Intrinsic Fluorescence Markers for Food Characteristics, Shelf Life, and Safety Estimation: Advanced Analytical Approach
Abstract
1. Fluorescence Spectroscopy in Relation to Food Analysis
2. Fluorescence Markers in Food
3. Applications of Fluorescence Spectroscopy in Food Analysis
3.1. Meat, Fish, and Eggs
3.2. Dairy Products
3.3. Fruits and Seeds
3.4. Honey
3.5. Biscuits
4. Future Directions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lawrence, R.C.; Gilles, J.; Creamer, L.K. The relation between cheese texture and flavour. N. Z. J. Dairy Sci. Technol. 1983, 18, 175–190. [Google Scholar]
- Truman, E.; Bischoff, M.; Elliott, C. Which literacy for health promotion: Health, food, nutrition or media? Health Promot. Int. 2020, 35, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.M.; Miller, D.P.; Morrissey, T.W. Food insecurity and child health. Pediatrics 2019, 144, e20190397. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Emerging Point-of-Care Technologies for Food Safety Analysis. Sensors 2019, 19, 817. [Google Scholar] [CrossRef] [PubMed]
- Karoui, R.; Blecker, C. Fluorescence spectroscopy measurement for quality assessment of food systems—A review. Food Bioprocess Technol. 2011, 4, 364–386. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Berlin, Germany, 2006; p. 954. [Google Scholar]
- Valeur, B. Molecular Fluorescence Principles and Applications; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2001; p. 381. [Google Scholar]
- Mendieta, J.; Díaz-Cruz, M.S.; Esteban, M.; Tauler, R. Multivariate curve resolution: A possible tool in the detection of intermediate structures in protein folding. Biophys. J. 1998, 74, 2876–2888. [Google Scholar] [PubMed]
- Hassouna, A.; Saharb, A.; Lakhald, L.; Aït-Kaddoure, A. Fluorescence spectroscopy as a rapid and non-destructive method for monitoring quality and authenticity of fish and meat products: Impact of different preservation conditions. LWT-Food Sci. Technol. 2019, 103, 279–292. [Google Scholar]
- Kumar, K.; Tarai, M.; Mishra, A.K. Unconventional steady-state fluorescence spectroscopy as an analytical technique for analyses of complex-multifluorophoric mixtures. TRAC Trends Anal. Chem. 2017, 97, 216–243. [Google Scholar] [CrossRef]
- Ramanujam, N. Fluorescence spectroscopy of neoplastic and non-neoplastic tissues. Neoplasia 2000, 2, 89. [Google Scholar] [CrossRef]
- Ladokhin, A.S. Fluorescence Spectroscopy in Peptide and Protein Analysis. In Encyclopedia of Analytical Chemistry; Mayers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 5762–5779. [Google Scholar]
- Sahar, A.; Boubellouta, T.; Portanguen, S.; Kondjoyan, A.; Dufour, É. Synchronous front-face fluorescence spectroscopy coupled with parallel factors (PARAFAC) analysis to study the effects of cooking time on meat. J. Food Sci. 2009, 74, E534–E539. [Google Scholar] [CrossRef]
- Sahar, A.; Dufour, É. Classification and characterization of beef muscles using front-face fluorescence spectroscopy. Meat Sci. 2015, 100, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Povlsen, V.T.; Sørensen, J. Application of Fluorescence Spectroscopy and Chemometrics in the Evaluation of Processed Cheese During Storage. J. Dairy Sci. 2003, 86, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Karoui, R.; Dufour, E. Dynamic testing rheology and fluorescence spectroscopy investigations of surface to centre differences in ripened soft cheeses. Int. Dairy J. 2003, 13, 973–985. [Google Scholar] [CrossRef]
- Kulmyrzaev, A.; Dufour, E.; Noel, Y.; Hanafi, M.; Karoui, R.; Qannari, E.M.; Mazerolles, G. Investigation at the molecular level of soft cheese quality and ripening by infrared and fluorescence spectroscopies and chemometrics relationships with rheology properties. Int. Dairy J. 2005, 15, 669–678. [Google Scholar] [CrossRef]
- Stanković, M.; Bartolić, D.; Mutavdžić, D.; Marković, S.; Grubić, S.; Jovanović, N.M.; Radotić, K. Estimation of honey bee colony infection with Nosema ceranae and Varroa destructor using fluorescence spectroscopy in combination with differential scanning calorimetry of honey samples. J. Apic. Res. 2021, 62, 507–513. [Google Scholar] [CrossRef]
- Karoui, R.; Schoonheydt, R.; Decuypere, E.; Nicolai, B.; De Baerdemaeker, J. Front face fluorescence spectroscopy as a tool for the assessment of egg freshness during storage at a temperature of 12.2 °C and 87% relative humidity. Anal. Chim. Acta 2007, 582, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Bartolić, D.; Stanković, M.; Mutavdžić, D.; Stanković, S.; Jovanović, D.; Radotić, K. Multivariate Curve Resolution—Alternate Least Square Analysis of Excitation-Emission Matrices for Maize Flour Contaminated with Aflatoxin B1. J. Fluoresc. 2018, 28, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.M.; Wold, J.P.; Mortensen, G. Light-induced changes in semi-hard cheese determined by fluorescence spectroscopy and chemometrics. Int. Dairy J. 2006, 16, 1483–1489. [Google Scholar] [CrossRef]
- Karoui, R.; Dufour, E.; De Baerdemaeker, J. Front face fluorescence spectroscopy coupled with chemometric tools for monitoring the oxidation of semi-hard cheeses throughout ripening. Food Chem. 2007, 101, 1305–1314. [Google Scholar] [CrossRef]
- Radotić, K.; Kalauzi, A.; Djikanović, D.; Jeremić, M.; Leblanc, R.M.; Cerovic, Z.G. Component analysis of the fluorescence spectra of a lignin model compound. J. Photochem. Photobiol. B 2006, 83, 1–10. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish spoilage mechanisms and preservation Techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Ammor, S.; Yaakoubi, K.; Chevallier, I.; Dufour, E. Identification by fluorescence spectroscopy of lactic acid bacteria isolated from a small-scale facility producing traditional dry sausages. J. Microbiol. Methods 2004, 59, e271–e281. [Google Scholar] [CrossRef] [PubMed]
- Aufartová, J.; Brabcová, I.; Torres-Padrón, M.E.; Solich, P.; Sosa-Ferrera, Z.; Santana-Rodríguez, J. Determination of fluoro-quinolones in fishes using microwave-assisted extraction combined with ultra-high performance liquid chromatography and fluorescence detection. J. Food Compos. Anal. 2017, 56, 140–146. [Google Scholar] [CrossRef]
- Durek, J.; Bolling, J.S.; Knorr, D.; Schwägele, F.; Schlüter, O. Effects of different storage conditions on quality related porphyrin fluorescence signatures of pork slices. Meat Sci. 2012, 90, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Gatellier, P.; Santé-Lhoutellier, V.; Portanguen, S.; Kondjoyan, A. Use of meat fluorescence emission as a marker of oxidation promoted by cooking. Meat Sci. 2009, 83, 651–656. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–684. [Google Scholar] [CrossRef]
- Veberg, A.; Vogt, G.; Wold, J.P. Fluorescence in aldehyde model systems related to lipid oxidation. LWT 2006, 39, 562–570. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; Liu, Q.; Chen, Q.; Zhang, H.; Xia, X.; Kong, B. Changes in myofibrillar protein gel quality of porcine longissimus muscle induced by its stuctural modification under different thawing methods. Meat Sci. 2019, 147, 108–115. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.W.; Pu, H.; Wei, Q. Principles and applications of spectroscopic techniques for evaluating food protein conformational changes: A review. Trends Food Sci. Technol. 2017, 67, 207–219. [Google Scholar] [CrossRef]
- Karoui, R.; Hassoun, A.; Ethuin, P. Front face fluorescence spectroscopy enables rapid differentiation of fresh and frozen-thawed sea bass (Dicentrarchus labrax) fillets. J. Food Eng. 2017, 202, 89–98. [Google Scholar] [CrossRef]
- Hemar, Y.; Gerbeaud, M.; Oliver, C.M.; Augustin, M.A. Original article investigation into the interaction between resveratrol and whey proteins using fluorescence spectroscopy. Int. J. Food. Sci. Technol. 2011, 46, 2137–2144. [Google Scholar] [CrossRef]
- Fagan, C.C.; Ferreira, T.G.; Payne, F.A.; O’Donnell, C.P.; O’Callaghan, D.J.; Castillo, M. Preliminary evaluation of endogenous milk fluorophores as tracer molecules for curd syneresis. J. Dairy Sci. 2011, 94, 5350–5358. [Google Scholar] [CrossRef] [PubMed]
- Herbert, S.; Riou, N.M.; Devaux, M.F.; Riaublanc, A.; Bouchet, B.; Galllant, D.J.; Dufour, E. Monitoring the identity and the structure of soft cheeses by fluorescence spectroscopy. Lait 2000, 80, 621–634. [Google Scholar] [CrossRef]
- Saito, Y.; Itakura, K.; Kuramoto, M.; Kaho, T.; Ohtake, N.; Hasegawa, H.; Suzuki, T.; Kondo, N. Prediction of protein and oil contents in soybeans using fluorescence excitation emission matrix. Food Chem. 2021, 365, 130403. [Google Scholar] [CrossRef]
- Zandomeneghi, M. Fluorescence of Cereal Flours. J. Agric. Food Chem. 1999, 47, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, H.; Taghavizad, R.; Majd, A. Origin of honey proteins and method for its quality control. Pak. J. Bot. 2010, 42, 3221–3228. [Google Scholar]
- Stanković, M.; Bartolić, D.; Šikoparija, B.; Spasojević, D.; Mutavdžić, D.; Natić, M.; Radotić, K. Variability Estimation of the Protein and Phenol Total Content in Honey Using Front Face Fluorescence Spectroscopy Coupled with MCR–ALS Analysis. J. Appl. Spectrosc. 2019, 86, 256–263. [Google Scholar] [CrossRef]
- Stanković, M.; Nikčević, M.; Radotić, K. Annual variation of proteins and phenols in honey of a bee society using fluorescence spectroscopy: A way to assess effects of antivarroa treatments on honey composition. Eur. Food. Technol. 2020, 246, 1515–1518. [Google Scholar] [CrossRef]
- Karoui, R.; Dufour, E.; Bosset, J.O. The use of front face fluorescence spectroscopy to classify the botanical origin of honey samples produced in Switzerland. Food Chem. 2007, 101, 314–323. [Google Scholar] [CrossRef]
- Milovanovic, P.; Hrncic, D.; Radotic, K.; Stankovic, M.; Mutavdzic, D.R.; Djonic, D.D.; Rasic-Markovic, A.; Djuric, D.M.; Stanojlovic, O.P.; Djuric, M.P. Moderate hyperhomocysteinemia induced by short-term dietary methionine overload alters bone microarchitecture and collagen features during growth. Life Sci. 2017, 191, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Egelandsdal, B.; Dingstad, G.; Tøgersen, G.; Lundby, F.; Langsrud, O. Autofluorescence quantifies collagen in sausage batters with a large variation in myoglobin content. Meat Sci. 2005, 69, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.M.; Wold, J.P. Fluorescence of muscle and connective tissue from cod and salmon. J. Agric. Food Chem. 2003, 51, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Pfündel, E.E.; Ghozlen, N.B.; Meyer, S.; Cerovic, Z.G. Investigating UV screening in leaves by two different types of portable UV fluorimeters reveals in vivo screening by anthocyanins and carotenoids. Photosynth. Res. 2007, 93, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Stober, F.; Lichtenthaler, H.K. Fluorescence emission spectra of plant leaves and plant constituents. Radiat. Environ. Biophys. 1991, 30, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Veberg, A.; Sørheim, O.; Moan, J.; Iani, V.; Juzenas, P.; Nilsen, A.N.; Wold, J.P. Measurement of lipid oxidation and porphyrins in high oxygen modified atmosphere and vacuum-packed minced Turkey and pork meat by fluorescence spectra and images. Meat Sci. 2006, 73, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Miquel Becker, E.; Christensen, J.; Frederiksen, C.S.; Haugaard, V.K. Front-face spectroscopy and chemometrics in analysis of yogurt: Rapid analysis of riboflavin. J. Dairy Sci. 2003, 86, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Sádecká, J.; Tóthová, J. Fluorescence Spectroscopy and Chemometrics in the Food Classification—A Review. Czech J. Food Sci. 2007, 25, 159–173. [Google Scholar] [CrossRef]
- Shaikh, S.; ODonnell, C. Applications of fluorescence spectroscopy in dairy processing: A review. Curr. Opin. Food Sci. 2017, 11, 16–24. [Google Scholar] [CrossRef]
- Boubellouta, T.; Galtier, V.; Dufour, E. Structural changes of milk components during acid-induced coagulation kinetics as studied by synchronous fluorescence and mid-infrared spectroscopy. Appl. Spectrosc. 2011, 65, 284–292. [Google Scholar] [CrossRef]
- Karoui, R.; Dufour, E.; De Baerdemaeker, J. Common components and specific weights analysis: A tool for monitoring the molecular structure of semi-hard cheese throughout ripening. Anal. Chim. Acta 2006, 572, 125–133. [Google Scholar] [PubMed]
- Ghnimi, H.; Karoui, R.; Attia, H.; Chénè, C.; Ennouri, M. Use of front face fluorescence spectroscopy coupled with multivariate data analysis for monitoring biscuits’ quality during aging. Food Sci. Nutr. 2022, 10, 4380–4393. [Google Scholar] [CrossRef] [PubMed]
- Lenhardt, L.; Zeković, I.; Dramićanin, T.; Milićević, B.; Burojević, J.; Dramićanin, M.D. Characterization of cereal flours by fluorescence spectroscopy coupled with PARAFAC. Food Chem. 2017, 229, 165–171. [Google Scholar] [CrossRef]
- Karoui, R.; Dufour, E.; Schoonheydt, R.; De Baerdemaeker, J. Characterisation of soft cheese by front face fluorescence spectroscopy coupled with chemometric tools: Effect of the manufacturing process and sampling zone. Food Chem. 2007, 100, 632–642. [Google Scholar] [CrossRef]
- Lenhardt, L.; Bro, R.; Zeković, I.; Dramićanin, T.; Dramićanin, M.D. Fluorescence spectroscopy coupled with PARAFAC and PLS DA for characterization and classification of honey. Food Chem. 2015, 175, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Bartolić, D.; Mutavdžić, D.; Carstensen, J.M.; Stanković, S.; Nikolić, M.; Radotić, K. Fluorescence spectroscopy and multispectral imaging for fingerprinting of aflatoxin-B1 contaminated (Zea mays L.) seeds: A preliminary study. Sci. Rep. 2022, 12, 4849. [Google Scholar] [CrossRef] [PubMed]
- Oto, N.; Oshita, S.; Makino, Y.; Kawagoe, Y.; Sugiyama, J.; Yoshimura, M. Nondestructive evaluation of ATP content and plate count on pork meat surface by fluorescence spectroscopy. Meat Sci. 2013, 93, 579–585. [Google Scholar] [CrossRef]
- Aït-Kaddour, A.; Boubellouta, T.; Chevallier, I. Development of a portable spectrofluorimeter for measuring the microbial spoilage of minced beef. Meat Sci. 2011, 88, 675–681. [Google Scholar] [CrossRef]
- Bai, Z.; Luo, Y.; Xu, W.; Gao, H.; Han, P.; Liu, T.; Wang, H.; Chen, A.; Huang, K. Development of a new fluorescence immunochromatography strip for detection of chloramphenicol residues in chicken muscles. J. Sci. Food Agric. 2013, 93, 3743–3747. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Liu, M.H.; Zhao, J.H.; Hong, Q. Determination of Gentamicin Residual in Duck Meat Using Fluorescence Analysis Method. Adv. Mater. Res. 2014, 1033–1034, 638–642. [Google Scholar] [CrossRef]
- Dufour, E.; Frencia, J.P.; Kane, E. Development of a rapid method based on front-face fluorescence spectroscopy for the monitoring of fish freshness. Food Res. Int. 2003, 36, 415–423. [Google Scholar] [CrossRef]
- Rahman, M.; Bui, M.V.; Shibata, M.; Nakazawa, N.; Rithu, M.N.A.; Yamashita, H.; Sadayasu, K.; Tsuchiyama, K.; Nakauchi, S.; Hagiwara, T.; et al. Rapid noninvasive monitoring of freshness variation in frozen shrimp using multidimensional fluorescence imaging coupled with chemometrics. Talanta 2021, 224, 121871. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.J.; Vazquez-Moreno, L.; Bermudez-Almada, M.D.C.; Guardado, R.B.; Ortega-Nieblas, M.J. Multiresidue Determination of Fluoroquinolones in Shrimp by Liquid Chromatography-Fluorescence-Mass Spectrometryn. J. AOAC Int. 2005, 88, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.J.; Jiang, H.Y.; Li, X.L.; Mi, T.J.; Li, A.C.; Shen, J.Z. Simultaneous Determination of Trace Levels of 10 Quinolones in Swine, Chicken, and Shrimp Muscle Tissues Using HPLC with Programmable Fluorescence Detection. J. Agric. Food Chem. 2007, 55, 3829–3834. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, D.K.; Munck, L.; Engelsen, S.B. Screening for dioxin contamination in fish oil by PARAFAC and N-PLSR analysis of fluorescence landscapes. J. Chemom. 2002, 16, 451–460. [Google Scholar] [CrossRef]
- Christensen, J.; Miquel Becker, E.M.; Frederiksen, C.S. Fluorescence spectroscopy and PARAFAC in the analysis of yogurt. Chemom. Intell. Lab. Syst. 2005, 75, 201–208. [Google Scholar] [CrossRef]
- Latchoumane, L.; Alary, K.; Minier, J.; Davrieux, F.; Lugan, R.; Chillet, M.; Roger, J.M. Front-Face Fluorescence Spectroscopy and Feature Selection for Fruit Classification Based on N-CovSel Method. Front. Anal. Sci. 2022, 2, 867527. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Lednev, V.N.; Sibirev, A.V.; Gudkov, S.V. The Use of Fluorescence Spectra for the detection of Scab and Rot in Fruit and Vegetable Crops. Front. Phys. 2021, 8, 640887. [Google Scholar] [CrossRef]
- Włodarskaa, K.; Pawlak-Lemańskaa, K.; Khmelinskiib, I.; Sikorska, E. Multivariate curve resolution—Alternating least squares analysis of the total synchronous fluorescence spectra: An attempt to identify polyphenols contribution to the emission of apple juices. Chemom. Intell. Lab. Syst. 2017, 164, 94–102. [Google Scholar]
- Agati, G.; D’Onofrio, C.; Ducci, E.; Cuzzola, A.; Remorini, D.; Tuccio, L.; Lazzini, F.; Mattii, G. Potential of a Multiparametric Optical Sensor for Determining in Situ the Maturity Components of Red and White Vitis vinifera Wine Grapes. J. Agric. Food Chem. 2013, 61, 12211–12218. [Google Scholar] [CrossRef]
- Ben Ghozlen, N.; Cerovic, Z.G.; Germain, C.; Toutain, S.; Latouche, G. Non-destructive optical monitoring of grape maturation by proximal sensing. Sensors 2010, 10, 10040–10068. [Google Scholar] [CrossRef]
- Quatela, A.; Gilmore, A.M.; Gall, K.E.S.; Sandros, M.; Csatorday, K.; Siemiarczuk, A.; Ben Yang, B.; Camenen, L. A-TEEMTM, a new molecular fingerprinting technique: Simultaneous absorbance-transmission and fluorescence excitation-emission matrix method. Methods Appl. Fluoresc. 2018, 6, 027002. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.E.J.; Gilmore, A.M.; Boss, P.K.; Pagay, V.; Jeffery, D.W. Machine learning for classifying and predicting grape maturity indices using absorbance and fluorescence spectra. Food Chem. 2023, 403, 134321. [Google Scholar] [CrossRef] [PubMed]
- Zandomeneghi, M.; Carbonaro, L.; Caffarata, C. Fluorescence of Vegetable Oils: Olive Oils. J. Agric. Food Chem. 2005, 53, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Lobo-Prieto, A.; Tena, N.; Aparicio-Ruiz, R.; García-González, D.L.; Sikorska, E. Monitoring Virgin Olive Oil Shelf-Life by Fluorescence Spectroscopy and Sensory Characteristics: A Multidimensional Study Carried Out under Simulated Market Conditions. Foods 2020, 9, 1846. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, K.; Zlatanov, M.; Eftimov, T.; Brabant, D.; Yosifova, S.; Halil, E.; Antova, G.; Angelova, M. Fluoresence Spectra from Vegetable Oils Using Violet and Blue Ld/Led Exitation And An Optical Fiber Spectrometer. Int. J. Food Prop. 2014, 17, 1211–1223. [Google Scholar] [CrossRef]
- Sikorska, E.; Górecki, T.; Khmelinskii, I.V.; Sikorski, M.; Kozio, J. Classification of edible oils using synchronous scanning fluorescence spectroscopy. Food Chem. 2005, 89, 217–225. [Google Scholar] [CrossRef]
- Bartolić, D.; Stanković, M.; Prokopijević, M.; Radotić, K. Effects of UV-A and UV-B Irradiation on Antioxidant Activity and Fluorescence Characteristics of Soybean (Glycine max L.) Seeds. Russ. J. Phys. Chem. A 2022, 96, 2797–2800. [Google Scholar] [CrossRef]
- Xue, S.S.; Tan, J. Rapid and non-destructive composition analysis of cereal flour blends by front-face synchronous fluorescence spectroscopy. J. Cereal Sci. 2022, 106, 103494. [Google Scholar] [CrossRef]
- Trautvetter, S.; Koelling-Speer, I.; Speer, K. Confirmation of phenolic acids andflavonoids in honeys by UPLC-MS. Apidologie 2009, 40, 140–150. [Google Scholar] [CrossRef]
- Ruoff, K.; Luginbu, W.; Künzli, R.; Bogdanov, S.; Olivier Bosset, J.; von der Ohe, K.; von der Ohe, W.; Amado, R. Authentication of the Botanical and Geographical Origin of Honey by Front-Face Fluorescence spectroscopy. J. Agric. Food Chem. 2006, 54, 6858–6866. [Google Scholar] [CrossRef]
- Stanković, M.; Prokopijević, M.; Šikoparija, B.; Nedić, N.; Andrić, F.; Polović, N.; Natić, M.; Radotić, K. Using Front-Face Fluorescence Spectroscopy and Biochemical Analysis of Honey to Assess a Marker for the Level of Varroa destructor Infestation of Honey Bee (Apis mellifera) Colonies. Foods 2023, 12, 629. [Google Scholar] [CrossRef]
- Hassoun, A. Exploring the Potential of Fluorescence Spectroscopy for the Discrimination between Fresh and Frozen-Thawed Muscle Foods. Photochem 2021, 1, 247–263. [Google Scholar] [CrossRef]
- Mahmudiono, T.; Saleh, R.O.; Widjaja, G.; Chen, T.C.; Yasin, G.; Thangavelu, L.; Altimari, U.S.; Chupradit, S.; Kadhim, M.M.; Marhoon, H.A. A review on material analysis of food safety based on fluorescence spectrum combined with artificial neural network technology. Food Sci. Technol. 2022, 42, e118721. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhao, Y.; Liu, J.; Guo, Y. A statistical analysis of the freshness of postharvest leafy vegetables with application of water based on chlorophyll fluorescence measurement. Inf. Process. Agric. 2017, 4, 269–274. [Google Scholar] [CrossRef]
- Agati, G.; Bilger, W.; Cerovic, Z. Fluorescence tools for sensing of quality-related phytochemicals in fruits and vegetables. In Sensor-Based Quality Assessment Systems for Fruits and Vegetables, 1st ed.; Taylor &Francis Group: London, UK, 2020; ISBN 978-1-77188-935-3. [Google Scholar]
- Sikorska, E.; Khmelinskii, I.; Sikorski, M. Fluorescence spectroscopy and imaging instruments for food quality evaluation. In Evaluation Technologies for Food Quality; Zhong, J., Wang, X., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Oxford, UK, 2019; pp. 491–533. [Google Scholar]



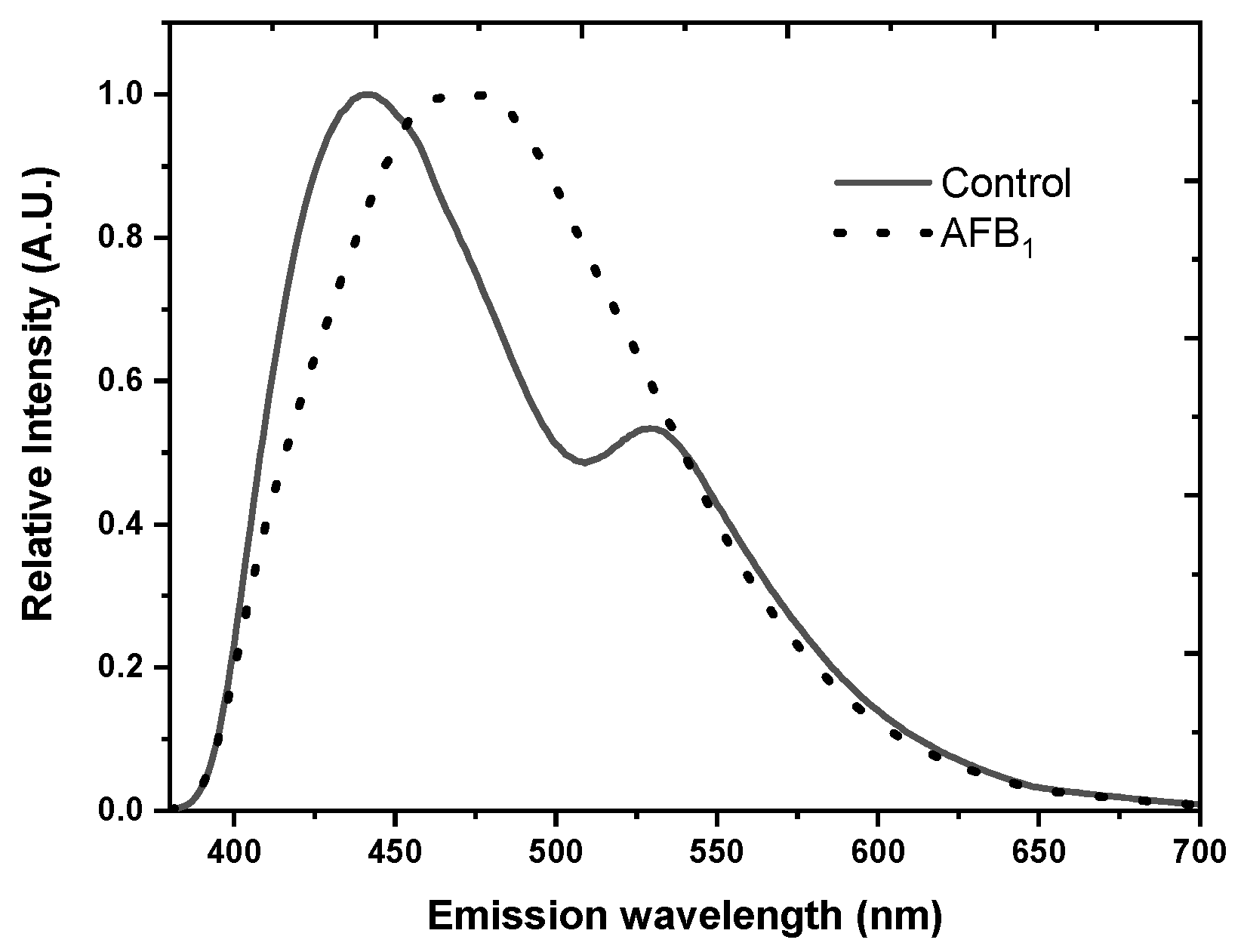
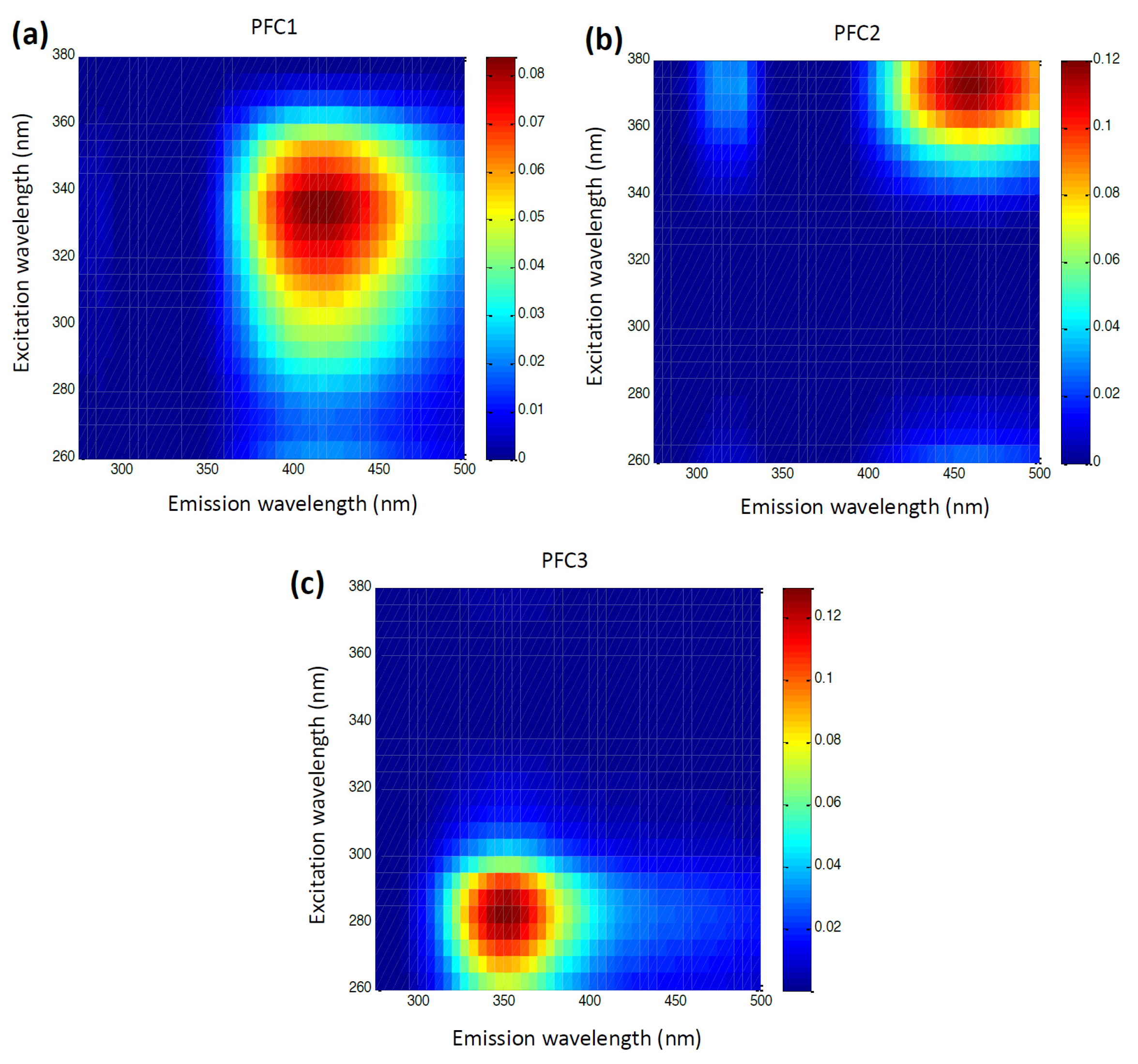
| Intrinsic Fluorophores | Excitation Maxima (nm) | Emission Maxima (nm) | References |
|---|---|---|---|
| Aminoacids | |||
| Tryptophan | 280 | 350 | [6,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] |
| Tyrosine | 275 | 305 | [11,12] |
| Phenylalanine | 260 | 280 | [11,12] |
| Proteins, enzymes, coenzymes | |||
| Proteins | 260–280 | 320–350 | [11,12,13,14,15,16,17,18,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] |
| Collagen | 325 | 400–470 | [11,44,45,46] |
| Elastin | 325 | 400–425 | [11] |
| Flavins | 380–450 | 515–535 | [19,27,28,29,34,35,47,48,49,50] |
| NADH, NADPH | 330–350 | 440–465 | [10,11,19,34,35,40,47] |
| Vitamins | |||
| Vitamin A | 320–330 | 470–510 | [10,15,16,17,19,34,37,51,52,53,54,55] |
| Vitamin B6 forms | 315–340 | 385–425 | [11,39,56] |
| Vitamin B12 | 250–275 | 305–335 | [11] |
| Vitamin C | 310–360 | 400–440 | [11] |
| Vitamin D | 390–425 | 450–480 | [11] |
| Vitamin K | 245 | 430–440 | [11] |
| Lipids | |||
| Lipofuscin | 340–400 | 350–600 | [11] |
| Maillard reaction products | 350 | 440 | [13,14,19,28,35,43,52,55,57,58] |
| Carotenoids | 450–480 | 525–580 | [47] |
| Phenolics compounds | |||
| Ferulic acid | 260 | 420 | [48] |
| Caffeic acid | 260 | 425 | [48] |
| Catechin, epicatechin, and vanilic acid | 278 | 360 | [41] |
| trans-Resveratrol | 330 | 375 | [35,41] |
| Aflatoxins | 360 | 400–460 | [20,59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radotić, K.; Stanković, M.; Bartolić, D.; Natić, M. Intrinsic Fluorescence Markers for Food Characteristics, Shelf Life, and Safety Estimation: Advanced Analytical Approach. Foods 2023, 12, 3023. https://doi.org/10.3390/foods12163023
Radotić K, Stanković M, Bartolić D, Natić M. Intrinsic Fluorescence Markers for Food Characteristics, Shelf Life, and Safety Estimation: Advanced Analytical Approach. Foods. 2023; 12(16):3023. https://doi.org/10.3390/foods12163023
Chicago/Turabian StyleRadotić, Ksenija, Mira Stanković, Dragana Bartolić, and Maja Natić. 2023. "Intrinsic Fluorescence Markers for Food Characteristics, Shelf Life, and Safety Estimation: Advanced Analytical Approach" Foods 12, no. 16: 3023. https://doi.org/10.3390/foods12163023
APA StyleRadotić, K., Stanković, M., Bartolić, D., & Natić, M. (2023). Intrinsic Fluorescence Markers for Food Characteristics, Shelf Life, and Safety Estimation: Advanced Analytical Approach. Foods, 12(16), 3023. https://doi.org/10.3390/foods12163023




