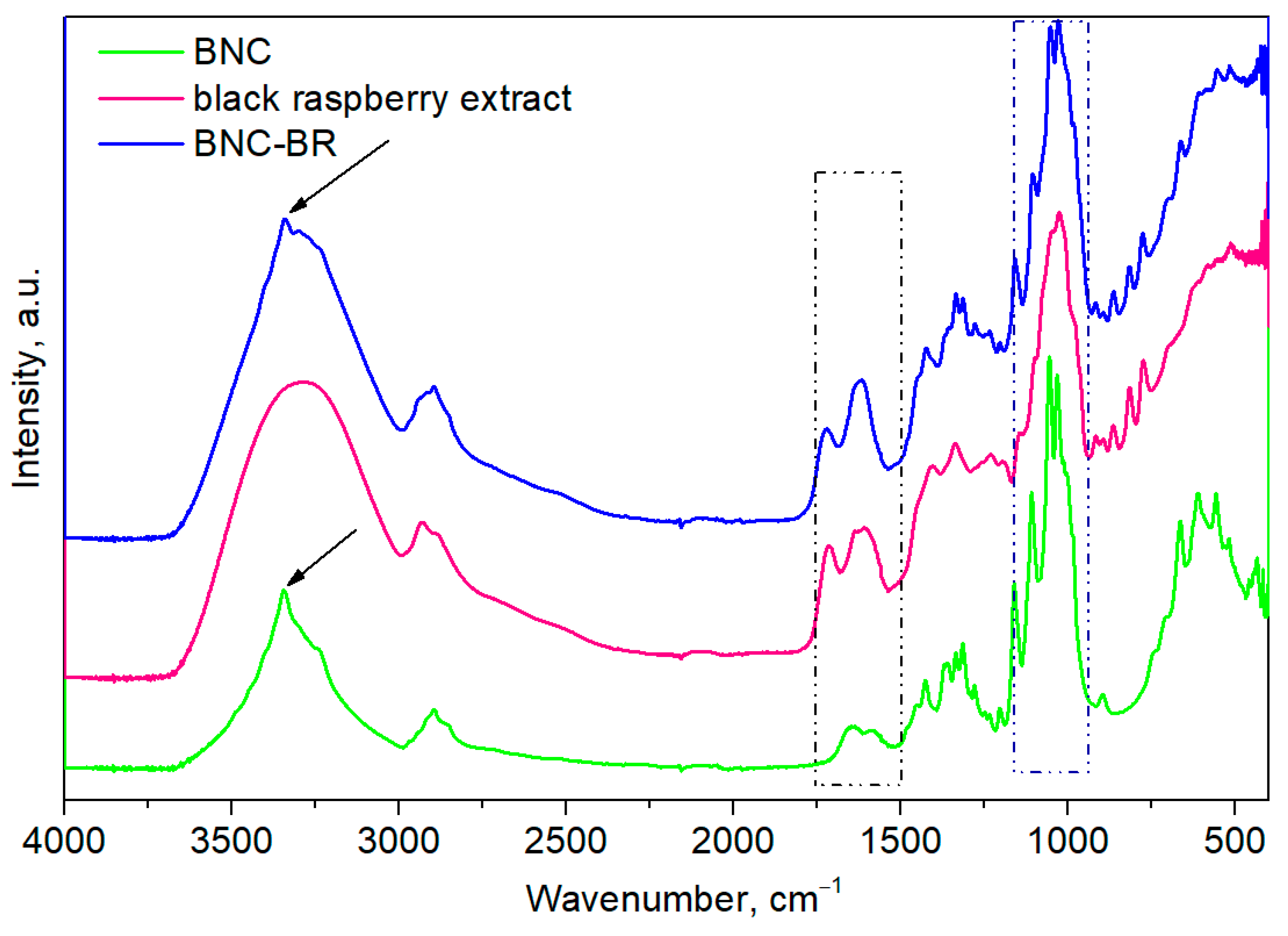
3.1. FTIR Analysis
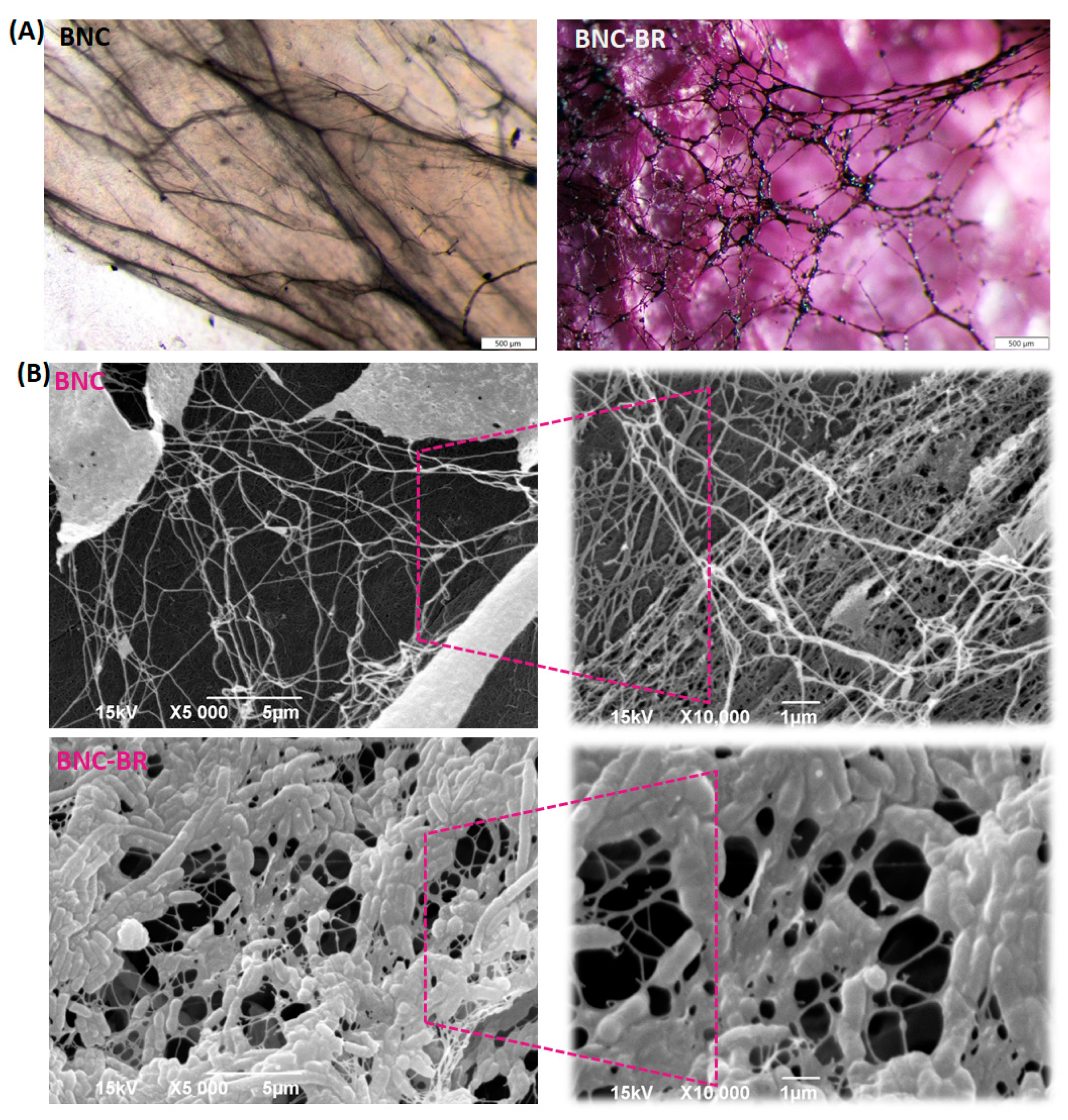
3.2. Optical Microscopy and SEM Analysis
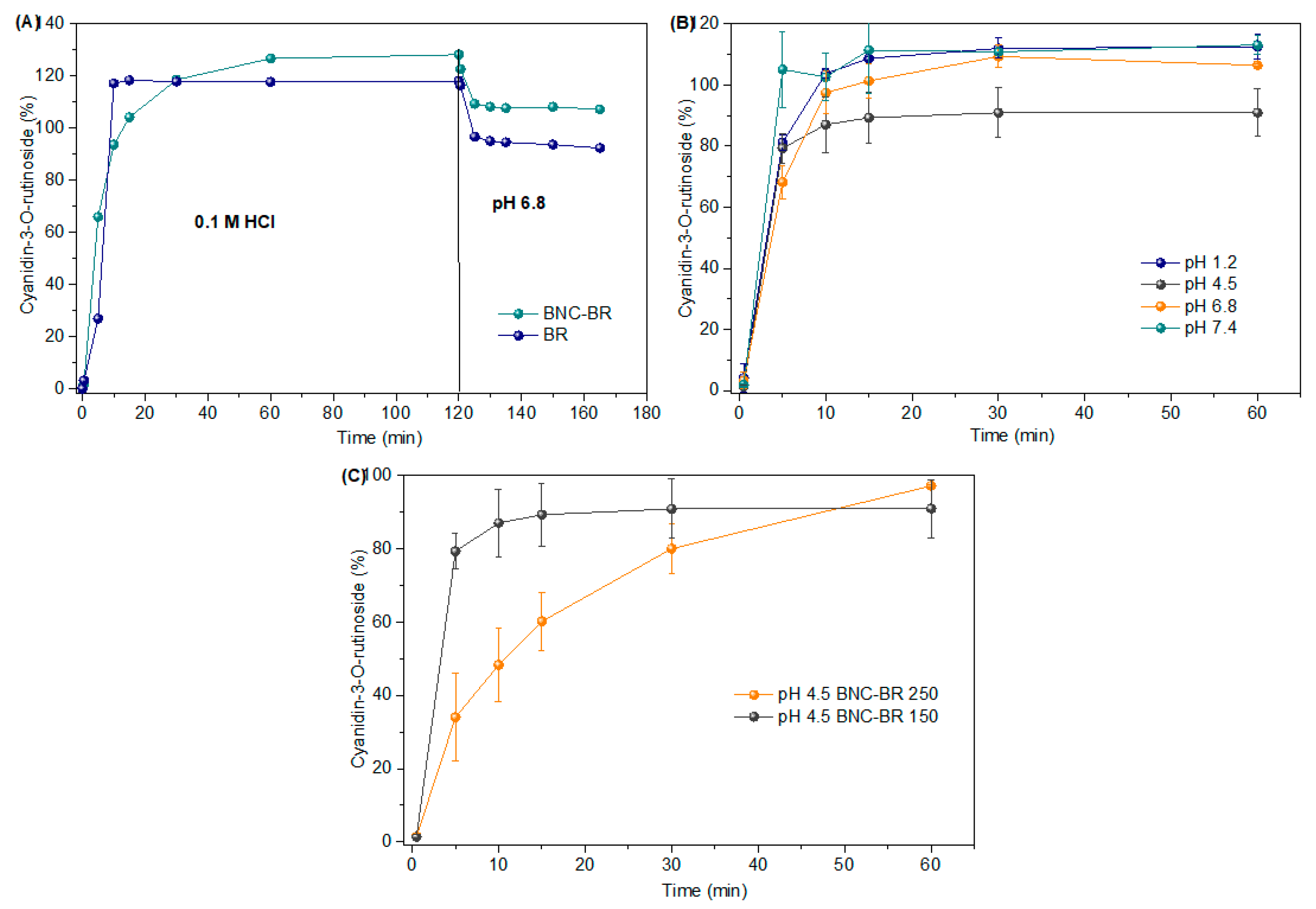
3.3. In Vitro Release of Cyanidin-3-O-Rutinoside
3.4. Total Bioactive Compound Content in Black Raspberry Pomace Extract
3.5. Antioxidant Activity of Black Raspberry Extract and BNC-BR
3.6. Antimicrobial Activity Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.; Bacabac, R.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Moniri, M.; Boroumand Moghaddam, A.; Azizi, S.; Abdul Rahim, R.; Bin Ariff, A.; Zuhainis Saad, W.; Navaderi, M.; Mohamad, R. Production and Status of Bacterial Cellulose in Biomedical Engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Thi, H.Y.; Kim, S.; Duy Nguyen, B.T.; Lim, D.; Kumar, S.; Lee, H.; Szekely, G.; Kim, J.F. Closing the Sustainable Life Cycle Loop of Membrane Technology via a Cellulose Biomass Platform. ACS Sustain. Chem. Eng. 2022, 10, 2532–2544. [Google Scholar] [CrossRef]
- Przygrodzka, K.; Charęza, M.; Banaszek, A.; Zielińska, B.; Ekiert, E.; Drozd, R. Bacterial Cellulose Production by Komagateibacter xylinus with the Use of Enzyme-Degraded Oligo- and Polysaccharides as the Substrates. Appl. Sci. 2022, 12, 12673. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose—A masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohyd. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed]
- de Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Li, W.; Shen, Y.; Liu, H.; Huang, X.; Xu, B.; Zhong, C.; Jia, S. Bioconversion of lignocellulosic biomass into bacterial nanocellulose: Challenges and perspectives. Green Chem. Eng. 2023, 4, 160–172. [Google Scholar] [CrossRef]
- Barja, F. Bacterial nanocellulose production and biomedical applications. J. Biomed. Res. 2021, 35, 310–317. [Google Scholar] [CrossRef]
- Ludwicka, K.; Jedrzejczak-Krzepkowska, M.; Kubiak, K.; Kolodziejczyk, M.; Pankiewicz, T.; Bielecki, S. Chapter 9—Medical and Cosmetic Applications of Bacterial NanoCellulose. In Bacterial Nanocellulose; Gama, M., Dourado, F., Bielecki, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 145–165. [Google Scholar]
- Almeida, T.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Bacterial Nanocellulose toward Green Cosmetics: Recent Progresses and Challenges. Int. J. Mol. Sci. 2021, 22, 2836. [Google Scholar] [CrossRef]
- Nugroho, D.A.; Aji, P. Characterization of Nata de Coco Produced by Fermentation of Immobilized Acetobacter xylinum. Agric. Agric. Sci. Procedia 2015, 3, 278–282. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Sohal, I.S.; Lorente, L.R.; Molina, R.M.; Pyrgiotakis, G.; Stevanovic, A.; Zhang, R.; McClements, D.J.; Geitner, N.K.; Bousfield, D.W.; et al. Reducing Intestinal Digestion and Absorption of Fat Using a Nature-Derived Biopolymer: Interference of Triglyceride Hydrolysis by Nanocellulose. ACS Nano 2018, 12, 6469–6479. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Chen, Y.; Guo, T.L.; Kong, F. Six weeks effect of different nanocellulose on blood lipid level and small intestinal morphology in mice. Int. J. Biol. Macromol. 2023, 228, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Kosiński, P.; Czarna, A.; Maliński, T. Rubus occidentalis (Rosaceae)—A new naturalized raspberry species in the Polish flora. Dendrobiology 2013, 71, 159–165. [Google Scholar] [CrossRef]
- Schulz, M.; Chim, J. Nutritional and bioactive value of Rubus berries. Food Biosci. 2019, 31, 100438. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Chemical Composition of Raspberry (Rubus spp.) Cultivars. In Nutritional Composition of Fruit Cultivars; Elsevier: Cambridge, MA, USA, 2016; Chapter 29. [Google Scholar]
- Lim, T.; Ryu, J.; Lee, K.; Park, S.Y.; Hwang, K.T. Protective Effects of Black Raspberry (Rubus occidentalis) Extract against Hypercholesterolemia and Hepatic Inflammation in Rats Fed High-Fat and High-Choline Diets. Nutrients 2020, 12, 2448. [Google Scholar] [CrossRef]
- Jeong, H.S.; Hong, S.J.; Cho, J.Y.; Lee, T.B.; Kwon, J.W.; Joo, H.J.; Park, J.H.; Yu, C.W.; Lim, D.S. Effects of Rubus occidentalis extract on blood pressure in patients with prehypertension: Randomized, double-blinded, placebo-controlled clinical trial. Nutrition 2016, 32, 461–467. [Google Scholar] [CrossRef]
- An, J.H.; Kim, D.L.; Lee, T.B.; Kim, K.J.; Kim, S.H.; Kim, N.H.; Kim, H.Y.; Choi, D.S.; Kim, S.G. Effect of Rubus occidentalis Extract on Metabolic Parameters in Subjects with Prediabetes: A Proof-of-concept, Randomized, Double-blind, Placebo-controlled Clinical Trial. Phytother. Res. 2016, 30, 1634–1640. [Google Scholar] [CrossRef]
- Renai, L.; Scordo, C.V.A.; Chiuminatto, U.; Ulaszewska, M.; Giordani, E.; Petrucci, W.A.; Tozzi, F.; Nin, S.; Del Bubba, M. Liquid Chromatographic Quadrupole Time-of-Flight Mass Spectrometric Untargeted Profiling of (Poly)phenolic Compounds in Rubus idaeus L. and Rubus occidentalis L. Fruits and Their Comparative Evaluation. Antioxidants 2021, 10, 704. [Google Scholar]
- Kula, M.; Krauze-Baranowska, M. Rubus occidentalis: The black raspberry—Its potential in the prevention of cancer. Nutr. Cancer 2016, 68, 18–28. [Google Scholar] [CrossRef]
- Tian, Q.; Aziz, R.M.; Stoner, G.; Schwartz, S.J. Anthocyanin determination in black raspberry (Rubus occidentalis) and biological specimens using liquid chromatography-electrospray ionization tandem mass spectrometry. J. Food Sci. 2005, 70, C43–C47. [Google Scholar] [CrossRef]
- Dossett, M.; Lee, J.; Finn, C.E. Characterization of a novel anthocyanin profile in wild black raspberry mutants: An opportunity for studying the genetic control of pigment and color. J. Funct. Foods 2011, 3, 207–214. [Google Scholar] [CrossRef]
- Struck, S.; Plaza, M.; Turner, C.; Rohm, H. Berry pomace—A review of processing and chemical analysis of its polyphenols. Int. J. Food Sci. Technol. 2016, 51, 1305–1318. [Google Scholar] [CrossRef]
- Brodowska, A. Raspberry pomace—Composition, properties and application. Eur. J. Biol. Res. 2017, 7, 86–96. [Google Scholar]
- Koutinas, A.A.; Yepez, B.; Kopsahelis, N.; Freire, D.M.G.; de Castro, A.M.; Papanikolaou, S.; Kookos, I.K. Techno-economic evaluation of a complete bioprocess for 2,3-butanediol production from renewable resources. Bioresour. Technol. 2016, 204, 55–64. [Google Scholar] [CrossRef]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa-Jr, A.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Miljkovic, M. Yugoslav Pharmacopoeia 2000 (Ph. Jug. V)—Adjusted Translation of European Pharmacopoeia 1997 (Ph. Eur. III) Pharmacopoeia, 5th ed.; Kovac, M., Ed.; Working Group for Harmonization and Analysis of Special Part with Monographs, Federal Institute for Health Protection and Improvement: Belgrade, Serbia, 2001.
- Kalogiannis, K.G.; Karnaouri, A.; Michailof, C.; Tzika, A.M.; Asimakopoulou, G.; Topakas, E.; Lappas, A.A. OxiOrganosolv: A novel acid free oxidative organosolv fractionation for lignocellulose fine sugar streams. Bioresour. Technol. 2020, 313, 123599. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Raba, J.; Mottola, H.A. Glucose Oxidase as an Analytical Reagent. Crit. Rev. Anal. Chem. 1995, 25, 1–42. [Google Scholar] [CrossRef]
- Council of Europe Platform on Ethics, Transparency and Integrity in Education. European Directorate for the Quality of Medicines and Health Care of the Council of Europe; European Medicines Agency: Strasbourg, France, 2019.
- Polli, J.E.; Rekhi, G.S.; Augsburger, L.L.; Shah, V.P. Methods to compare dissolution profiles and a rationale for wide dissolution specifications for metoprolol tartrate tablets. J. Pharm. Sci. 1997, 86, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Kolundžić, M.; Grozdanić, N.Đ.; Dodevska, M.; Milenković, M.; Sisto, F.; Miani, A.; Farronato, G.; Kundaković, T. Antibacterial and cytotoxic activities of wild mushroom Fomes fomentarius (L.) Fr., Polyporaceae. Ind. Crops Prod. 2016, 79, 110–115. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Jacek, P.; Dourado, F.; Gama, M.; Bielecki, S. Molecular aspects of bacterial nanocellulose biosynthesis. Microb. Biotechnol. 2019, 12, 633–649. [Google Scholar] [CrossRef]
- Jeremic, S.; Djokic, L.; Ajdačić, V.; Božinović, N.; Pavlovic, V.; Manojlović, D.D.; Babu, R.; Senthamaraikannan, R.; Rojas, O.; Opsenica, I.; et al. Production of bacterial nanocellulose (BNC) and its application as a solid support in transition metal catalysed cross-coupling reactions. Int. J. Biol. Macromol. 2019, 129, 351–360. [Google Scholar] [CrossRef]
- Abol-Fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.E.-H.B. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020, 10, 3491. [Google Scholar] [CrossRef]
- Dar, M.A.; Dhole, N.P.; Xie, R.; Pawar, K.D.; Ullah, K.; Rahi, P.; Pandit, R.S.; Sun, J. Valorization Potential of a Novel Bacterial Strain, Bacillus altitudinis RSP75, towards Lignocellulose Bioconversion: An Assessment of Symbiotic Bacteria from the Stored Grain Pest, Tribolium castaneum. Microorganisms 2021, 9, 1952. [Google Scholar] [CrossRef]
- Dar, M.A.; Syed, R.; Pawar, K.D.; Dhole, N.P.; Xie, R.; Pandit, R.S.; Sun, J. Evaluation and characterization of the cellulolytic bacterium, Bacillus pumilus SL8 isolated from the gut of oriental leafworm Spodoptera litura: An assessment of its potential value for lignocellulose bioconversion. Environ. Technol. Innov. 2022, 27, 102459. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Dikshit, P.K.; Kim, B.S. Green in situ immobilization of gold and silver nanoparticles on bacterial nanocellulose film using Punica granatum peels extract and their application as reusable catalysts. Int. J. Biol. Macromol. 2022, 205, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Dima, S.-O.; Panaitescu, D.-M.; Orban, C.; Ghiurea, M.; Doncea, S.-M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.-A.; Trică, B.; et al. Bacterial Nanocellulose from Side-Streams of Kombucha Beverages Production: Preparation and Physical-Chemical Properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Ali, H.G.M.; Eliwa, N.E.R. Phytochemical screening, anthocyanins and antimicrobial activities in some berries fruits. J. Food Meas. Charact. 2019, 13, 911–920. [Google Scholar] [CrossRef]
- Abba, M.; Ibrahim, Z.; Chong, C.S.; Zawawi, N.A.; Kadir, M.R.A.; Yusof, A.H.M.; Razak, S.I.A. Transdermal Delivery of Crocin Using Bacterial Nanocellulose Membrane. Fiber Polym. 2019, 20, 2025–2031. [Google Scholar] [CrossRef]
- Ataide, J.A.; de Carvalho, N.M.; Rebelo, M.d.A.; Chaud, M.V.; Grotto, D.; Gerenutti, M.; Rai, M.; Mazzola, P.G.; Jozala, A.F. Bacterial Nanocellulose Loaded with Bromelain: Assessment of Antimicrobial, Antioxidant and Physical-Chemical Properties. Sci. Rep. 2017, 7, 18031. [Google Scholar] [CrossRef]
- Vachanont, T.; Torgbo, S.; Sukyai, P. Release Kinetic Model and Antimicrobial Activity of Freeze-Dried Curcumin-loaded Bacterial Nanocellulose Composite. Polym. Sci. A 2020, 62, 218–227. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, W.; Luo, J.; Deng, D. Multifunctional nano-cellulose composite films with grape seed extracts and immobilized silver nanoparticles. Carbohyd. Polym. 2019, 205, 447–455. [Google Scholar] [CrossRef]
- Pham, T.N.; Quoc Toan, T.; Lam, T.; Vu-Quang, H.; Vo, D.-V.; Tran, V.; Bui Le, M. Anthocyanins extraction from Purple Sweet Potato (Ipomoea batatas (L.) Lam): The effect of pH values on natural color. IOP Conf. Ser. Mater. Sci. Eng. 2019, 542, 012031. [Google Scholar]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.; Lim, S. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Rakic, V.; Ulrih, N. Influence of pH on color variation and stability of cyanidin and cyanidin 3-O-β-glucopyranoside in aqueous solution. CyTA-J. Food 2021, 19, 174–182. [Google Scholar] [CrossRef]
- Taokaew, S.; Nunkaew, N.; Siripong, P.; Phisalaphong, M. Characteristics and anticancer properties of bacterial cellulose films containing ethanolic extract of mangosteen peel. J. Biomater. Sci. Polym. Ed. 2014, 25, 907–922. [Google Scholar] [CrossRef]
- Bakr, E.A.; Gaber, M.; Saad, D.R.; Salahuddin, N. Comparative study between two different morphological structures based on polylactic acid, nanocellulose and magnetite for co-delivery of flurouracil and curcumin. Int. J. Biol. Macromol. 2023, 230, 123315. [Google Scholar] [CrossRef]
- Peres, M.; Nigoghossian, K.; Primo, F.; Saska, S.; Capote, T.; Scarel-Caminaga, R.; Messaddeq, Y.; Ribeiro, S.; Tedesco, A. Bacterial Cellulose Membranes as a Potential Drug Delivery System for Photodynamic Therapy of Skin Cancer. J. Braz. Chem. Soc. 2016, 27, 1949–1959. [Google Scholar] [CrossRef]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Song, L.; Yang, Z.; Qiu, M.; Wang, J.; Shi, S. Anthocyanins: Promising Natural Products with Diverse Pharmacological Activities. Molecules 2021, 26, 3807. [Google Scholar] [CrossRef]
- Shin, D.; Chae, K.S.; Choi, H.R.; Lee, S.J.; Gim, S.W.; Kwon, G.T.; Lee, H.T.; Song, Y.C.; Kim, K.J.; Kong, H.S.; et al. Bioactive and pharmacokinetic characteristics of pre-matured black raspberry, Rubus occidentalis. Ital. J. Food Sci. 2018, 30, 428–439. [Google Scholar]
- Ryu, D.; Koh, E. Stability of anthocyanins in bokbunja (Rubus occidentalis L.) under in vitro gastrointestinal digestion. Food Chem. 2018, 267, 157–162. [Google Scholar]
- Ozgen, M.; Wyzgoski, F.J.; Tulio, A.Z.; Gazula, A.; Miller, A.R.; Scheerens, J.C.; Reese, R.N.; Wright, S.R. Antioxidant Capacity and Phenolic Antioxidants of Midwestern Black Raspberries Grown for Direct Markets Are Influenced by Production Site. HortScience 2008, 43, 2039–2047. [Google Scholar] [CrossRef]
- Almeida, T.; Moreira, P.; Sousa, F.J.; Pereira, C.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Bioactive Bacterial Nanocellulose Membranes Enriched with Eucalyptus globulus Labill. Leaves Aqueous Extract for Anti-Aging Skin Care Applications. Materials 2022, 15, 1982. [Google Scholar] [PubMed]
- Sukhtezari, S.; Almasi, H.; Pirsa, S.; Zandi, M.; Pirouzifard, M. Development of bacterial cellulose based slow-release active films by incorporation of Scrophularia striata Boiss. extract. Carbohydr. Polym. 2017, 156, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Ossowicz-Rupniewska, P.; Rakoczy, R.; Konopacki, M.; Perużyńska, M.; Droździk, M.; Makuch, E.; Duchnik, W.; Kucharski, Ł.; Wenelska, K.; et al. Bacterial Cellulose Membrane Containing Epilobium angustifolium L. Extract as a Promising Material for the Topical Delivery of Antioxidants to the Skin. Int. J. Mol. Sci. 2021, 22, 6269. [Google Scholar]
| Mathematical Model | Equation | Parameter |
|---|---|---|
| Zero-order | F—fraction of the dissolved active compound at time t; K0—kinetic constant; t—time period of release of the active compound [35] | |
| First-order | F—fraction of the dissolved active compound at time t; K1—kinetic constant; t—time period of release of the active compound [35] | |
| Higuchi model | F—fraction of the dissolved active compound at time t; KH—Higuchi dissolution constant; t—time period of release of the active compound [36] | |
| Korsmeyer-Peppas | F—fraction of the dissolved active compound at time t; Kp—kinetic constant; t—time period of release of the active compound; n—release exponent indicating the mechanism of active compound release [37] |
| Correlation Coefficients (R2) | n | ||||
|---|---|---|---|---|---|
| Zero-Order | First-Order | Higuchi | Korsmayer-Peppas | ||
| pH 1.2 | 0.2279 | 0.2870 | 0.4923 | 0.6175 | 0.0847 |
| pH 4.5 | 0.2554 | 0.3904 | 0.5548 | 0.6988 | 0.0478 |
| pH 6.8 | 0.2138 | 0.2096 | 0.4400 | 0.5220 | 0.0904 |
| pH 7.4 | 0.1256 | 0.3578 | 0.2707 | 0.6653 | 0.0204 |
| Compound | Content * | Assay | Result |
|---|---|---|---|
| Total phenolic content | 77.25 ± 0.23 mg GAE/g | DPPH | 60.51 ± 0.18 µg/mL |
| Total anthocyanin content | 27.42 ± 0.32 mg CGE/g | FRAP | 1.66 ± 0.03 mmol Fe2+/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponjavic, M.; Filipovic, V.; Topakas, E.; Karnaouri, A.; Zivkovic, J.; Krgovic, N.; Mudric, J.; Savikin, K.; Nikodinovic-Runic, J. Two-Step Upcycling Process of Lignocellulose into Edible Bacterial Nanocellulose with Black Raspberry Extract as an Active Ingredient. Foods 2023, 12, 2995. https://doi.org/10.3390/foods12162995
Ponjavic M, Filipovic V, Topakas E, Karnaouri A, Zivkovic J, Krgovic N, Mudric J, Savikin K, Nikodinovic-Runic J. Two-Step Upcycling Process of Lignocellulose into Edible Bacterial Nanocellulose with Black Raspberry Extract as an Active Ingredient. Foods. 2023; 12(16):2995. https://doi.org/10.3390/foods12162995
Chicago/Turabian StylePonjavic, Marijana, Vuk Filipovic, Evangelos Topakas, Anthi Karnaouri, Jelena Zivkovic, Nemanja Krgovic, Jelena Mudric, Katarina Savikin, and Jasmina Nikodinovic-Runic. 2023. "Two-Step Upcycling Process of Lignocellulose into Edible Bacterial Nanocellulose with Black Raspberry Extract as an Active Ingredient" Foods 12, no. 16: 2995. https://doi.org/10.3390/foods12162995
APA StylePonjavic, M., Filipovic, V., Topakas, E., Karnaouri, A., Zivkovic, J., Krgovic, N., Mudric, J., Savikin, K., & Nikodinovic-Runic, J. (2023). Two-Step Upcycling Process of Lignocellulose into Edible Bacterial Nanocellulose with Black Raspberry Extract as an Active Ingredient. Foods, 12(16), 2995. https://doi.org/10.3390/foods12162995












