Characterization of the Key Aroma Compounds in Different Yeast Proteins by GC-MS/O, Sensory Evaluation, and E-Nose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Sensory Evaluation
2.3. Electronic Nose Analysis
2.4. Aroma Extraction by Headspace Solid-Phase Microextraction (HS-SPME)
2.5. GC-MS and GC-MS/O
2.6. Qualitative and Quantitative Methods
2.7. Addition Experiment
2.8. Statistical Analysis
3. Results and Discussion
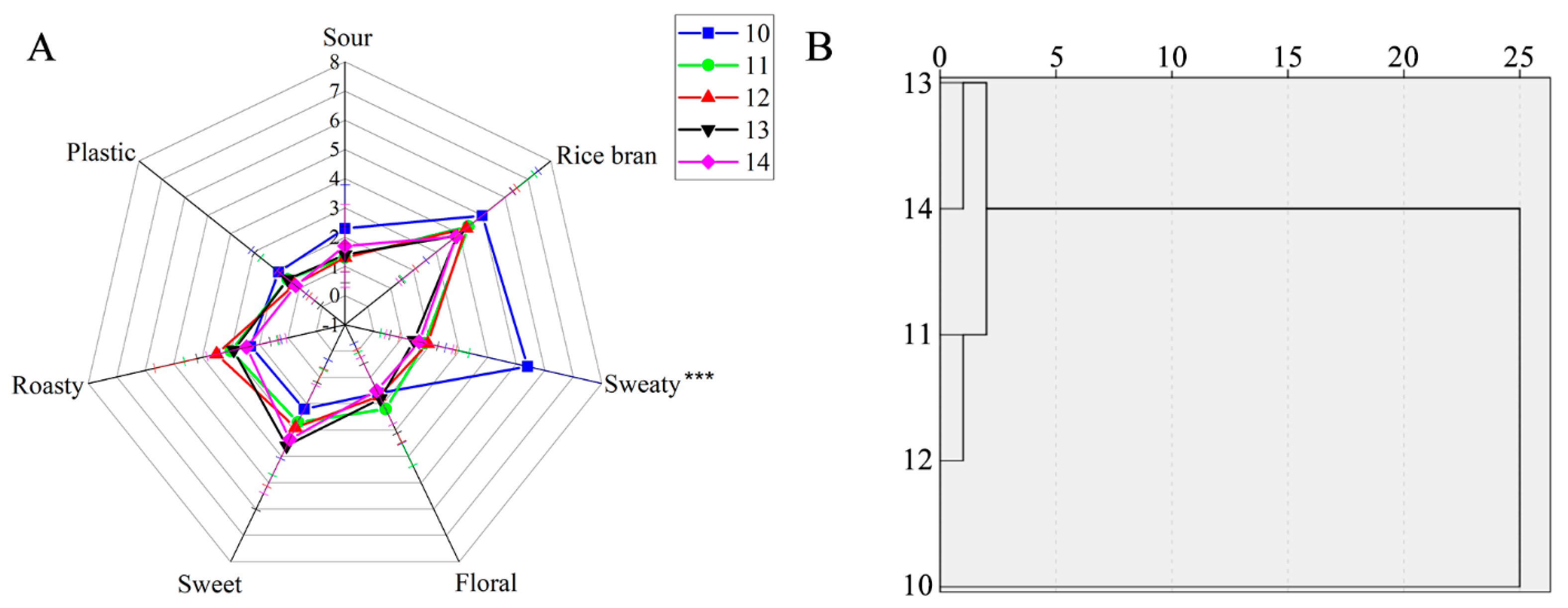
3.1. Sensory Evaluation
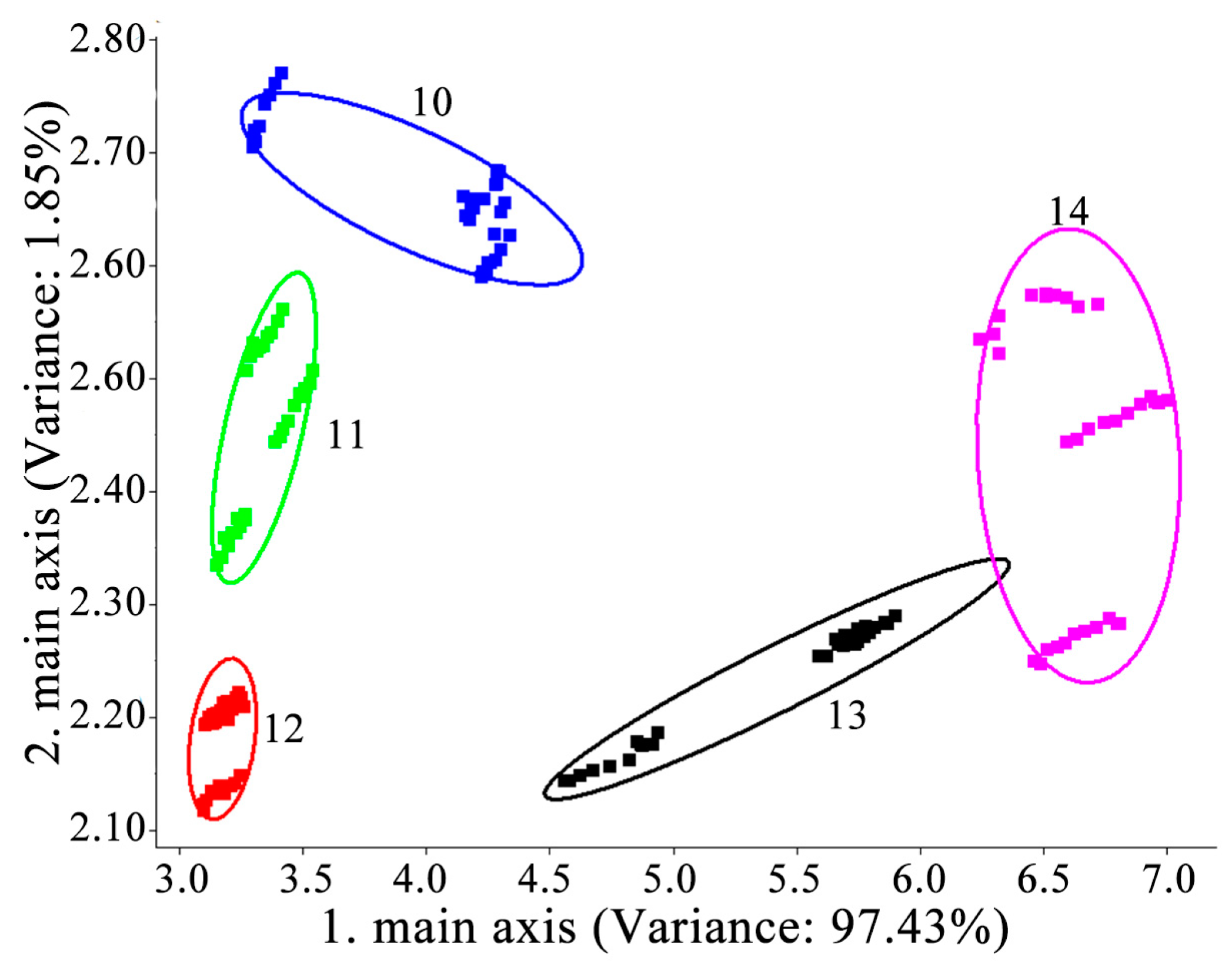
3.2. Sensor Array Response to YPs
3.3. Volatile Compound Analysis
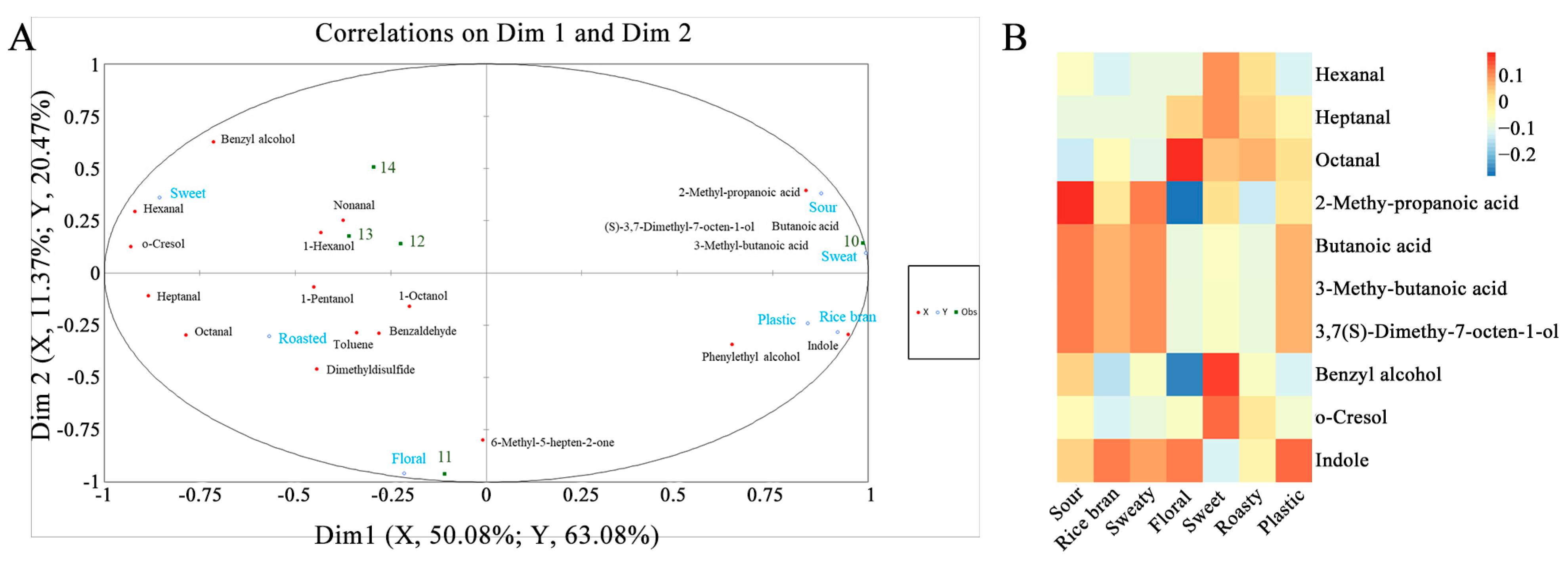
3.4. Relationship between Volatile Compounds and Sensory Evaluation
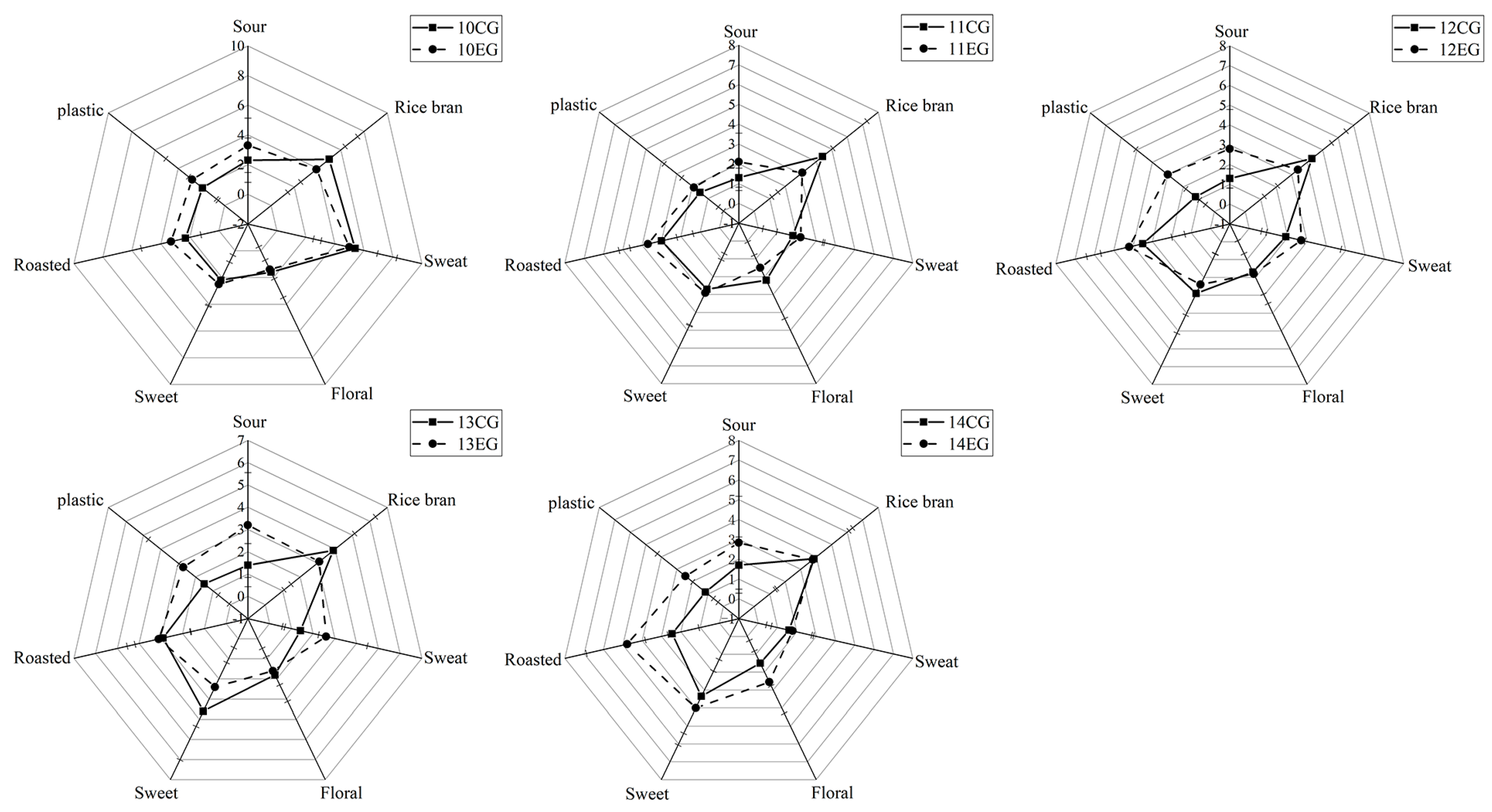
3.5. Aroma Addition Experiment Analyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aslankoohi, E.; Herrera-Malaver, B.; Rezaei, M.N.; Steensels, J.; Courtin, C.M.; Verstrepen, K.J. Non-conventional yeast strains increase the aroma complexity of bread. PLoS ONE 2016, 11, e0165126. [Google Scholar] [CrossRef] [PubMed]
- Carrau, F.; Gaggero, C.; Aguilar, P.S. Yeast diversity and native vigor for flavor phenotypes. Trends Biotechnol. 2015, 33, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat analogues: Health promising sustainable meat substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Pu, D.D.; Zhang, J.C.; Hao, Z.L.; Zhao, X.X.; Sun, B.G.; Zhang, Y.Y. Identification of novel umami peptides in chicken breast soup through a sensory-guided approach and molecular docking to the T1R1/T1R3 taste receptor. J. Agr. Food Chem. 2023, 71, 7803–7811. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Sato, Y.; Okada, M.; Yamaguchi, S. Cyclodextrins produced by cyclodextrin glucanotransferase mask beany off-aromas in plant-based meat analogs. PLoS ONE 2022, 17, e0269278. [Google Scholar] [CrossRef]
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial protein: Future sustainable food supply route with low environmental footprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef]
- Pacheco, M.T.B.; Caballero-Cordoba, G.M.; Sgarbieri, V.C. Composition and nutritive value of yeast biomass and yeast protein concentrates. J. Nutr. Sci. Vitaminol. 1997, 43, 601–612. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast protein as an easily accessible food source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an alternative and valuable source of nutritional and bioactive compounds for humans. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.P.; Hubinger, M.D. Spent brewer’s yeast as a source of high added value molecules: A systematic review on its characteristics, processing and potential applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef]
- Ma, J.; Sun, Y.; Meng, D.; Zhou, Z.; Zhang, Y.; Yang, R. Yeast proteins: The novel and sustainable alternative protein in food applications. Trends Food Sci. Technol. 2023, 135, 190–201. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Q.; Zhuang, J.; Feng, T.; Ho, C.T.; Song, S. Characterization of aroma-active compounds in four yeast extracts using instrumental and sensory techniques. J. Agric. Food Chem. 2019, 68, 267–278. [Google Scholar] [CrossRef]
- Mahadevan, K.; Farmer, L. Key odor impact compounds in three yeast extract pastes. J. Agric. Food Chem. 2006, 54, 7242–7250. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, P.; Chen, E.; Song, H.; Li, P.; Li, K.; Xiong, J. Investigating characteristics and possible origins of off-odor substances in various yeast extract products. J. Food Biochem. 2020, 44, e13184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, H.; Li, P.; Yao, J.; Xiong, J. Determination of potential off-flavour in yeast extract. LWT-Food Sci. Technol. 2017, 82, 184–191. [Google Scholar] [CrossRef]
- Raza, A.; Song, H.; Begum, N.; Raza, J.; Iftikhar, M.; Li, P.; Li, K. Direct classification of volatile organic compounds in heat-treated glutathione-enriched yeast extract by headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS). Food Anal. Methods 2020, 13, 2279–2289. [Google Scholar] [CrossRef]
- Pu, D.; Zhang, Y.; Zhang, H.; Sun, B.; Ren, F.; Chen, H.; Tang, Y. Characterization of the key aroma compounds in traditional hunan smoke-cured pork leg (Larou, THSL) by aroma extract dilution analysis (AEDA), odor activity value (OAV), and sensory evaluation experiments. Foods 2020, 9, 413. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Yang, C.J.; Ding, W.; Ma, L.J.; Jia, R. Discrimination and characterization of different intensities of goaty aroma in goat milk by means of an electronic nose. J. Dairy Sci. 2015, 98, 55–67. [Google Scholar] [CrossRef]
- Zhou, X.; Chong, Y.; Ding, Y.; Gu, S.; Liu, L. Determination of the effects of different washing processes on aroma characteristics in silver carp mince by MMSE–GC–MS, e-nose and sensory evaluation. Food Chem. 2016, 207, 205–213. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, W.; Yu, H.; Yuan, J.; Tian, H. Characterization of major odor-active compounds responsible for nutty aroma in Cheddar cheese according to Chinese taste. Flavour Fragr. J. 2021, 36, 171–181. [Google Scholar] [CrossRef]
- Gancarz, M.; Malaga-Toboła, U.; Oniszczuk, A.; Tabor, S.; Oniszczuk, T.; Gawrysiak-Witulska, M.; Rusinek, R. Detection and measurement of aroma compounds with the electronic nose and a novel method for MOS sensor signal analysis during the wheat bread making process. Food Bioprod. Process 2021, 127, 90–98. [Google Scholar] [CrossRef]
- Yin, M.; Shao, S.; Zhou, Z.; Chen, M.; Zhong, F.; Li, Y. Characterization of the Key Aroma Compounds in Dog Foods by Gas Chromatography–Mass Spectrometry, Acceptance Test, and Preference Test. J. Agric. Food Chem. 2020, 68, 9195–9204. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Shan, Y.; Zhang, L.; Sun, B.; Zhang, Y. Identification and inhibition of the key off-odorants in duck broth by means of the sensomics approach and binary odor mixture. J. Agric. Food Chem. 2022, 70, 13367–13378. [Google Scholar] [CrossRef] [PubMed]
- Smit, B.A.; Engels, W.J.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef]
- Thongwong, A.; Fernando, L.N.; Grün, I.U.; Clarke, A.D. Reduction of Warmed-Over Aroma Volatiles from Freeze-Dried Lean by Supercritical CO2 Extraction. J. Food Sci. 1999, 64, 387–389. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, J.; Zang, M.; Zhang, K.; Li, D.; Li, X. Aroma Profile Analysis of Instant and Traditional Lanzhou Beef Bouillons Using HS-SPME-GC/MS, Electronic Nose and Electronic Tongue. Bioengineering 2022, 9, 582. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, Z.; Liu, S.; Zhu, S.; Li, G.; Zhong, F.; Mao, J. Formation pathways and precursors of furfural during Zhenjiang aromatic vinegar production. Food Chem. 2021, 354, 129503. [Google Scholar] [CrossRef]
- Brenner, M.W.; Khan, A.A. Furfural and beer color as indices of beer aroma deterioration. J. Am. Soc. Brew. Chem. 1976, 34, 14–19. [Google Scholar]
- Zhang, S.; Li, C.; Wu, J.; Peng, S.; Wu, W.; Liao, L. Properties investigations of rape stalks fermented by different salt concentration: Effect of volatile compounds and physicochemical indexes. Food Chem. X 2023, 18, 100746. [Google Scholar] [CrossRef]
- Wei, Y.; Zou, W.; Shen, C.H.; Yang, J.G. Basic aroma types and component characteristics of Chinese traditional liquors: A review. J. Food Sci. 2020, 85, 4096–4107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, X.; Hayat, K.; Duhoranimana, E.; Zhang, X.; Xia, S.; Yu, J.; Xing, F. Characterization of odor-active compounds of chicken broth and improved aroma by thermal modulation in electrical stewpots. Food Res. Int. 2018, 109, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Fu, Y.; Liu, Y.; Huang, J.; Chen, J.; Yin, J.; Jin, S.; Sun, W.; Xu, Y. Comparative analysis of different grades of Tieguanyin oolong tea based on metabolomics and sensory evaluation. LWT 2023, 174, 114423. [Google Scholar] [CrossRef]
- Patton, S. The methyl ketones of blue cheese and their relation to its aroma. J. Dairy Sci. 1950, 33, 680–684. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Wang, B.; Song, H.; Zou, T. Screening of the volatile compounds in fresh and thermally treated watermelon juice via headspace-gas chromatography-ion mobility spectrometry and comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry analysis. LWT 2021, 137, 110478. [Google Scholar] [CrossRef]
- Song, X.; Wang, G.; Zhu, L.; Zheng, F.; Ji, J.; Sun, J.; Li, H.; Huang, M.; Zhao, Q.; Zhao, M.; et al. Comparison of two cooked vegetable aroma compounds, dimethyl disulfide and methional, in Chinese Baijiu by a sensory-guided approach and chemometrics. LWT 2021, 146, 111427. [Google Scholar] [CrossRef]
- Li, R.; Chen, K.; Li, L.; Zhao, S.; Guo, C.; Wang, X.; Zhang, J.; Liang, M. Identification of key odor compounds in the burnt smell of upper tobacco leaves through the molecular sensory science technique. Sci. Asia 2023, 49, 290–296. [Google Scholar] [CrossRef]
- McKay, M.; Bauer, F.F.; Panzeri, V.; Buica, A. Investigation of olfactory interactions of low levels of five off-flavour causing compounds in a red wine matrix. Food Res. Int. 2020, 128, 108878. [Google Scholar] [CrossRef]
| Array | Sensor Types | Properties of Different Arrays |
|---|---|---|
| S1 | W1C | Aromatic compounds, benzene |
| S2 | W5S | Sensitive to nitrogen oxides |
| S3 | W3C | Ammonia, aromatic compounds |
| S4 | W6S | Selective mainly for hydrogen |
| S5 | W5C | Alkanes, aromatic compounds |
| S6 | W1S | Short-chain alkanes, such as methane |
| S7 | W1W | Sulfur organic compound |
| S8 | W2S | Sensitive to alcohols, ethers, aldehydes, and ketones |
| S9 | W2W | Aromatic compounds, sulfur organic compounds |
| S10 | W3S | Sensitive to long-chain alkanes |
| No. | RI a | Compounds | CAS | Relative Concentration (μg/kg) b | Aroma Quality | ||||
|---|---|---|---|---|---|---|---|---|---|
| #10 | #11 | #12 | #13 | #14 | |||||
| Aldehydes | |||||||||
| 1 | 1089/1081 | Hexanal | 66-25-1 | 59.06 ± 17.68 b | 129.25 ± 38.98 a | 195.11 ± 86.30 a | 186.40 ± 45.36 a | 173.49 ± 11.42 a | Green, grass |
| 2 | 1174/1181 | Heptanal | 111-71-7 | 76.75 ± 3.88 c | 935.69 ± 76.68 a | 557.69 ± 98.11 b | 1176.58 ± 325.61 b | 983.97 ± 112.90 a | Citrus, green |
| 3 | 1270/1284 | Octanal | 124-13-0 | 48.34 ± 18.40 c | 248.47 ± 55.19 a | 164.89 ± 28.62 b | 311.21 ± 47.77 a | 179.78 ± 69.25 b | Citrus, fat, green |
| 4 | 1388/1368 | Nonanal | 124-19-6 | 94.94 ± 31.36 b | 361.59 ± 38.03 a | 161.99 ± 96.95 b | 374.64 ± 58.93 a | 306.62 ± 66.73 a | Green, lemon |
| 5 | 1458/1450 | Furfural | 98-01-1 | 10.20 ± 6.24 b | 8.05 ± 3.67 b | 8.01 ± 1.66 b | 16.97 ± 8.45 a | 19.55 ± 4.14 a | Baked potatoes, bread |
| 6 | 1503/1504 | Benzaldehyde | 100-52-7 | 940.80 ± 391.75 b | 2230.07 ± 389.90 a | 1695.19 ± 671.23 b | 3042.13 ± 1297.22 a | 2287.81 ± 177.01 a | Bitter almond, green |
| 7 | 1528/1523 | (Z)-2-Nonenal | 60784-31-8 | 1.37 ± 0.00 d | 2.84 ± 0.00 a | 1.79 ± 0.00 b | 1.64 ± 0.06 c | Green, fatty | |
| 8 | 1667/1625 | Benzeneacetaldehyde | 122-78-1 | 11.99 ± 7.70 | Floral, honey, sweet | ||||
| Alcohols | |||||||||
| 9 | 1249/1261 | 1-Pentanol | 71-41-0 | 9.13 ± 1.06 b | 4.17 ± 0.12 c | 14.86 ± 3.33 a | 13.17 ± 1.99 a | Fruit, solvent, sweet | |
| 10 | 1349/1345 | 1-Hexanol | 111-27-3 | 10.83 ± 3.19 c | 37.74 ± 2.41 b | 27.50 ± 6.69 b | 54.37 ± 16.52 a | 61.03 ± 5.25 a | Grassy, marzipan-like |
| 11 | 1455/1454 | 1-Heptanol | 111-70-6 | 35.45 ± 26.09 b | 117.34 ± 43.38 a | 74.94 ± 31.79 a | 61.84 ± 3.38 b | Green, fatty | |
| 12 | 1564/1558 | 1-Octanol | 111-87-5 | 16.09 ± 6.12 b | 50.62 ± 9.69 a | 48.05 ± 19.79 a | 66.62 ± 29.42 a | 59.19 ± 3.89 a | Creamy, sweet |
| 13 | 1640/1656 | Furfuryl alcohol | 98-00-0 | 8.38 ± 0.00 | 7.35 ± 2.07 | Bread, sweet, roasty | |||
| 14 | 1767/1767 | (3S)-3,7-Dimethyloct-7-en-1-ol | 6812-78-8 | 26.37 ± 9.79 a | 6.28 ± 1.13 b | 2.17 ± 0.93 b | Floral, sweet | ||
| 15 | 1879/1863 | Benzyl alcohol | 100-51-6 | 6.34 ± 2.51 b | 10.90 ± 0.75 a | 9.52 ± 3.87 a | 13.28 ± 6.25 a | 9.78 ± 1.06 a | Sweet, floral |
| 16 | 1873/1895 | Phenylethyl Alcohol | 60-12-8 | 25.34 ± 6.41 a | 26.43 ± 0.98 a | 5.16 ± 2.41 b | 26.54 ± 12.31 a | 11.38 ± 1.27 b | Fruit, honey, sweet |
| Ketones | |||||||||
| 17 | 1283/1281 | 2-Octanone | 111-13-7 | 187.80 ± 136.86 a | 64.20 ± 0.00 b | 34.04 ± 0.38 b | Fat, fragrant, mold | ||
| 18 | 1365/1325 | 6-Methyl-5-hepten-2-one | 110-93-0 | 19.33 ± 7.49 b | 76.58 ± 2.03 b | 52.23 ± 21.59 b | 154.37 ± 39.67 a | 157.69 ± 34.33 a | Citrus, sweet, strawberry |
| 19 | 1387/1364 | 2-Nonanone | 821-55-6 | 8.71 ± 1.21 b | 15.30 ± 7.60 b | 92.55 ± 60.54 a | 27.99 ± 6.58 b | 14.84 ± 7.56 b | Fruit, green |
| 20 | 1489/1479 | 2-Decanone | 693-54-9 | 4.55 ± 0.57 b | 39.40 ± 26.59 a | 6.75 ± 2.03 b | 5.62 ± 2.00 b | Fat, fruity | |
| Acids | |||||||||
| 21 | 1557/1631 | 2-Methyl-propanoic acid | 79-31-2 | 6.37 ± 4.99 | Sour, butter, sweat | ||||
| 22 | 1630/1685 | Butanoic acid | 107-92-6 | 47.46 ± 33.10 | Butter, cheese, sour | ||||
| 23 | 1633/1694 | 3-Methyl-butanoic acid | 503-74-2 | 22.50 ± 15.50 | Sweaty, cheese | ||||
| Others | |||||||||
| 24 | 1146/1136 | 3-Carene | 14.49 ± 1.00 b | 22.86 ± 6.49 a | 13.75 ± 1.45 b | 31.49 ± 9.35 a | Woody | ||
| 25 | 1071/1071 | Dimethyl disulfide | 624-92-0 | 9.87 ± 7.80 | 10.40 ± 10.73 | 5.54 ± 3.15 | Cabbage, garlic, onion | ||
| 26 | 1934/1990 | o-Cresol | 95-48-7 | 4.79 ± 2.36 c | 53.51 ± 3.76 a | 48.59 ± 19.35 b | 89.40 ± 40.25 a | 76.07 ± 7.59 a | Musty, plastic, medicinal |
| 27 | 2412/2404 | Indole | 120-72-9 | 943.40 ± 386.62 a | 325.33 ± 35.15 b | 4.46 ± 4.65 c | Burnt, mothball | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Pu, D.; Shi, Y.; Sun, B.; Guo, H.; Li, K.; Zhang, Y. Characterization of the Key Aroma Compounds in Different Yeast Proteins by GC-MS/O, Sensory Evaluation, and E-Nose. Foods 2023, 12, 3136. https://doi.org/10.3390/foods12163136
Chen J, Pu D, Shi Y, Sun B, Guo H, Li K, Zhang Y. Characterization of the Key Aroma Compounds in Different Yeast Proteins by GC-MS/O, Sensory Evaluation, and E-Nose. Foods. 2023; 12(16):3136. https://doi.org/10.3390/foods12163136
Chicago/Turabian StyleChen, Jiahui, Dandan Pu, Yige Shi, Baoguo Sun, Hui Guo, Ku Li, and Yuyu Zhang. 2023. "Characterization of the Key Aroma Compounds in Different Yeast Proteins by GC-MS/O, Sensory Evaluation, and E-Nose" Foods 12, no. 16: 3136. https://doi.org/10.3390/foods12163136





