Investigating the Impact of Dietary Fibers on Mycotoxin Bioaccessibility during In Vitro Biscuit Digestion and Metabolites Identification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
Dietary Fibers Employed for Biscuits Preparation
2.2. Biscuit Recipe and Preparation
2.3. Sample Treatment
2.3.1. Mycotoxin Extraction
2.3.2. In Vitro Digestion
2.4. Liquid Chromatography-Mass Spectrometry Parameters
2.5. Analytical Method Validation
2.6. Color Measurements
2.7. Statistical Analysis
2.8. UHPLC-Q-Orbitrap-MS for Non-Targeted Analysis
3. Results and Discussion
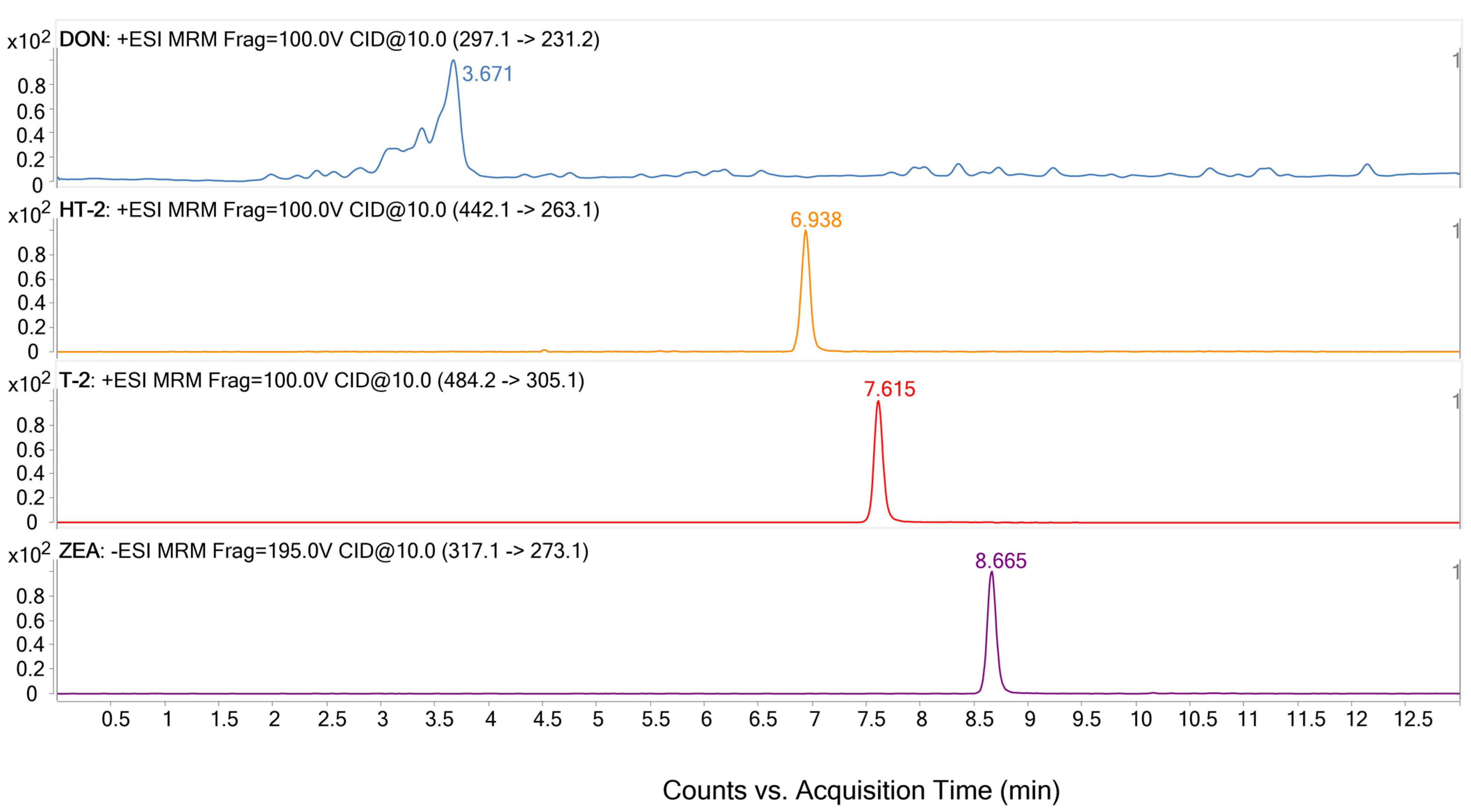
3.1. LC-QqQ-MS Optimization
3.2. Evaluation of Dietary Fibers as Blinders to Mycotoxins
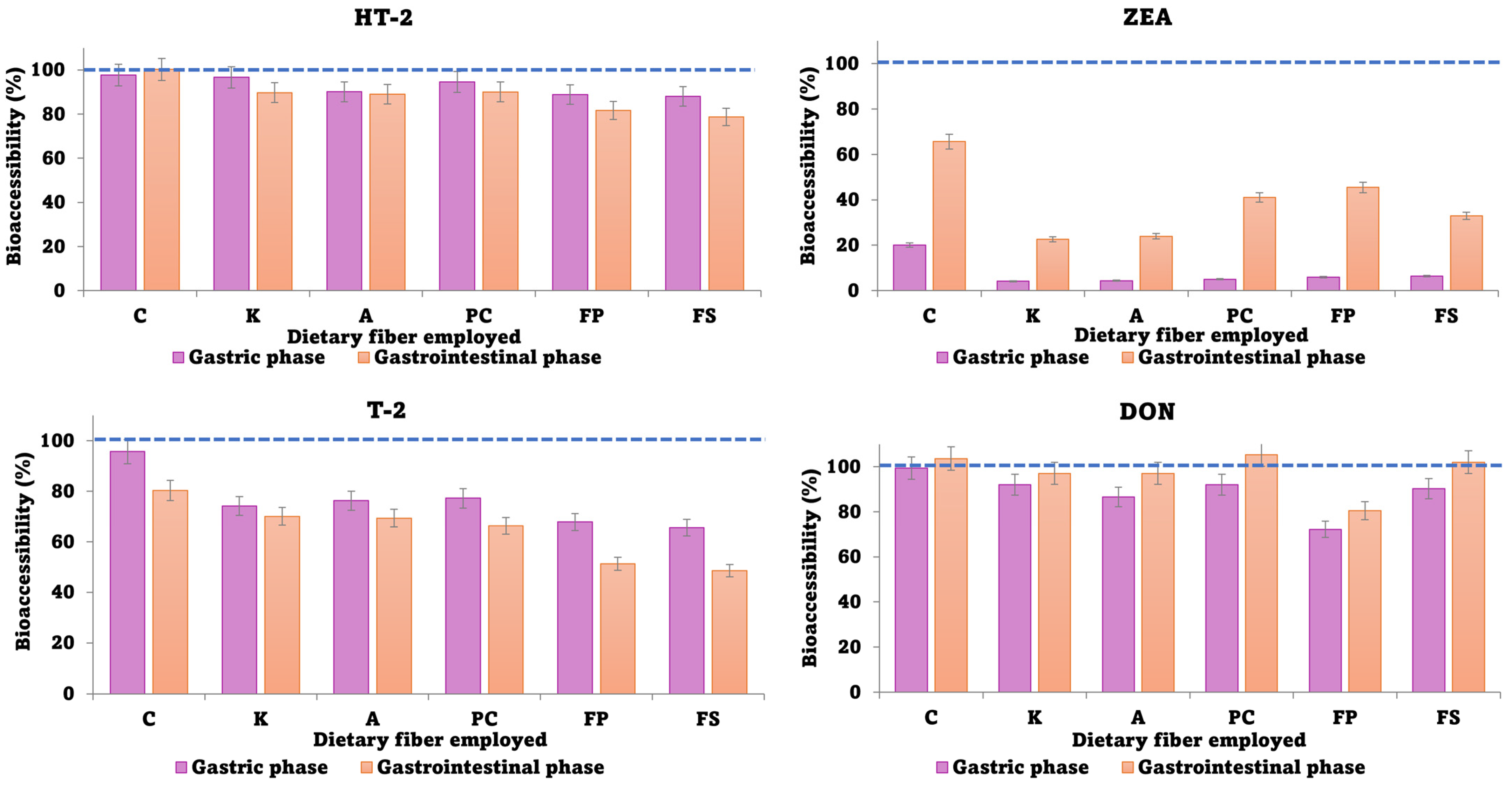
3.3. Bioaccessibility of Mycotoxins after In Vitro Digestion of Biscuits
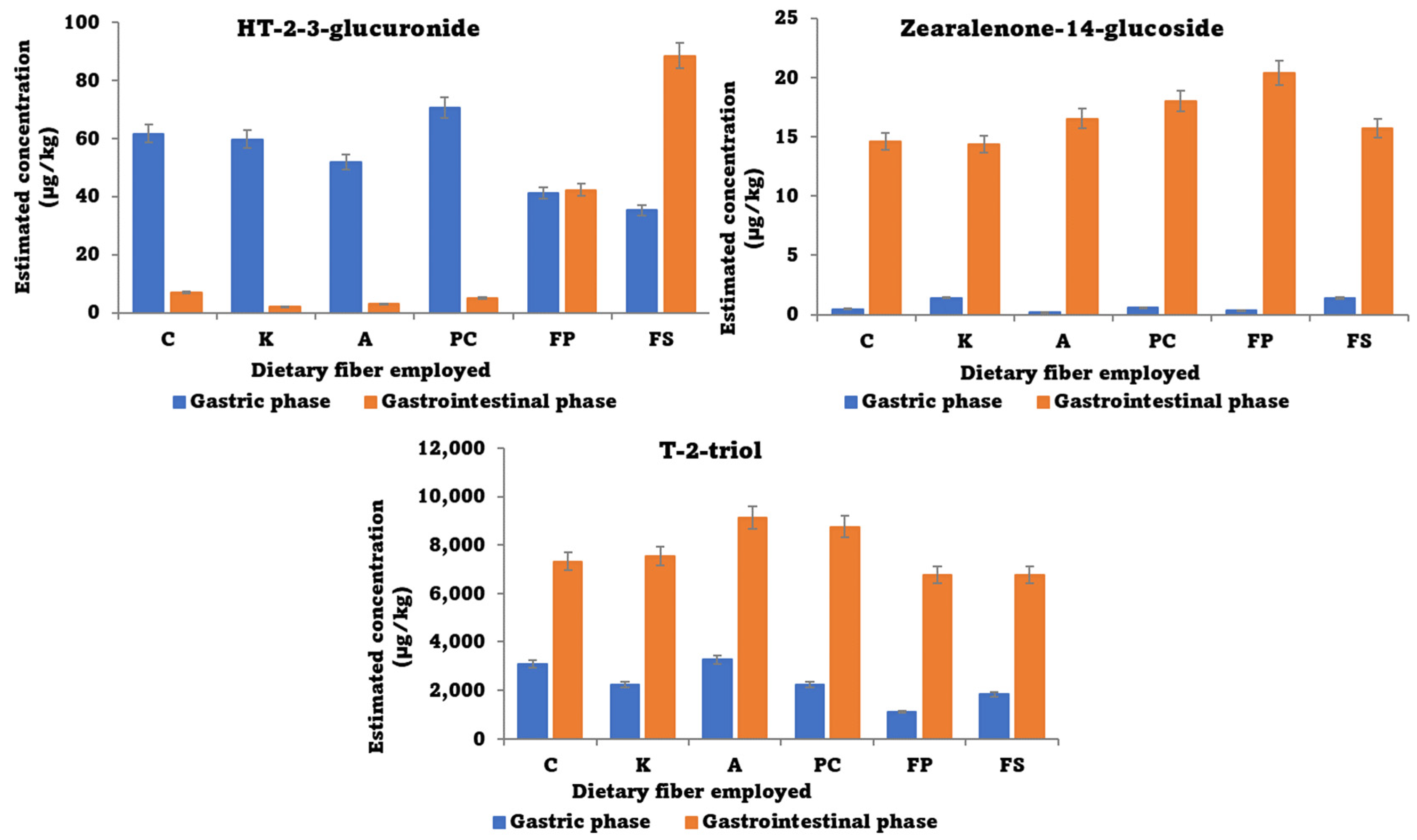
3.4. Non-Targeted Analysis for Metabolite Search
4. Future Perspectives and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, V.N.; Viktorová, J.; Ruml, T. Mycotoxins: Biotransformation and Bioavailability Assessment Using Caco-2 Cell Monolayer. Toxins 2020, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.M.C.; Cunha, S.C.; Faria, M.A.; Ferreira, I.M.P.L.V.O. Aflatoxins in Food and Feed: Occurrence, Legislation and Mitigation Strategies. In Aflatoxins: Biochemistry, Toxicology, Public Health, Policies and Modern Methods of Analysis; Ed. Nova Science Publishers, Inc.: New York, NY, USA, 2019; pp. 287–316. [Google Scholar]
- Lizárraga-Paulín, E.G.; Moreno-Martínez, E.; Torres-Pacheco, I. Novel Methods for Preventing and Controlling Aflatoxins in Food: A Worldwide Daily Challenge. In Aflatoxins; Miranda-Castro, S.P., Ed.; IntechOpen: Rijeka, Croatia, 2013; p. Ch. 5. [Google Scholar]
- Bui-Klimke, T.R.; Guclu, H.; Kensler, T.W.; Yuan, J.M.; Wu, F. Aflatoxin Regulations and Global Pistachio Trade: Insights from Social Network Analysis. PLoS ONE 2014, 9, e92149. [Google Scholar] [CrossRef]
- Wu, F.; Guclu, H. Aflatoxin Regulations in a Network of Global Maize Trade. PLoS ONE 2012, 7, e45151. [Google Scholar] [CrossRef]
- Gupta, R.; Doss, R.; Lall, R.; Srivastava, A.; Sinha, A. Trichothecenes and Zearalenone. In Reproductive and Developmental Toxicology; Academic Press: Cambridge, MA, USA, 2022; pp. 1003–1016. [Google Scholar]
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and Metabolism of Deoxynivalenol in Animals and Humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Su, Y.; Li, T. An Update on T2-Toxins: Metabolism, Immunotoxicity Mechanism and Human Assessment Exposure of Intestinal Microbiota. Heliyon 2022, 8, e10012. [Google Scholar] [CrossRef] [PubMed]
- Rocha, O.; Ansari, K.; Doohan, F.M. Effects of Trichothecene Mycotoxins on Eukaryotic Cells: A Review. Food Addit. Contam. 2005, 22, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A Review of Recent Innovative Strategies for Controlling Mycotoxins in Foods. Food Control 2023, 144, 109350. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Wang, J.; Shan, A.; Xu, L. Changes in Intestinal Barrier Functions and Gut Microbiota in Rats Exposed to Zearalenone. Ecotoxicol. Environ. Saf. 2020, 204, 111072. [Google Scholar] [CrossRef]
- Bbosa, G.S.; Lubega, A.; Ogwal-Okeng, J. Review of the Biological and Health Effects of Aflatoxins on Body Organs and Body Systems. In Aflatoxins; Kitya, D., Ed.; IntechOpen: Rijeka, Croatia, 2013; p. Ch. 12. [Google Scholar]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In Vitro Bioaccessibility Assessment as a Prediction Tool of Nutritional Efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef]
- Meca, G.; Meneghelli, G.; Ritieni, A.; Mañes, J.; Font, G. Influence of Different Soluble Dietary Fibers on the Bioaccessibility of the Minor Fusarium Mycotoxin Beauvericin. Food Chem. Toxicol. 2012, 50, 1362–1368. [Google Scholar] [CrossRef]
- Holanda, D.M.; Kim, S.W. Mycotoxin Occurrence, Toxicity, and Detoxifying Agents in Pig Production with an Emphasis on Deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Haider, N.N.; Summo, C.; Coldea, T.E.; George, S.S.; Altemimi, A.B. Biscuit Contaminants, Their Sources and Mitigation Strategies: A Review. Foods 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.M.C.; Cunha, S.C.; Faria, M.A.; Martins, Z.E.; Ferreira, I.M.P.L.V.O. Influence of Oven and Microwave Cooking with the Addition of Herbs on the Exposure to Multi-Mycotoxins from Chicken Breast Muscle. Food Chem. 2019, 276, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, J.; Font, G.; Mañes, J.; Ferrer, E. Mitigation of Enniatins in Edible Fish Tissues by Thermal Processes and Identification of Degradation Products. Food Chem. Toxicol. 2017, 101, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Assunção, R.; Martins, C.; Dupont, D.; Alvito, P. Patulin and Ochratoxin A Co-Occurrence and Their Bioaccessibility in Processed Cereal-Based Foods: A Contribution for Portuguese Children Risk Assessment. Food Chem. Toxicol. 2016, 96, 205–214. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Havenaar, R.; Visconti, A. Assessment of the Multi-Mycotoxin-Binding Efficacy of a Carbon/Aluminosilicate-Based Product in an in Vitro Gastrointestinal Model. J. Agric. Food Chem. 2007, 55, 4810–4819. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Havenaar, R.; Visconti, A. Assessing the Zearalenone-Binding Activity of Adsorbent Materials during Passage through a Dynamic in Vitro Gastrointestinal Model. Food Chem. Toxicol. 2003, 41, 1283–1290. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Havenaar, R.; Visconti, A. Evaluation of the Intestinal Absorption of Deoxynivalenol and Nivalenol by an in Vitro Gastrointestinal Model, and the Binding Efficacy of Activated Carbon and Other Adsorbent Materials. Food Chem. Toxicol. 2004, 42, 817–824. [Google Scholar] [CrossRef]
- Greco, D.; D’Ascanio, V.; Santovito, E.; Logrieco, A.F.; Avantaggiato, G. Comparative Efficacy of Agricultural By-Products in Sequestering Mycotoxins. J. Sci. Food Agric. 2019, 99, 1623–1634. [Google Scholar] [CrossRef]
- López-Ruiz, R.; Marin-Saez, J.; Cunha, S.C.; Fernandes, A.; de Freitas, V.; Viegas, O.; Ferreira, I.M.P.L.V.O. Fibre Enrichment of Cookies to Mitigate Acrylamide Formation and Gastrointestinal Bioaccessibility. LWT 2023, 182, 114835. [Google Scholar] [CrossRef]
- Gustafsson, J.; Landberg, M.; Bátori, V.; Åkesson, D.; Taherzadeh, M.J.; Zamani, A. Development of Bio-Based Films and 3D Objects from Apple Pomace. Polymers 2019, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Bátori, V.; Jabbari, M.; Åkesson, D.; Lennartsson, P.R.; Taherzadeh, M.J.; Zamani, A. Production of Pectin-Cellulose Biofilms: A New Approach for Citrus Waste Recycling. Int. J. Polym. Sci. 2017, 2017, 9732329. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinto, E.; Almeida, A.A.; Pinho, O.; Ferreira, I.M.P.L.V.O. Fibre Fortification of Wheat Bread: Impact on Mineral Composition and Bioaccessibility. Food Funct. 2017, 8, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.R.; Viegas, O.; Páscoa, R.N.M.J.; Ferreira, I.M.P.L.V.O.; Rangel, A.O.S.S.; Lopes, J.A. In-Line Monitoring of the Coffee Roasting Process with near Infrared Spectroscopy: Measurement of Sucrose and Colour. Food Chem. 2016, 208, 103–110. [Google Scholar] [CrossRef] [PubMed]
- López-Ruiz, R.; Romero-González, R.; Garrido Frenich, A. Simultaneous Determination of Polar Pesticides in Human Blood Serum by Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometer. J. Pharm. Biomed. Anal. 2020, 190, 113492. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- European Commission SANTE 11312/2021; Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. European Commision: Bruselles, Belgium, 2022.
- Ashe, T. DELTA E, DELTA H, DELTA T: WHAT DOES IT MEAN? Delta. Color Manag. Qual. Output 2014, 1–4. [Google Scholar] [CrossRef]
- Cunha, S.C.; Sá, S.V.M.; Fernandes, J.O. Multiple Mycotoxin Analysis in Nut Products: Occurrence and Risk Characterization. Food Chem. Toxicol. 2018, 114, 260–269. [Google Scholar] [CrossRef]
- Wang, P.; Sun, G.; Lu, P.; Liu, Y.; Zhu, Y.; Chen, F. Mitigation Effects of High Methoxyl Pectin on Acrylamide Formation in the Maillard Model System. Food Chem. 2022, 378, 132095. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, Characterisation, and Application of Pectin from Tropical and Sub-Tropical Fruits: A Review. Food Rev. Int. 2022, 38, 282–312. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Fakhri, Y.; Sant’Ana, A.S. Impact of Unit Operations during Processing of Cereal-Based Products on the Levels of Deoxynivalenol, Total Aflatoxin, Ochratoxin A, and Zearalenone: A Systematic Review and Meta-Analysis. Food Chem. 2018, 268, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Pascari, X.; Maul, R.; Kemmlein, S.; Marin, S.; Sanchis, V. The Fate of Several Trichothecenes and Zearalenone during Roasting and Enzymatic Treatment of Cereal Flour Applied in Cereal-Based Infant Food Production. Food Control 2020, 114, 107245. [Google Scholar] [CrossRef]
- Kuchenbuch, H.S.; Becker, S.; Schulz, M.; Cramer, B.; Humpf, H.U. Thermal Stability of T-2 and Ht-2 Toxins during Biscuit-and Crunchy Muesli-Making and Roasting. Food Addit. Contam Part A Chem. Anal. Control Expo. Risk Assess 2018, 35, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, A.; Meca, G.; Font, G.; Ruiz, M.J. Bioaccessibility of Enniatins A, A1, B, and B1 in Different Commercial Breakfast Cereals, Cookies, and Breads of Spain. J. Agric. Food Chem. 2013, 61, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.M.C.; Faria, M.A.; Cunha, S.C.; Miladinovic, B.; Ferreira, I.M. Transport of Mycotoxins across Human Gastric NCI–N87 and Intestinal Caco-2 cell Models. Food Chem. Toxicol. 2019, 131, 110595. [Google Scholar] [CrossRef] [PubMed]
- Tamura, C.; Nakauma, M.; Furusawa, H.; Kadota, T.; Kamata, Y.; Nishijima, M.; Itoh, S.; Sugita-Konishi, Y. Formulation of a Pectin Gel That Efficiently Traps Mycotoxin Deoxynivalenol and Reduces Its Bioavailability. Carbohydr. Polym. 2013, 93, 747–752. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, E.; Monaci, L.; Mackie, A.; Salt, L.; Visconti, A. Bioaccessibility of T-2 and HT-2 Toxins in Mycotoxin Contaminated Bread Models Submitted to in Vitro Human Digestion. Innov. Food Sci. Emerg. Technol. 2014, 22, 248–256. [Google Scholar] [CrossRef]
- Aoudia, N.; Tangni, E.K.; Larondelle, Y. Distribution of Ochratoxin A in Plasma and Tissues of Rats Fed a Naturally Contaminated Diet Amended with Micronized Wheat Fibres: Effectiveness of Mycotoxin Sequestering Activity. Food Chem. Toxicol. 2008, 46, 871–878. [Google Scholar] [CrossRef]
- Harris, P.J.; Sasidharan, V.K.; Roberton, A.M.; Triggs, C.M.; Blakeney, A.B.; Ferguson, L.R. Adsorption of a Hydrophobic Mutagen to Cereal Brans and Cereal Bran Dietary Fibres. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1998, 412, 323–331. [Google Scholar] [CrossRef]
- Lu, Q.; Sui, M.; Luo, Y.W.; Luo, J.Y.; Yang, M.H. Further Insight into the Potential Toxicity of Zearalenone-14-Glucoside Based on Toxicokinetics, Tissue Distribution, Transformation, and Excretion in Rats. Ecotoxicol. Environ. Saf. 2022, 246, 114184. [Google Scholar] [CrossRef]
- Narváez, A.; Izzo, L.; Pallarés, N.; Castaldo, L.; Rodríguez-Carrasco, Y.; Ritieni, A. Human Biomonitoring of T-2 Toxin, t-2 Toxin-3-Glucoside and Their Metabolites in Urine through High-Resolution Mass Spectrometry. Toxins 2021, 13, 869. [Google Scholar] [CrossRef] [PubMed]
- Paris, M.P.K.; Schweiger, W.; Hametner, C.; Stückler, R.; Muehlbauer, G.J.; Varga, E.; Krska, R.; Berthiller, F.; Adam, G. Zearalenone-16- o -Glucoside: A New Masked Mycotoxin. J. Agric. Food Chem. 2014, 62, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.S.; Schulz, M.; Focke, C.; Becker, S.; Cramer, B.; Humpf, H.U. Glucosylation of T-2 and HT-2 Toxins Using Biotransformation and Chemical Synthesis: Preparation, Stereochemistry, and Stability. Mycotoxin Res. 2018, 34, 159–172. [Google Scholar] [CrossRef]
- Lu, Q.; Qin, J.A.; Fu, Y.W.; Luo, J.Y.; Lu, J.H.; Logrieco, A.F.; Yang, M.H. Modified Mycotoxins in Foodstuffs, Animal Feed, and Herbal Medicine: A Systematic Review on Global Occurrence, Transformation Mechanism and Analysis Methods. TrAC Trends Anal. Chem. 2020, 133, 116088. [Google Scholar] [CrossRef]
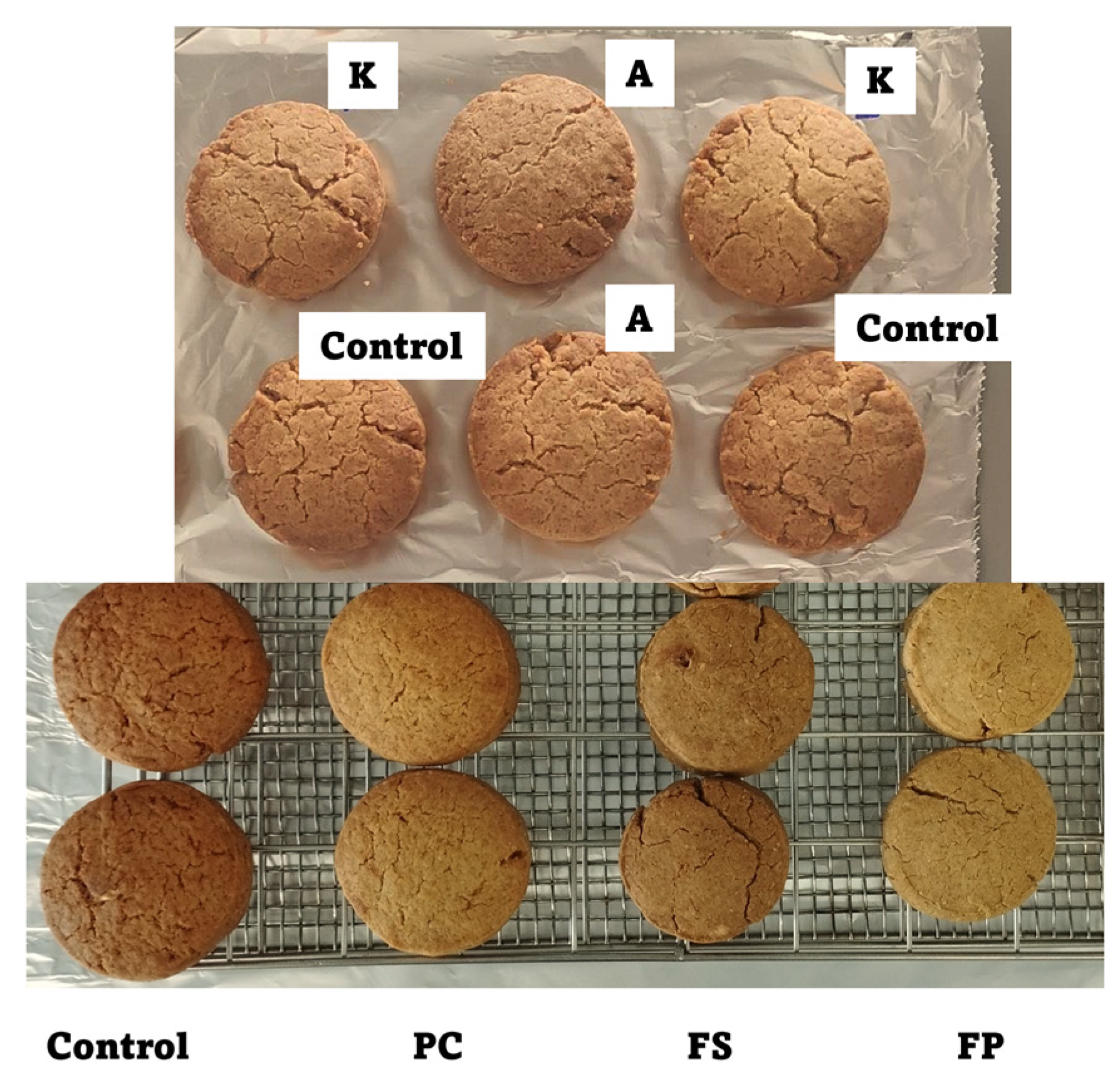
| Ingredients | Recipe with A, K, PC | Recipe with FS | Recipe with FP |
|---|---|---|---|
| Flour | 475 g | 150 g | 345 g |
| NH4HCO3 | 2 g | 2 g | 2 g |
| NaHCO3 | 9 g | 9 g | 9 g |
| Sugar | 136 g | - | 136 g |
| Salt | 4 g | 4 g | 4 g |
| Malt extract | 20 g | 20 g | 20 g |
| Glucose-fructose syrup | 100 g | - | 100 g |
| Sunflower oil | 117 g | 117 g | 117 g |
| Water | 75 mL | 185 mL | 175 mL |
| Dietary fiber | 25 g | 350 g | 155 g |
| Analyte | Precursor Ion (m/z) | Product Ion (m/z) | Cone Voltage (V) | Collision Energy (eV) | Ionization Mode | Retention Time (min) |
|---|---|---|---|---|---|---|
| DON | 297.1 | 249.1 | 100 | 10 | Positive | 3.67 |
| 231.2 | 100 | 10 | ||||
| HT-2 | 442.1 | 263.1 | 100 | 10 | Positive | 6.93 |
| 214.9 | 100 | 10 | ||||
| T-2 | 484.2 | 305.1 | 100 | 10 | Positive | 7.62 |
| 215.1 | 100 | 10 | ||||
| ZEA | 317.1 | 273.1 | 195 | 10 | Negative | 8.66 |
| 175.0 | 195 | 20 | ||||
| 13C15DON | 310.1 | 278.8 | 150 | 10 | Negative | 3.11 |
| 250.3 | 150 | 10 |
| Compounds | Linearity | Matrix Effect a | Recoveries (%) | Intraday Precision c (% RSD) | Interday Precision c (% RSD) | LOQ (µg/kg) | LOD (µg/kg) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LOQ b | 500 µg/kg | LOQ b | 500 µg/kg | LOQ b | 500 µg/kg | ||||||
| DON | B | 0.9950 | −610 | 79 | 85 | 12 | 8 | 16 | 10 | 50 | 25 |
| GP | 0.9918 | −520 | 82 | 89 | 10 | 9 | 20 | 15 | 100 | 50 | |
| GIP | 0.9955 | −555 | 65 | 69 | 18 | 15 | 17 | 12 | 100 | 50 | |
| ZEA | B | 0.9994 | 31 | 118 | 120 | 4 | 2 | 3 | 2 | 2.5 | 0.5 |
| GP | 0.9965 | 40 | 102 | 102 | 5 | 4 | 4 | 3 | 10 | 2 | |
| GIP | 0.9945 | 65 | 91 | 96 | 8 | 5 | 8 | 4 | 10 | 2 | |
| HT-2 | B | 0.9951 | −6 | 72 | 95 | 6 | 8 | 8 | 5 | 7.5 | 2.5 |
| GP | 0.9900 | −15 | 78 | 78 | 12 | 9 | 9 | 6 | 10 | 5 | |
| GIP | 0.9917 | −26 | 82 | 85 | 10 | 11 | 11 | 7 | 10 | 5 | |
| T-2 | B | 0.9980 | −14 | 71 | 85 | 5 | 3 | 3 | 2 | 2.5 | 0.5 |
| GP | 0.9899 | −5 | 68 | 68 | 10 | 5 | 5 | 3 | 10 | 2 | |
| GIP | 0.9902 | −18 | 85 | 96 | 9 | 6 | 6 | 1 | 10 | 2 | |
| Analytes | ||||
|---|---|---|---|---|
| Dietary Fibers | DON | HT-2 | T-2 | ZEA |
| Biscuits Concentration µg/kg | ||||
| C | 497.0 (5) | 507.0 (5) | 504.0 (3) | 502.3 (2) |
| K | 489.3 (6) | 490.3 (9) | 495.0 (2) | 295.0 (3) |
| A | 502.1 (5) | 488.0 (12) | 490.2 (13) | 475.0 (15) |
| PC | 501.3 (2) | 514.2 (14) | 511.3 (10) | 499.3 (1) |
| FP | 499.2 (1) | 495.3 (4) | 505.2 (7) | 498.0 (5) |
| FS | 500.9 (3) | 508.4 (10) | 501.4 (1) | 490.0 (4) |
| Gastric Phase, Concentration µg/kg | ||||
| C | 496.7 (2) | 488.2 (18) | 478.4 (9) | 100.7 (1) |
| K | 460.2 (5) | 483.2 (6) | 370.9 (10) | 20.4 (3) |
| A | 433.0 (8) | 450.6 (8) | 381.0 (10) | 30.9 (5) |
| PC | 460.2 (12) | 472.9 (12) | 385.9 (13) | 25.1 (5) |
| FP | 360.9 (10) | 444.3 (13) | 338.8 (4) | 29.4 (4) |
| FS | 451.1 (8) | 439.8 (5) | 328.0 (8) | 35.6 (3) |
| p-value 1 | 0.00542 | 0.00468 | 0.00009 | 0.00000 |
| Gastrointestinal Phase, Concentration µg/kg | ||||
| C | 518.3 (13) | 501.1 (5) | 401.3 (7) | 329.9 (12) |
| K | 485.4 (5) | 448.5 (6) | 311.2 (6) | 113.0 (5) |
| A | 485.2 (7) | 465.1 (8) | 346.6 (9) | 169.4 (6) |
| PC | 527.1 (8) | 503.4 (10) | 331.5 (5) | 205.3 (10) |
| FP | 402.2 (11) | 408.3 (4) | 256.3 (10) | 226.4 (8) |
| FS | 510.3 (4) | 393.2 (3) | 242.6 (5) | 185.4 (7) |
| p-value 1 | 0.04470 | 0.00928 | 0.00131 | 0.00025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Ruiz, R.; Marin-Saez, J.; Cunha, S.C.; Fernandes, A.; de Freitas, V.; Viegas, O.; Ferreira, I.M.P.L.V.O. Investigating the Impact of Dietary Fibers on Mycotoxin Bioaccessibility during In Vitro Biscuit Digestion and Metabolites Identification. Foods 2023, 12, 3175. https://doi.org/10.3390/foods12173175
López-Ruiz R, Marin-Saez J, Cunha SC, Fernandes A, de Freitas V, Viegas O, Ferreira IMPLVO. Investigating the Impact of Dietary Fibers on Mycotoxin Bioaccessibility during In Vitro Biscuit Digestion and Metabolites Identification. Foods. 2023; 12(17):3175. https://doi.org/10.3390/foods12173175
Chicago/Turabian StyleLópez-Ruiz, Rosalía, Jesús Marin-Saez, Sara. C. Cunha, Ana Fernandes, Victor de Freitas, Olga Viegas, and Isabel M. P. L. V. O. Ferreira. 2023. "Investigating the Impact of Dietary Fibers on Mycotoxin Bioaccessibility during In Vitro Biscuit Digestion and Metabolites Identification" Foods 12, no. 17: 3175. https://doi.org/10.3390/foods12173175
APA StyleLópez-Ruiz, R., Marin-Saez, J., Cunha, S. C., Fernandes, A., de Freitas, V., Viegas, O., & Ferreira, I. M. P. L. V. O. (2023). Investigating the Impact of Dietary Fibers on Mycotoxin Bioaccessibility during In Vitro Biscuit Digestion and Metabolites Identification. Foods, 12(17), 3175. https://doi.org/10.3390/foods12173175








