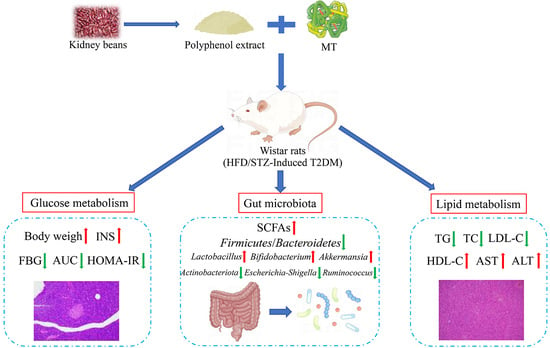
Metallothionein–Kidney Bean Polyphenol Complexes Showed Antidiabetic Activity in Type 2 Diabetic Rats by Improving Insulin Resistance and Regulating Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Polyphenol from Kidney Beans Using the Ultrasound-Assisted Method
2.3. Preparation of the MT–Kidney Bean Polyphenol Complex
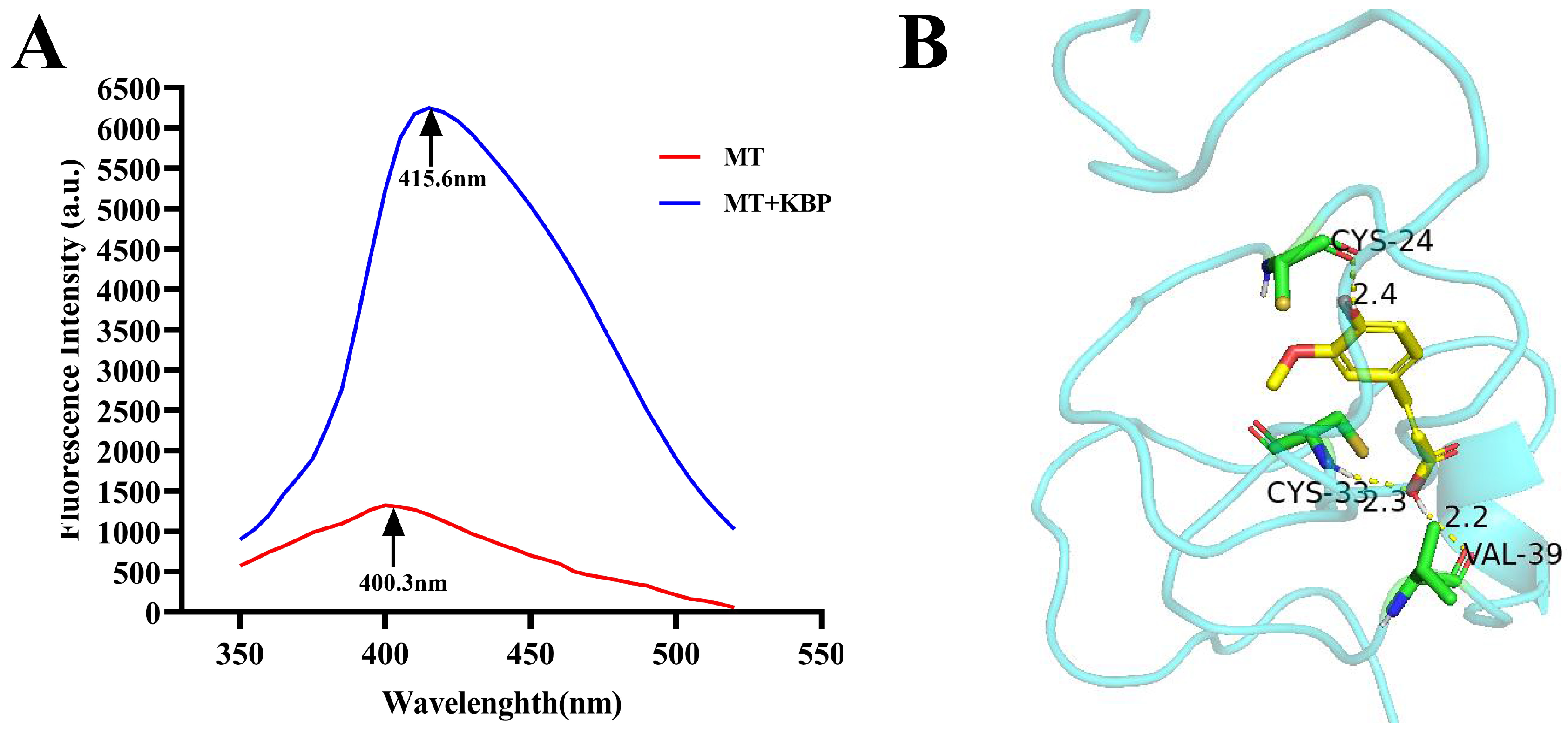
2.4. Interaction between MT and Kidney Bean Polyphenol
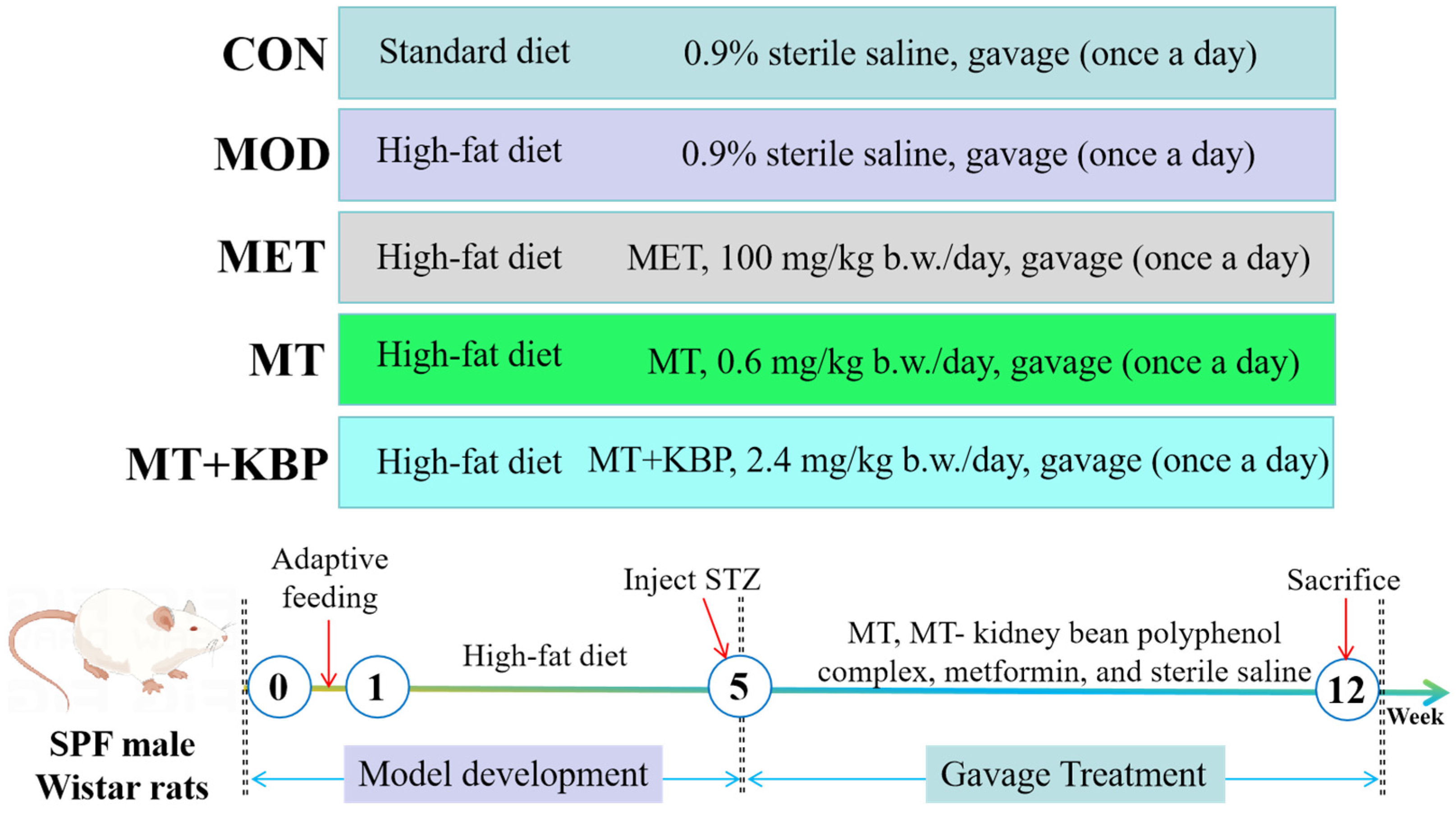
2.5. Animal and Experimental Design
2.6. Sample Collection and Biochemical Analysis
2.7. Histopathological Analysis
2.8. Determination of SCFAs
2.9. Analysis of Intestinal Microorganisms by the 16SrRNA Amplification Sequence
2.10. Statistical Analysis
3. Results
3.1. Interaction between MT and Kidney Bean Polyphenol
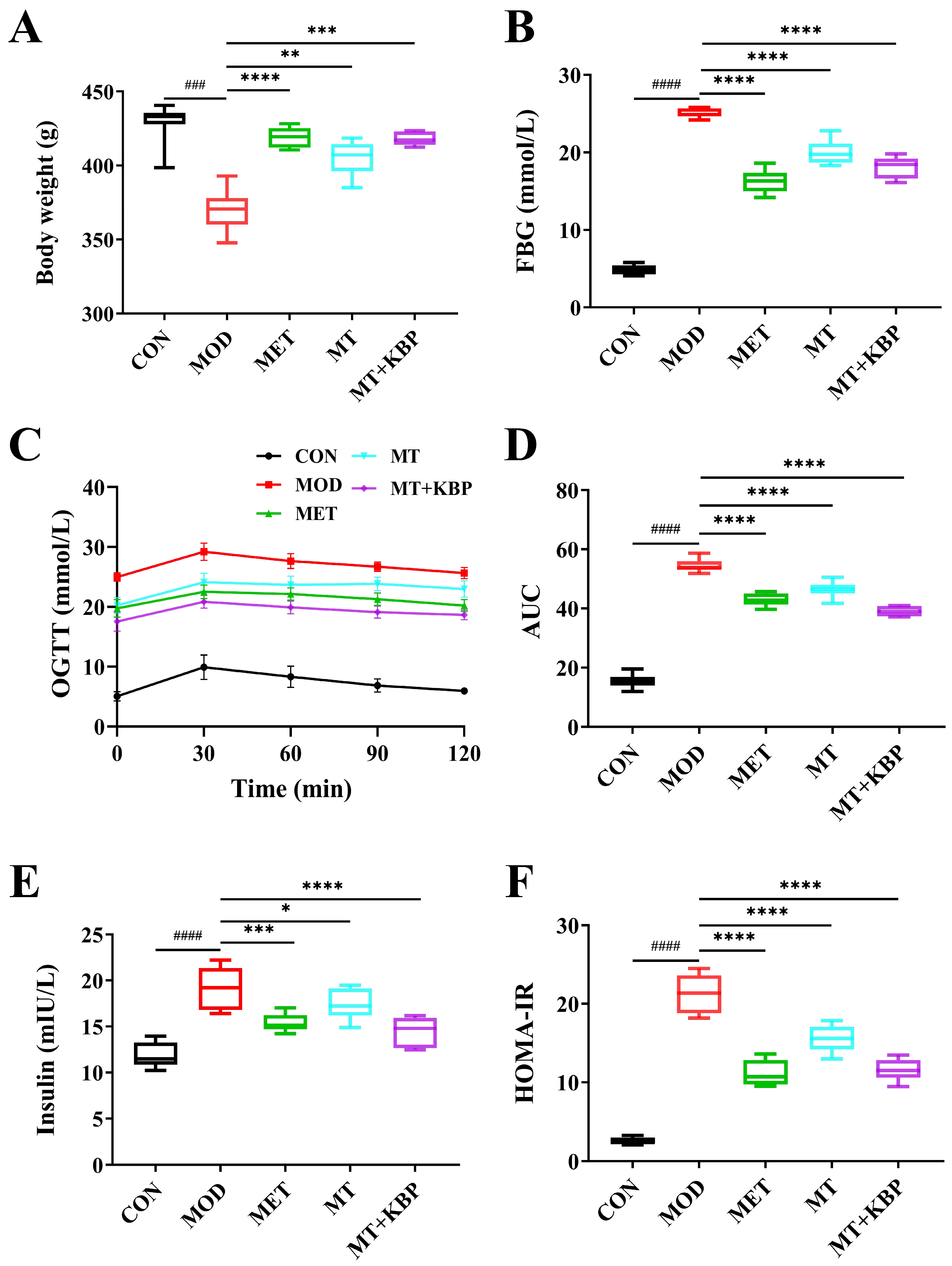
3.2. Effect of the MT–Kidney Bean Polyphenol Complex on the Blood Glucose Level of Rats with T2DM
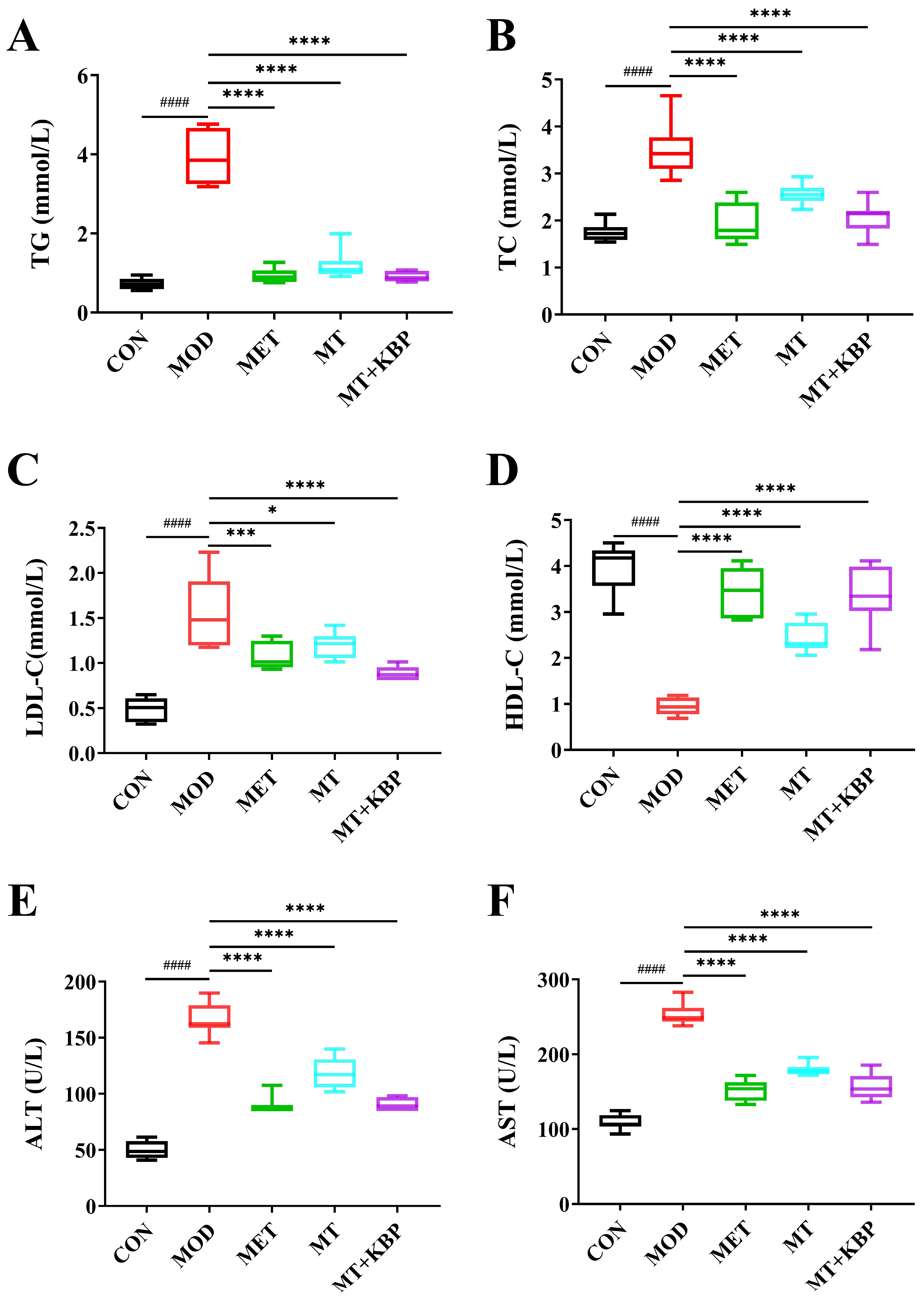
3.3. Effect of the MT–Kidney Bean Polyphenol Complex on Biochemical Indicators in rats with T2DM
3.4. Effects of the MT–Kidney Bean Polyphenol Complex on Histopathology of Pancreas and Liver in Rats with T2DM
3.5. Effect of the MT–Kidney Bean Polyphenol Complex on SCFAs in the Intestinal Contents of Rats with T2DM
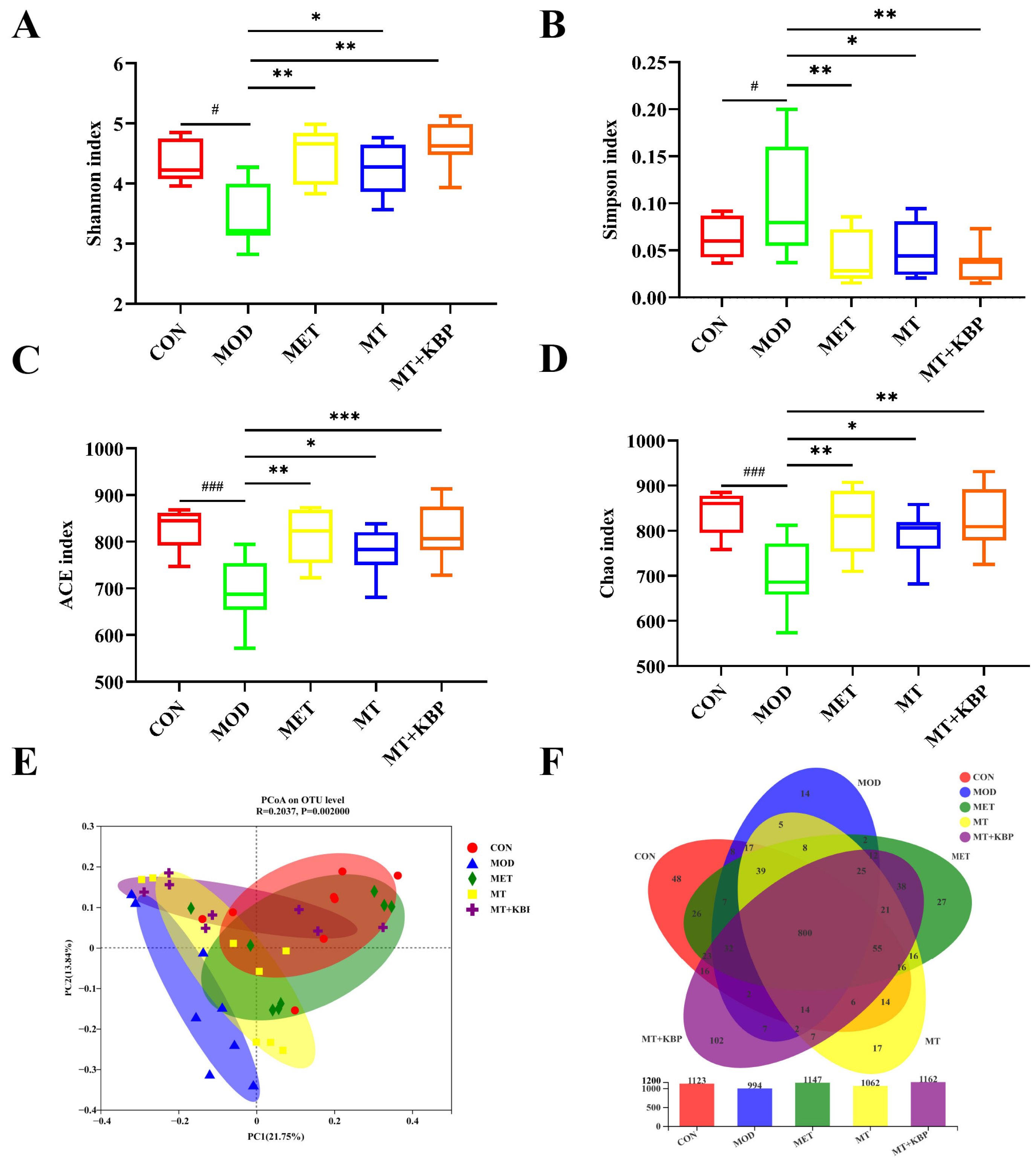
3.6. Effects of the MT–Kidney Bean Polyphenol Complex on the Diversity of the Gut Microbiota in Rats with T2DM
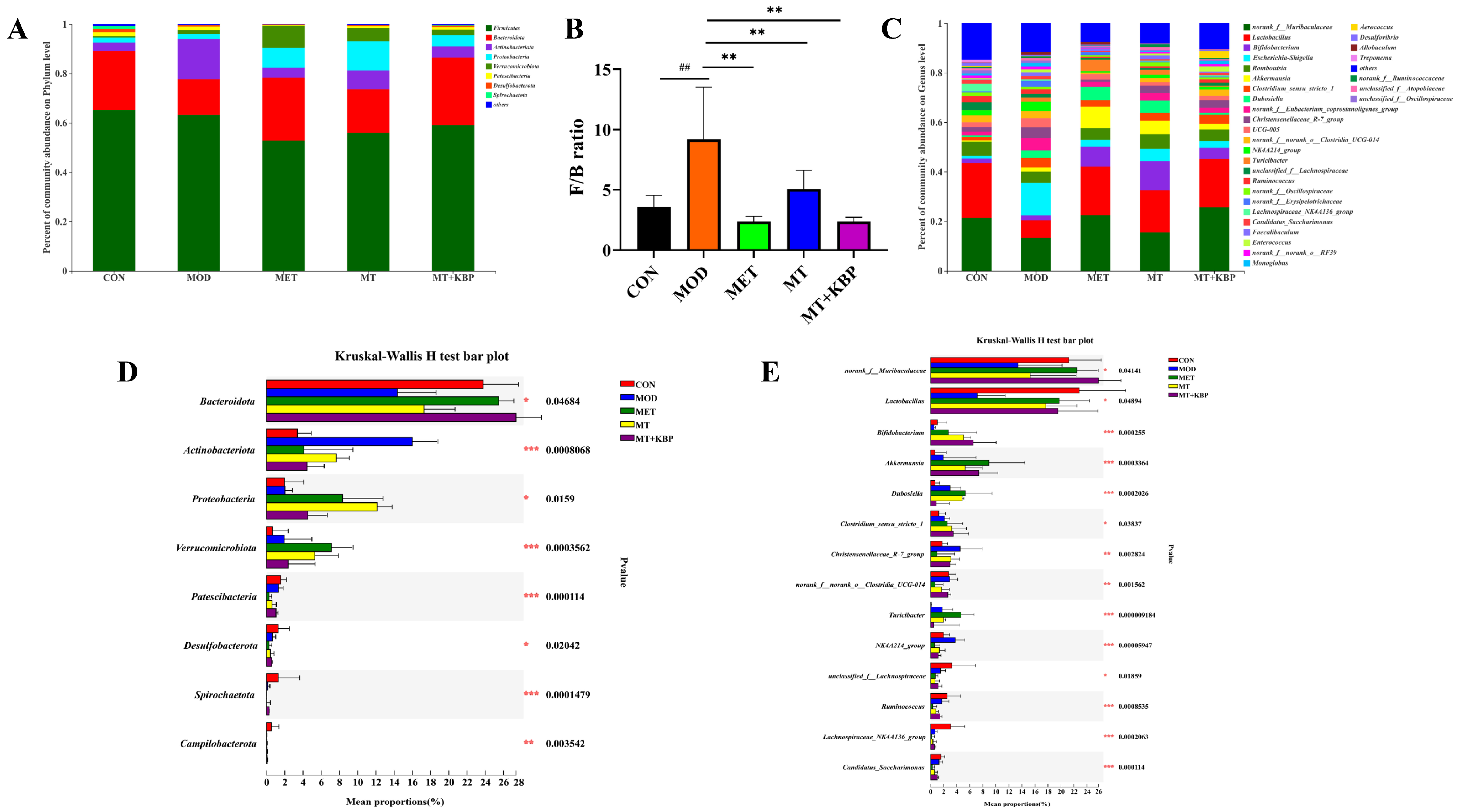
3.7. Effects of the MT–Kidney Bean Polyphenol Complex on the Composition of Gut Microbiota in Rats with T2DM
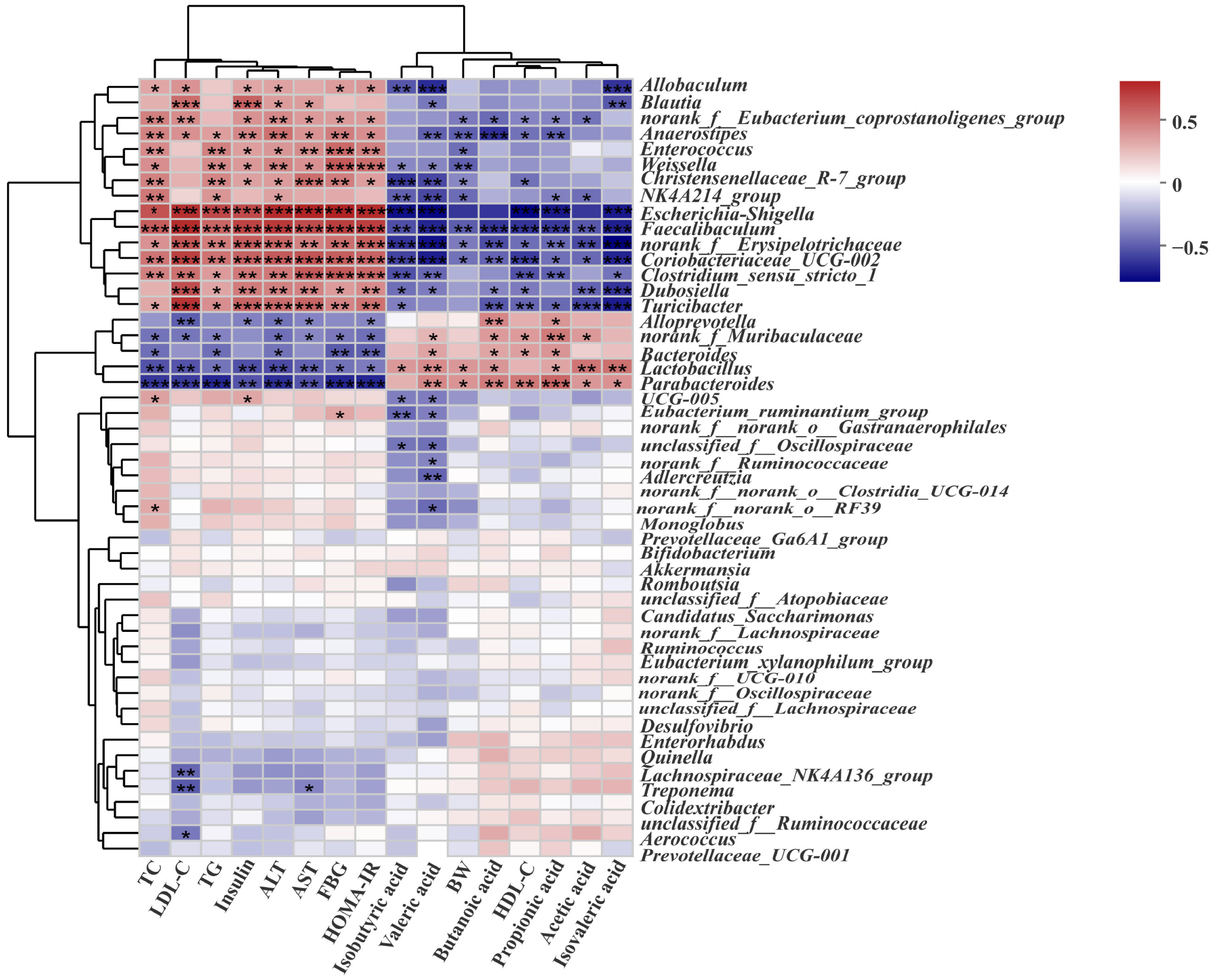
3.8. Correlation Analysis between the Biochemical Indicators and Gut Microbiota in Rats with T2DM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Fang, Q.; Nie, Q.; Hu, J.; Yang, C.; Huang, T.; Li, H.; Nie, S. Hypoglycemic and Hypolipidemic Mechanism of Tea Polysaccharides on Type 2 Diabetic Rats via Gut Microbiota and Metabolism Alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Chen, R.; Chen, G. Personalized Nutrition for People with Diabetes and at Risk of Diabetes Has Begun. J. Future Foods 2022, 2, 193–202. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 Diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Moucheraud, C.; Lenz, C.; Latkovic, M.; Wirtz, V.J. The Costs of Diabetes Treatment in Low- and Middle-Income Countries: A Systematic Review. BMJ Glob. Health 2019, 4, e001258. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Siavash, M.; Tabbakhian, M.; Sabzghabaee, A.; Razavi, N. Severity of Gastrointestinal Side Effects of Metformin Tablet Compared to Metformin Capsule in Type 2 Diabetes Mellitus Patients. J. Res. Pharm. Pract. 2017, 6, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Lacaille-Dubois, M.A.; Wagner, H. New Perspectives for Natural Triterpene Glycosides as Potential Adjuvants. Phytomedicine 2017, 37, 49–57. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional Interactions between the Gut Microbiota and Host Metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Zhang, Q.; Zhang, J.; Liu, S.; Li, C.; Wang, L. Glycolipid Metabolism and Metagenomic Analysis of the Therapeutic Effect of a Phenolics-Rich Extract from Noni Fruit on Type 2 Diabetic Mice. J. Agric. Food Chem. 2022, 70, 2876–2888. [Google Scholar] [CrossRef]
- Huang, W.; Guo, H.L.; Deng, X.; Zhu, T.T.; Xiong, J.F.; Xu, Y.H.; Xu, Y. Short-Chain Fatty Acids Inhibit Oxidative Stress and Inflammation in Mesangial Cells Induced by High Glucose and Lipopolysaccharide. Exp. Clin. Endocrinol. Diabetes 2017, 125, 98–105. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of Dietary Polyphenols on Gut Microbiota, Their Metabolites and Health Benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L. Introduction to Metallothionein. Methods Enzym. 1991, 205, 3–7. [Google Scholar] [CrossRef]
- Margoshes, M.; Vallee, B.L. A Cadmium Protein from Equine Kidney Cortex. J. Am. Chem. Soc. 1957, 79, 4813–4814. [Google Scholar] [CrossRef]
- Francisco, P.; Melgar, L.M.; Díaz, S.; Martín-González, A.; Gutiérrez, J.C. The Tetrahymena Metallothionein Gene Family: Twenty-One New CDNAs, Molecular Characterization, Phylogenetic Study and Comparative Analysis of the Gene Expression under Different Abiotic Stressors. BMC Genom. 2016, 17, 346. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Iglesias, H.; Alvarez, L.; García, M.; Petrash, C.; Sanz-Medel, A.; Coca-Prados, M. Metallothioneins (MTs) in the Human Eye: A Perspective Article on the Zinc-MT Redox Cycle. Metallomics 2014, 6, 201–208. [Google Scholar] [CrossRef]
- Beattie, J.H.; Wood, A.M.; Newman, A.M.; Bremner, I.; Choo, K.H.A.; Michalska, A.E.; Duncan, J.S.; Trayhurn, P. Obesity and Hyperleptinemia in Metallothionein (-I and-II) Null Mice. Proc. Natl. Acad. Sci. USA 1998, 95, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional Quality of Legumes, and Their Role in Cardiometabolic Risk Prevention: A Review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. A Review of the Nutritional Value of Legumes and Their Effects on Obesity and Its Related Co-Morbidities. Obes. Rev. 2014, 15, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Dhull, S.B.; Sandhu, K.S.; Kaur, M.; Purewal, S.S. Kidney Bean (Phaseolus vulgaris) Starch: A Review. Legum. Sci. 2020, 2, e52. [Google Scholar] [CrossRef]
- Nolan, R.; Shannon, O.M.; Robinson, N.; Joel, A.; Houghton, D.; Malcomson, F.C. It’s No Has Bean: A Review of the Effects of White Kidney Bean Extract on Body Composition and Metabolic Health. Nutrients 2020, 12, 1398. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary Polyphenols as Antidiabetic Agents: Advances and Opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Rocha, B.A.M.; Teixeira, C.S.; Silva-Filho, J.C.; Nóbrega, R.B.; Alencar, D.B.; Nascimento, K.S.; Freire, V.N.; Gottfried, C.J.S.; Nagano, C.S.; Sampaio, A.H.; et al. Structural Basis of ConM Binding with Resveratrol, an Anti-Inflammatory and Antioxidant Polyphenol. Int. J. Biol. Macromol. 2015, 72, 1136–1142. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Y.T.; Feng, Y.C.; Wang, C.Y.; Zhang, D.J. Potential Effects of Mung Bean Protein and a Mung Bean Protein-Polyphenol Complex on Oxidative Stress Levels and Intestinal Microflora in Aging Mice. Food Funct. 2022, 13, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, D.; Li, B.; Lund, M.N.; Xing, Y.; Wang, Y.; Li, F.; Cao, X.; Liu, Y.; Chen, X.; et al. Engineering Polyphenols with Biological Functions via Polyphenol-Protein Interactions as Additives for Functional Foods. Trends Food Sci. Technol. 2021, 110, 470–482. [Google Scholar] [CrossRef]
- Zhang, S.; Sheng, Y.N.; Feng, Y.C.; Diao, J.J.; Wang, C.Y.; Zhang, D.J. Changes in Structural and Functional Properties of Globulin–Polyphenol Complexes in Mung Beans: Exploration under Different Interaction Ratios and Heat Treatment Conditions. Int. J. Food Sci. Technol. 2022, 57, 1920–1935. [Google Scholar] [CrossRef]
- Li, Z.; Jia, R.; Wu, J.; Lin, L.; Ou, Z.; Liao, B.; Zhang, L.; Zhang, X.; Song, G.; Zhao, M. Sargassum Fusiforme Polysaccharide Partly Replaces Acarbose against Type 2 Diabetes in Rats. Int. J. Biol. Macromol. 2021, 170, 447–458. [Google Scholar] [CrossRef]
- Wang, X.; Xu, B.; Wang, Y. The Effect of Metallothioneins from Yeast on Mercury Expelling and Kidney Damage Renovating in Chronic Mercury-Treated Mice. J. Chin. Inst. Food Sci. Technol. 2019, 19, 41–47. [Google Scholar]
- Gong, P.; Xiao, X.; Wang, S.; Shi, F.; Liu, N.; Chen, X.; Yang, W.; Wang, L.; Chen, F. Hypoglycemic Effect of Astragaloside IV via Modulating Gut Microbiota and Regulating AMPK/SIRT1 and PI3K/AKT Pathway. J. Ethnopharmacol. 2021, 281, 114558. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.R.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Fl-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, C.; Wan, C.; Li, Y.; Lu, X.; Lin, Y.; Gao, J. Two Laminaria Japonica Polysaccharides with Distinct Structure Characterization Affect Gut Microbiota and Metabolites in Hyperlipidemic Mice Differently. Food Res. Int. 2022, 159, 111615. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Liu, C.S.; Hu, Y.N.; Luo, Z.Y.; Chen, F.L.; Yuan, L.X.; Tan, X.M. Coix Seed Polysaccharides Alleviate Type 2 Diabetes Mellitus via Gut Microbiota-Derived Short-Chain Fatty Acids Activation of IGF1/PI3K/AKT Signaling. Food Res. Int. 2021, 150, 110717. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liang, L.; Yu, G.; Li, Q. Pumpkin Polysaccharide Modifies the Gut Microbiota during Alleviation of Type 2 Diabetes in Rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, D.; Xu, X.; Dai, J.; Lao, G.; Zhang, S.; Xu, X.; Dinnyés, A.; Xiong, Y.; Sun, Q. Myofibrillar Protein-Chlorogenic Acid Complexes Ameliorate Glucose Metabolism via Modulating Gut Microbiota in a Type 2 Diabetic Rat Model. Food Chem. 2023, 409, 135195. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Rajasekhar, M.D.; Kondeti, V.K.; Fatima, S.S.; Kumar, E.G.T.; Swapna, S.; Ramesh, B.; Rao, C.A. Antihyperglycemic and Antihyperlipidemic Activities of Methanol:Water (4:1) Fraction Isolated from Aqueous Extract of Syzygium alternifolium Seeds in Streptozotocin Induced Diabetic Rats. Food Chem. Toxicol. 2010, 48, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Qian, D.W.; Jiang, S.; Shang, E.X.; Zhu, Z.H.; Duan, J.A. Scutellariae Radix and Coptidis Rhizoma Improve Glucose and Lipid Metabolism in T2DM Rats via Regulation of the Metabolic Profiling and MAPK/PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 3634. [Google Scholar] [CrossRef]
- Prihatin, J.; Narulita, E.; Mufidah, L.; Kurniawan, A.; Wulandari, D.; Hariyadi, S. Antihyperglycaemic and Tissue-Repair Effects of Myrmeleon formicarius Extract in Streptozotocin-Induced Diabetic Mice. J. Taibah Univ. Med. Sci. 2019, 14, 149–155. [Google Scholar] [CrossRef]
- Gong, P.; Wang, X.; Liu, M.; Wang, M.; Wang, S.; Guo, Y.; Chang, X.; Yang, W.; Chen, X.; Chen, F. Hypoglycemic Effect of a Novel Polysaccharide from Lentinus edodes on STZ-Induced Diabetic Mice via Metabolomics Study and Nrf2/HO-1 Pathway. Food Funct. 2022, 13, 3036–3049. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Chambers, E.S.; Hill, T.; Maldonado, I.R.; Liu, B.; Bewick, G.; Morrison, D.J.; Preston, T.; Wallis, G.A.; Tedford, C.; et al. The Diet-Derived Short Chain Fatty Acid Propionate Improves Beta-Cell Function in Humans and Stimulates Insulin Secretion from Human Islets in Vitro. Diabetes Obes. Metab. 2017, 19, 257–265. [Google Scholar] [CrossRef]
- Shi, Z.; Zhu, Y.; Teng, C.; Yao, Y.; Ren, G.; Richel, A. Anti-Obesity Effects of α-Amylase Inhibitor Enriched-Extract from White Common Beans (Phaseolus vulgaris L.) Associated with the Modulation of Gut Microbiota Composition in High-Fat Diet-Induced Obese Rats. Food Funct. 2020, 11, 1624–1634. [Google Scholar] [CrossRef]
- Pang, W.; Wang, D.; Zuo, Z.; Wang, Y.; Sun, W.; Zhang, N.; Zhang, D. Kidney Bean Fermented Broth Alleviates Hyperlipidemic by Regulating Serum Metabolites and Gut Microbiota Composition. Nutrients 2022, 14, 3202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yang, T.; Xu, W.; Huang, Y.; Ran, L.; Yan, Y.; Mi, J.; Lu, L.; Sun, Y.; Zeng, X.; et al. The Polysaccharides from the Fruits of Lycium barbarum L. Confer Anti-Diabetic Effect by Regulating Gut Microbiota and Intestinal Barrier. Carbohydr. Polym. 2022, 291, 119626. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, W.; Xin, X.; Li, X.; Liu, D. Extract of Ice Plant (Mesembryanthemum crystallinum) Ameliorates Hyperglycemia and Modulates the Gut Microbiota Composition in Type 2 Diabetic Goto-Kakizaki Rats. Food Funct. 2019, 10, 3252–3261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ning, Y.; Yuan, C.; Cui, B.; Liu, G.; Zhang, Z. The Protective Mechanism of a Debranched Corn Starch/Konjac Glucomannan Composite against Dyslipidemia and Gut Microbiota in High-Fat-Diet Induced Type 2 Diabetes. Food Funct. 2021, 12, 9273–9285. [Google Scholar] [CrossRef]
- Qu, L.; Ren, J.; Huang, L.; Pang, B.; Liu, X.; Liu, X.; Li, B.; Shan, Y. Antidiabetic Effects of Lactobacillus Casei Fermented Yogurt through Reshaping Gut Microbiota Structure in Type 2 Diabetic Rats. J. Agric. Food Chem. 2018, 66, 12696–12705. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Ma, Q.; Zhai, R.; Xie, X.; Chen, T.; Zhang, Z.; Liu, H.; Nie, C.; Yuan, X.; Tu, A.; Tian, B.; et al. Hypoglycemic Effects of Lycium barbarum Polysaccharide in Type 2 Diabetes Mellitus Mice via Modulating Gut Microbiota. Front. Nutr. 2022, 9, 91627. [Google Scholar] [CrossRef]
- Dabelea, D.; Stafford, J.M.; Mayer-Davis, E.J.; D’Agostino, R.; Dolan, L.; Imperatore, G.; Linder, B.; Lawrence, J.M.; Marcovina, S.M.; Mottl, A.K.; et al. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed during Childhood and Adolescence with Complications during Teenage Years and Young Adulthood. JAMA-J. Am. Med. Assoc. 2017, 317, 825–835. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. Pharmacology and Therapeutic Implications of Current Drugs for Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2016, 12, 566–592. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary Polyphenols and Type 2 Diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Shiroma, E.J.; Nam, J.K.; Duregon, E.; Vieira Ligo Teixeira, C.; Price, N.L.; Bernier, M.; Camandola, S.; Vaughan, K.L.; Colman, R.J.; et al. Fasting Blood Glucose as a Predictor of Mortality: Lost in Translation. Cell Metab. 2021, 33, 2189–2200. [Google Scholar] [CrossRef]
- Nanditha, A.; Susairaj, P.; Raghavan, A.; Vinitha, R.; Satheesh, K.; Nair, D.R.; Jeyaraj, S.; Snehalatha, C.; Ramachandran, A. Concordance in Incidence of Diabetes among Persons with Prediabetes Detected Using Either Oral Glucose Tolerance Test or Glycated Haemoglobin. Prim. Care Diabetes 2022, 16, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xiao, D.; Liu, W.; Song, Y.; Zou, B.; Li, L.; Li, P.; Cai, Y.; Liu, D.; Liao, Q.; et al. Intake of Ganoderma Lucidum Polysaccharides Reverses the Disturbed Gut Microbiota and Metabolism in Type 2 Diabetic Rats. Int. J. Biol. Macromol. 2020, 155, 890–902. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Zhang, L.; Chen, Y.; Wu, T.; Liu, R.; Sui, W.; Zhu, Q.; Zhang, M. Licorice Extract Ameliorates Hyperglycemia through Reshaping Gut Microbiota Structure and Inhibiting TLR4/NF-ΚB Signaling Pathway in Type 2 Diabetic Mice. Food Res. Int. 2022, 153, 110945. [Google Scholar] [CrossRef] [PubMed]
- Bahar, E.; Akter, K.M.; Lee, G.H.; Lee, H.Y.; Rashid, H.O.; Choi, M.K.; Bhattarai, K.R.; Hossain, M.M.M.; Ara, J.; Mazumder, K.; et al. β-Cell Protection and Antidiabetic Activities of Crassocephalum crepidioides (Asteraceae) Benth. S. Moore Extract against Alloxan-Induced Oxidative Stress via Regulation of Apoptosis and Reactive Oxygen Species (ROS). BMC Complement. Altern. Med. 2017, 17, 179. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Tudoreanu, L.; Ștefan, G. Plant Polyphenols Mechanisms of Action on Insulin Resistance and against the Loss of Pancreatic Beta Cells. Crit. Rev. Food Sci. Nutr. 2021, 62, 325–352. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Yang, X.; Wang, Z.; Yao, X.; Guo, Y. Enhanced Bioavailability and Anti-Hyperglycemic Activity of Young Apple Polyphenols by Complexation with Whey Protein Isolates. J. Food Sci. 2022, 87, 1257–1267. [Google Scholar] [CrossRef]
- Aronoff, S.; Rosenblatt, S.; Braithwaite, S.; Egan, J.; Mathisen, A.L.; Schneider, R.L. Pioglitazone Hydrochloride Monotherapy Improves Glycemic Control in the Treatment of Patients with Type 2 Diabetes: A 6-Month Randomized Placebo-Controlled Dose-Response Study. Diabetes Care 2000, 23, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Thakur, K.; Chen, G.-H.; Hu, F.; Zhang, J.-G.; Zhang, H.-B.; Wei, Z.-J. Metabolic Effect of 1-Deoxynojirimycin from Mulberry Leaves on Db/Db Diabetic Mice Using Liquid Chromatography-Mass Spectrometry Based Metabolomics. J. Agric. Food Chem. 2017, 65, 4658–4667. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, M.J.; Khattab, M.H.; Jairoun, A.A. Association of Serum Calcium Level with Waist Circumference and Other Biochemical Health-Care Predictors among Patients with Type 2 Diabetes. J. Pharm. Bioallied Sci. 2019, 11, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Honka, M.-J.; Latva-Rasku, A.; Bucci, M.; Virtanen, K.A.; Hannukainen, J.C.; Kalliokoski, K.K.; Nuutila, P. Insulin Stimulated Glucose Uptake in Skeletal Muscle, Adipose Tissue and Liver: A Positron Emission Tomography Study. Eur. J. Endocrinol. 2018, 178, 523–531. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Intestinal Microbiota and Their Metabolic Contribution to Type 2 Diabetes and Obesity. J. Diabetes Metab. Disord. 2021, 20, 1855–1870. [Google Scholar] [CrossRef]
- Guo, Z.; Pan, J.; Zhu, H.; Chen, Z.Y. Metabolites of Gut Microbiota and Possible Implication in Development of Diabetes Mellitus. J. Agric. Food Chem. 2022, 70, 5945–5960. [Google Scholar] [CrossRef]
- Besten, G.; Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Weitkunat, K.; Stuhlmann, C.; Postel, A.; Rumberger, S.; Fankhänel, M.; Woting, A.; Petzke, K.J.; Gohlke, S.; Schulz, T.J.; Blaut, M.; et al. Short-Chain Fatty Acids and Inulin, but Not Guar Gum, Prevent Diet-Induced Obesity and Insulin Resistance through Differential Mechanisms in Mice. Sci. Rep. 2017, 7, 6109. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Yin, J.; Nie, S. Multiomics Approach to Explore the Amelioration Mechanisms of Glucomannans on the Metabolic Disorder of Type 2 Diabetic Rats. J. Agric. Food Chem. 2021, 69, 2632–2645. [Google Scholar] [CrossRef] [PubMed]
- Knip, M.; Siljander, H. The Role of the Intestinal Microbiota in Type 1 Diabetes Mellitus. Nat. Rev. Endocrinol. 2016, 12, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa Roxburghii Tratt Fruit Attenuates Hyperglycemia and Hyperlipidemia and Regulates Colon Microbiota in Diabetic Db/Db Mice. J. Agric. Food Chem. 2020, 68, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, Y.; Yu, D.; Jin, L.; Gong, X.; Zhang, B. Perilla Oil Regulates Intestinal Microbiota and Alleviates Insulin Resistance through the PI3K/AKT Signaling Pathway in Type-2 Diabetic KKAy Mice. Food Chem. Toxicol. 2020, 135, 110965. [Google Scholar] [CrossRef] [PubMed]
- Al-Jameel, S.S. Association of Diabetes and Microbiota: An Update. Saudi J. Biol. Sci. 2021, 28, 4446–4454. [Google Scholar] [CrossRef]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Vilela, V.; Dudonné, S.; Pilon, G.; Fournier, M.; Lecours, M.A.; Desjardins, Y.; Roy, D.; et al. A Polyphenol-Rich Cranberry Extract Reverses Insulin Resistance and Hepatic Steatosis Independently of Body Weight Loss. Mol. Metab. 2017, 6, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J.; Miousse, I.R.; Nookaew, I.; Ewing, L.E.; Skinner, C.M.; Jenjaroenpun, P.; Wongsurawat, T.; Kennon-McGill, S.; Avula, B.; Bae, J.Y.; et al. Decaffeinated Green Tea Extract Does Not Elicit Hepatotoxic Effects and Modulates the Gut Microbiome in Lean B6C3F1 Mice. Nutrients 2019, 11, 776. [Google Scholar] [CrossRef]
- Li, L.; Tian, Y.; Zhang, S.; Feng, Y.; Wang, H.; Cheng, X.; Ma, Y.; Zhang, R.; Wang, C. Regulatory Effect of Mung Bean Peptide on Prediabetic Mice Induced by High-Fat Diet. Front. Nutr. 2022, 9, 913016. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and Cultivation Study of Muribaculaceae Reveals Novel Species, Host Preference, and Functional Potential of This yet Undescribed Family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two Routes of Metabolic Cross-Feeding between Bifidobacterium Adolescentis and Butyrate-Producing Anaerobes from the Human Gut. Appl. Environ. Microbiol. 2006, 72, 3593–3599. [Google Scholar] [CrossRef]
- Du, H.; Wang, Q.; Li, T.; Ren, D.; Yang, X. Grape Seed Proanthocyanidins Reduced the Overweight of C57BL/6J Mice through Modulating Adipose Thermogenesis and Gut Microbiota. Food Funct. 2021, 12, 8467–8477. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Angarita, L.; Morillo, V.; Navarro, C.; Martínez, M.S.; Chacín, M.; Torres, W.; Rajotia, A.; Rojas, M.; Cano, C.; et al. Microbiota and Diabetes Mellitus: Role of Lipid Mediators. Nutrients 2020, 12, 3039. [Google Scholar] [CrossRef]
- Zhang, C.H.; Sheng, J.Q.; Sarsaiya, S.; Shu, F.X.; Liu, T.T.; Tu, X.Y.; Ma, G.Q.; Xu, G.L.; Zheng, H.X.; Zhou, L.F. The Anti-Diabetic Activities, Gut Microbiota Composition, the Anti-Inflammatory Effects of Scutellaria–Coptis Herb Couple against Insulin Resistance-Model of Diabetes Involving the Toll-like Receptor 4 Signaling Pathway. J. Ethnopharmacol. 2019, 237, 202–214. [Google Scholar] [CrossRef]
- Li, H.; Shi, J.; Zhao, L.; Guan, J.; Liu, F.; Huo, G.; Li, B. Lactobacillus Plantarum KLDS1.0344 and Lactobacillus Acidophilus KLDS1.0901 Mixture Prevents Chronic Alcoholic Liver Injury in Mice by Protecting the Intestinal Barrier and Regulating Gut Microbiota and Liver-Related Pathways. J. Agric. Food Chem. 2021, 69, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Obanda, D.; Page, R.; Guice, J.; Raggio, A.M.; Husseneder, C.; Marx, B.; Stout, R.W.; Welsh, D.A.; Taylor, C.M.; Luo, M.; et al. CD Obesity-Prone Rats, but Not Obesity-Resistant Rats, Robustly Ferment Resistant Starch without Increased Weight or Fat Accretion. Obesity 2018, 26, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Liu, C.; Chen, M.; Zou, J.; Zhang, Z.; Cui, X.; Jiang, S.; Shang, E.; Qian, D.; Duan, J. Scutellariae Radix and Coptidis Rhizoma Ameliorate Glycolipid Metabolism of Type 2 Diabetic Rats by Modulating Gut Microbiota and Its Metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 303–317. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, H.; Tian, J.; Shu, C.; Sun, R.; Li, B.; Meng, X. Protective Effects of α-Casein or β-Casein on the Stability and Antioxidant Capacity of Blueberry Anthocyanins and Their Interaction Mechanism. LWT 2019, 115, 108434. [Google Scholar] [CrossRef]
- Zang, Z.; Chou, S.; Tian, J.; Lang, Y.; Shen, Y.; Ran, X.; Gao, N.; Li, B. Effect of Whey Protein Isolate on the Stability and Antioxidant Capacity of Blueberry Anthocyanins: A Mechanistic and in Vitro Simulation Study. Food Chem. 2021, 336, 127700. [Google Scholar] [CrossRef]
- Ribnicky, D.M.; Roopchand, D.E.; Poulev, A.; Kuhn, P.; Oren, A.; Cefalu, W.T.; Raskin, I. Artemisia dracunculus L. Polyphenols Complexed to Soy Protein Show Enhanced Bioavailability and Hypoglycemic Activity in C57BL/6 Mice. Nutrition 2014, 30, 4–10. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary Protein-Phenolic Interactions: Characterization, Biochemical-Physiological Consequences, and Potential Food Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 3589–3615. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y.L. Interaction of Whey Proteins with Phenolic Derivatives under Neutral and Acidic PH Conditions. J. Food Sci. 2017, 82, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, Z.; Zhao, J.; Liu, Y. The Effect of Non-Covalent Interaction of Chlorogenic Acid with Whey Protein and Casein on Physicochemical and Radical-Scavenging Activity of in Vitro Protein Digests. Food Chem. 2018, 268, 334–341. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Z.; Pang, W.; Sun, W.; Lu, B.; Zou, L.; Zhang, D.; Wang, Y. Metallothionein–Kidney Bean Polyphenol Complexes Showed Antidiabetic Activity in Type 2 Diabetic Rats by Improving Insulin Resistance and Regulating Gut Microbiota. Foods 2023, 12, 3139. https://doi.org/10.3390/foods12163139
Zuo Z, Pang W, Sun W, Lu B, Zou L, Zhang D, Wang Y. Metallothionein–Kidney Bean Polyphenol Complexes Showed Antidiabetic Activity in Type 2 Diabetic Rats by Improving Insulin Resistance and Regulating Gut Microbiota. Foods. 2023; 12(16):3139. https://doi.org/10.3390/foods12163139
Chicago/Turabian StyleZuo, Zhaohang, Weiqiao Pang, Wei Sun, Baoxin Lu, Liang Zou, Dongjie Zhang, and Ying Wang. 2023. "Metallothionein–Kidney Bean Polyphenol Complexes Showed Antidiabetic Activity in Type 2 Diabetic Rats by Improving Insulin Resistance and Regulating Gut Microbiota" Foods 12, no. 16: 3139. https://doi.org/10.3390/foods12163139
APA StyleZuo, Z., Pang, W., Sun, W., Lu, B., Zou, L., Zhang, D., & Wang, Y. (2023). Metallothionein–Kidney Bean Polyphenol Complexes Showed Antidiabetic Activity in Type 2 Diabetic Rats by Improving Insulin Resistance and Regulating Gut Microbiota. Foods, 12(16), 3139. https://doi.org/10.3390/foods12163139