Essential Oils and Their Combination with Lactic Acid Bacteria and Bacteriocins to Improve the Safety and Shelf Life of Foods: A Review
Abstract
:1. Introduction
| Botanical/Common Name | Plant Product | Bioactivity in Food Model/Product Quality | Reference(s) |
|---|---|---|---|
| Artemisia dracunculus L. (Tarragon) | * EO | Antimicrobial in beef burger/flavor enhancer in meat products | [63] |
| Allium sativum (Garlic) | EO | Antibacterial/in poultry meat | [64] |
| Allium schoenoprasum (Chives) | Diallyl sulfides | Inhibits the growth of foodborne pathogens | [65] |
| Anethum graveolens (Dill) | EO | Antimicrobial in dairy products/improves the physico-chemical and sensory characteristics of yogurt | [66] |
| Brassica nigra (Black mustard) | Extracts combined with oregano, Syzygium, and cinnamon | Antimicrobial, antioxidant/chicken meat/improve sensory attributes | [67] |
| Carum carvi (Caraway) | * EO | Improves the quality of dry-fermented sausages/reduces the level of sodium nitrite in dry-fermented sausages | [68] |
| Citrus aurantifolia (Lime) | * EO, Limonene, β-Pinene, γ-Terpinene, and Citral | Antimicrobial | [69] |
| Crocus sativus (Saffron) | Stigma powder | Antimicrobial, antioxidant/chicken breast meat/improves physico-chemical characteristics of chicken meat | [70] |
| Curcuma longa (Turmeric) | Rhizome extract/curcumin | Oxidative stability of meat increases the shelf life and quality in meat. | [71,72] |
| Cuminum cyminum (Cumin) | * EO, cuminal | Antibacterial/meet protection/prolongs the shelf life | [73] |
| Cymbopogon citratus (Lemon grass) | * EO combined with ginger EO | Antimicrobial in fresh chicken meat/extends the shelf life of chicken meat for 9 days at a temperature of 2–7 °C | [74] |
| Foeniculum vulgare (Fennel) | * EO | Antioxidant and antimicrobial nanocoating of fennel EO in meat/fish packaging/improves the antioxidant and antimicrobial properties of coatings | [75] |
| Hyssopus officinalis (Hyssop) | * EO combined with coriander EO | Prolongs the shelf life of ground beef/preserve in vacuum-packed meat | [76] |
| Kaempferia galanga (Kencur) | Extract | Antibacterial activity in poultry products/cell membranes damage in food pathogen bacteria | [77] |
| Laurus nobilis (Bay) | * EO/leaf extract | Antibacterial/increase the shelf life of lamb meat/increases the shelf life of lamb meat | [78] |
| Lippia graveolens (Mexican oregano) | EO mixed with basil EO | Microencapsulated EOs increases the shelf life of refrigerated meat products and positive effect on the sensory properties | [79] |
| Mentha piperita (Mint) | * EO | Prolongs the shelf life of beef meat/extends the shelf life | [80,81] |
| Melissa officinalis (Balm) | * EO combined with thyme EO | Antimicrobial/may protect the chicken meat from decomposition during storage and extends the shelf life | [82] |
| Ocimum basilicum (Basil) | * EO with aloe vera | Extend the shelf-life of strawberry fruit and preserve post-harvest quality Combinations of EOs of O. basilicum, Cymbopogon nardus and C. flexuosus increased banana shelf life by up to 21 days/control post-harvest diseases, and extended storage life. | [83,84] |
| Murraya koenigii (Curry leaf) | Leaf powder | Antioxidant/cooked goat meat/inhibitor of oxidation products in raw ground and cooked goat meat | [85] |
| Myristica fragrans (Nutmeg) | * EO | Antibacterial and antioxidant/improves the color stability and sensory properties of beef slices | [86] |
| Nigella sativa (Black cumin) | EO | EO prolongs the shelf life and improves the sensory quality of fresh fish fillets | [87] |
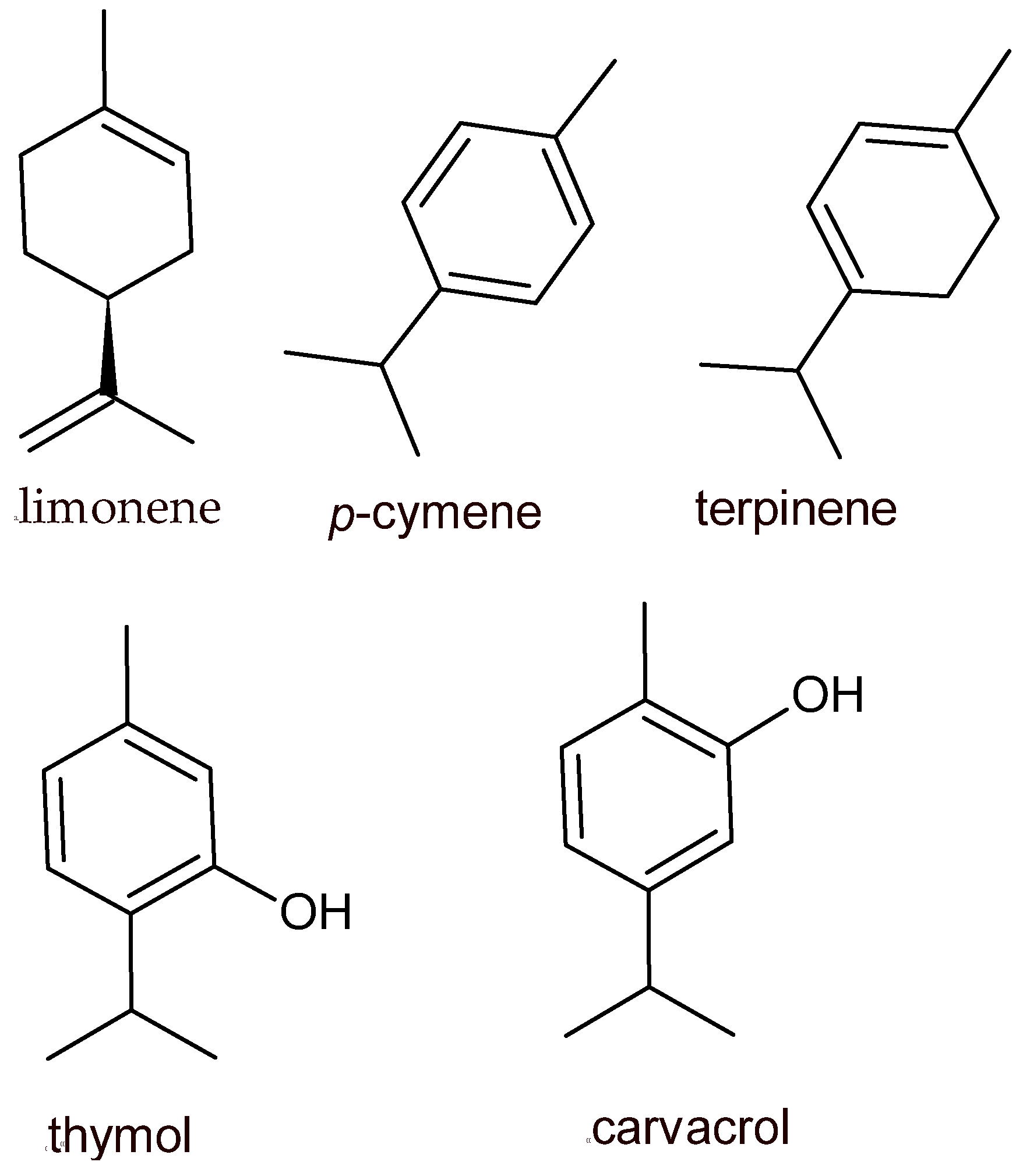
| Origanum vulgare (Oregano) | * EO/carvacrol | Antibacterial/increases the shelf life of pork, antimicrobial oregano oil nanoemulsions in fresh lettuce, antibacterial in vacuum-packed minced beef | [23,88,89] |
| Pimpinella anisum (Anise) | * EO | Antimicrobial/antioxidant/prolongs the shelf life of chicken fillets | [90] |
| Rhus coriaria (Sumac) | Water extract | Antimicrobial effect/extends shelf life of refrigerated raw broiler wings, improving sensory quality, and color | [91] |
| Rosmarinus officinalis (Rosemary) | * EO | Antimicrobial against meat pathogens/extends the shelf life of beef stored at 4 °C for 20 days | [92] |
| Salvia officinalis (Sage) | * EO Ethanolic extracts Hydro-ethanol extract Oil and ethanol extracts | Prolongs the shelf life and compositional quality of fish burgers (4 months at frozen storage) Antioxidant/extends the shelf life of mayonnaise during storage Prolongs the shelf life of trout fillets Antibacterial/prolongs the storage stability of vacuum-packed low pressure chicken meat | [93,94,95,96] |
| Satureja montana (Winter savory) | EO/supercritical extracts | Antioxidants in pre-cooked pork chops/extends the shelf life | [97] |
| Thymus vulgaris (Thyme) | * EO | Antimicrobial/prolongs the shelf life of gilthead seabream extends the shelf life of oranges for fresh use and juice processing | [47,98] |
| Thymus capitatus (Headed Savory) | EO | Antimicrobial/improves the microbial and sensory quality of beef meat | [99] |
| Trachyspermum ammi (Ajwan) | EO | Antibacterial/chitosan-based film with EO improves the safety of chicken fillet stored for 12 days at refrigerated temperature | [100] |
| Zingiber officinale (Ginger) | * EO | Antimicrobial/extends the shelf life of minced meat | [101] |
2. Use of Essential Oils and Biocontrol in Minimally Processed Fruit and Vegetables
3. Application of Essential Oils as Meat and Dairy Preservatives
3.1. Antimicrobials as Meat Preservatives
| Microbial Target | Meat/Meat Products | EO or Combinations | Concentration Used/Product Benefits | Reference(s) |
|---|---|---|---|---|
| Listeria monocytogenes | Ham | Oregano and Cinnamon cassia EOs | 500 ppm/inhibit the growth of food pathogens with no effect on sensory attributes | [151] |
| Enterobacteriaceae | Lamb meat | Cinnamon cassia EO | (0.5%) (w/w)/reducing bacteria growth but worse color stability | [152] |
| S. enteritidis, Listeria monocytogenes, St. auresu | Marinated pork loin | Oregano, rosemary, and juniper EO | (0.02–0.03%) (w/w)/extends shelf life, Improve sensory characteristics | [160] |
| Salmonella typhimurium | Pork meat | Micromeria dalmatica EO | 0.15 mg/mL/reduces bacterial growth | [175] |
| E. coli, Salmonella sp. | Chicken breast meat | Thyme and balm EO | (0.5%) (w/w)/extends the shelf life | [82] |
| E. coli | Chicken hamburgers | Thyme EO | 0.02 and 0.05 w/w/provides oxidative and microbial stability | [154] |
| Salmonella, Listeria and E. coli | Sausages | Oreganum virens EO | EO incorporated in active film/extends the shelf life and sensory properties | [158] |
| psychrotrophics, Brochothrix thermosphacta, Pseudomonas | Beef meat | Rosemary EO | 4% (w/w)/extends the shelf-life of refrigerated beef meat | [92] |
| L. monocytogenes | Pork meat | Oregano EO + bacteriocin | 50 µL/100 g/synergistic effect of EO and bacteriocins prolongs the shelf life for two weeks while under storage at 4 °C | [172] |
| L. monocytogenes | Minced fish | Thyme EO + Nisin | EO 0.4% nisin (1000 IU/g)/control bacteria growth and improve sensory properties | [173] |
| L. monocytogenes | Shrimps | Salvia, Citrus, Mentha, Thymus EO+ bacLP17 | EOs 8-128 µL/mL; bacLP17 16 µL/mL /improve organoleptic properties and reduce growth of L. monocytogenes | [174] |
| Pseudomonas spp., LAB and B. thermosphacta | Marinated beef | Thymol and carvacrol | 0.4% and 0.8% (w/w)/extends the shelf life | [176] |
| Salmonella typhimurium, Listeria monocytogenes, and Escherichia coli O157:H7 | Beef slices | Thymol | (0.5%) (w/w)/inhibited growth aerobic bacteria inactivate coliform bacteria | [177] |
| Salmonella enteritidis, Campylobacter and E. coli | Breast fillets and wings | Marinade with thyme and orange EOs | (0.5%) (w/w)/inhibit microbes | [178] |
3.2. Essential Oils as Dairy Preservatives
| Combined Antimicrobial | |||||||
|---|---|---|---|---|---|---|---|
| Dairy Food Application | Target Microorganisms | Nisin Concentration | Antimicrobial Type | Antimicrobial Concentration | Other Treatment | Activity Reported | Reference |
| Unpasteurized cow milk | Escherichia coli, S. aureus | 0.008 mg/mL | Magnesium oxide nanoparticles | 2 mg/mL without heat | NA * | MgO NP in combination with nisin lead to damage to the cell membrane, causing the pathogen’s death | [215] |
| 0.5 mg/mL | Heat (60 °C) | ||||||
| Pasteurized milk | S. aureus, L. monocytogenes | 16 µg/mL | Perilla oil | 1 mg/mL | NA * | Synergistic effect resulting in higher cell wall damage when nisin and perilla oil were used in combination | [216] |
| S. aureus | 8 mg/mL | Cinnamaldehyde | 0.25 mg/mL | NA * | Synergistic effect with increased antimicrobial activity against S. aureus | [217] | |
| 0.37 and 0.75 µg/mL | Phage-encoded endolysin LysH5 | 7.5 and 15 U/mL | NA * | Synergistic effect of the absence of S. aureus was reached only with the combination of the two antimicrobials | [218] | ||
| 8 µg/mL | p-Anisaldehyde | NA * | Synergistic effect demonstrated | [219] | |||
| 1.5 µg/mL | Bacteriophage Φ35 Bacteriophage Φ88 | 1:1 cocktail of phages Φ35 and Φ88 at 103 pfu/mL | NA * | nisin activity, which can induce surface changes that can impair bacteriophage activity | [220] | ||
| UHT whole milk | S. aureus | 400, 600, 800, and 1200 AU/mL | Bovicin HC5 | 400, 600, 800, and 1200 AU/mL | NA * | Bovicin and nisin combinations were effective in inhibiting Listeria and S. aureus at lower concentrations than when used alone | [221] |
| L. monocytogenes, | |||||||
| Listeria innocua, | |||||||
| UHT processed 2% reduced-fat milk and whole milk | L. monocytogenes | 250 and 500 IU/mL | Lactobionic acid | 10 mg/mL | NA * | LBA increased the synergistic effect between nisin and thymol against L. monocytogenes but not E. coli | [207] |
| Thymol | 1–2 mg/mL | ||||||
| UHT skimmed milk with 0.04% fat | L. monocytogenes, S. aureus, E. coli O157:H7, Salmonella enterica, Yersinia enterocolitica, Aeromonas hydrophila, Campylobacter jejuni | 100 IU/mL | Reuterin | 8 AU6/mL | NA * | Nisin and reuterin showed a synergistic effect in milk at refrigerated temperatures causing the complete deactivation of Listeria and S. aureus | [205] |
| Cow milk | Staphylococcus aureus, Listeria monocytogenes | 1/4 MIC | Thymol, eugenol, carvacrol, and cinnamaldehyde | 1/4 MIC | NA * | Nisin combination with phenolic compounds showed a synergistic effect | [222] |
| Whole, low, and skimmed milk | L. monocytogenes | 62.5, 125, 250, and 500 IU/mL | Cone EO of Metasequoia glyptostroboides | 1 and 2% | NA * | Synergistic effect of nisin and cone EO against listeria in whole, low, and skimmed milk | [223] |
| Leaf EO of Metasequoia glyptostroboides | 1, 2, and 5% | NA * | Remarkable synergism of leaf EO and nisin against listeria in whole, low, and skimmed milk | [197] | |||
| Whole (3.5%), low (1%), and skimmed (no fat content) milk | 62.5, 125, 250, and 500 IU/mL | Garlic shoot juice | 2.5 and 5% | NA * | Synergistic anti-listerial activity of nisin and garlic shoot juice | [224] | |
| Chocolate milk | 25 µg/mL | Thymol | 100 µg/mL | NA * | Enhanced antilisterial activity by the combination of nisin with carvacrol or cinnamaldehyde | [225] | |
| Carvacrol | 304 µg/mL | ||||||
| trans-cinnamaldehyde | 327.6 µg/mL | ||||||
| Reconstituted powdered infant milk formula | Cronobacter sakazakii | 60 µM and 250 µg/mL | Carvacrol | 300 µg/mL | NA * | Bioengineered nisin variants showed an increased antimicrobial activity compared to nisin A and an enhanced synergistic effect with carvacrol | [226] |
| Citric acid | 30 mM | ||||||
| Homogenized UHT skimmed milk | S. aureus | 1 to 20 IU/mL | Lysozyme | 300 to 5000 IU/mL | High-intensity pulsed-electric field: 120 to 1200 µs | Synergistic effect | [227] |
| Enterocin AS-48 (AS-48) | 28 AU/mL | ||||||
| Iranian youghurt (Doogh) | E. coli O157:H7 | 250 and 500 IU/mL | Ziziphora clinopodioides Essential Oil | 5 mg/mL | NA * | Nisin and EO combination reduced E. coli population, showing a synergistic effect | [228] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The Antimicrobial Efficacy of Plant Essential Oil Combinations and Interactions with Food Ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef]
- Gutierrez, J.; Rodriguez, G.; Barry-Ryan, C.; Bourke, P. Efficacy of Plant Essential Oils against Foodborne Pathogens and Spoilage Bacteria Associated with Ready-to-Eat Vegetables: Antimicrobial and Sensory Screening. J. Food Prot. 2008, 71, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Zahin, M.; Ahmad, I.; Owais, M.; Khan, M.S.A.; Bansal, S.S.; Farooq, S. Antifungal Activity of Medicinal Plant Extracts and Phytocompounds: A Review. In Combating Fungal Infections Problems and Remedy; Springer: Berlin/Heidelberg, Germany, 2010; pp. 449–484. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Tufail, T.; Ahmad, A. Use of Natural Antimicrobial Agents: A Safe Preservation Approach. In Active Antimicrobial Food Packaging; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Davidson, P.M.; Critzer, F.J.; Matthew Taylor, T. Naturally Occurring Antimicrobials for Minimally Processed Foods. Annu. Rev. Food Sci. Technol. 2013, 4, 163–190. [Google Scholar] [CrossRef]
- Moreira, M.R.; Ponce, A.G.; Del Valle, C.E.; Roura, S.I. Inhibitory Parameters of Essential Oils to Reduce a Foodborne Pathogen. LWT Food Sci. Technol. 2005, 38, 565–570. [Google Scholar] [CrossRef]
- Weerakkody, N.S.; Caffin, N.; Turner, M.S.; Dykes, G.A. In Vitro Antimicrobial Activity of Less-Utilized Spice and Herb Extracts against Selected Food-Borne Bacteria. Food Control 2010, 21, 1408–1414. [Google Scholar] [CrossRef]
- Adu, A.A.; Sudiana, I.K.; Martini, S. The Effect of Nitrite Food Preservatives Added to Se’i Meat on the Expression of Wild-Type P53 Protein. Open Chem. 2020, 18, 559–564. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food—Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wu, L.; Guan, W. Dietary Nitrates, Nitrites, and Nitrosamines Intake and the Risk of Gastric Cancer: A Meta-Analysis. Nutrients 2015, 7, 9872–9895. [Google Scholar] [CrossRef] [PubMed]
- Crowe, W.; Elliott, C.T.; Green, B.D. A Review of the In Vivo Evidence Investigating the Role of Nitrite Exposure from Processed Meat Consumption in the Development of Colorectal Cancer. Nutrients 2019, 11, 2673. [Google Scholar] [CrossRef]
- Schwarz, U.I.; Seemann, D.; Oertel, R.; Miehlke, S.; Kuhlisch, E.; Fromm, M.F.; Kim, R.B.; Bailey, D.G.; Kirch, W. Grapefruit Juice Ingestion Significantly Reduces Talinolol Bioavailability. Clin. Pharmacol. Ther. 2005, 77, 291–301. [Google Scholar] [CrossRef]
- Geraci, A.; Di Stefano, V.; Di Martino, E.; Schillaci, D.; Schicchi, R. Essential Oil Components of Orange Peels and Antimicrobial Activity. Nat. Prod. Res. 2017, 31, 653–659. [Google Scholar] [CrossRef]
- Deng, W.; Liu, K.; Cao, S.; Sun, J.; Zhong, B.; Chun, J. Chemical Composition, Antimicrobial, Antioxidant, and Antiproliferative Properties of Grapefruit Essential Oil Prepared by Molecular Distillation. Molecules 2020, 25, 217. [Google Scholar] [CrossRef]
- Ou, M.C.; Liu, Y.H.; Sun, Y.W.; Chan, C.F. The Composition, Antioxidant and Antibacterial Activities of Cold-Pressed and Distilled Essential Oils of Citrus paradisi and Citrus grandis (L.) Osbeck. Evid. Based Complement. Altern. Med. 2015, 2015, 804091. [Google Scholar] [CrossRef]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical Composition and Bioactivity of Different Oregano (Origanum Vulgare) Extracts and Essential Oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Upadhyaya, K. Characteristic Features and Comparative Analysis of Essential Oil Composition of Selected Genus of Ocimum sanctum L. through GC-MS. J. Vector Borne Dis. 2023, 60, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Babovic, N.; Djilas, S.; Jadranin, M.; Vajs, V.; Ivanovic, J.; Petrovic, S.; Zizovic, I. Supercritical Carbon Dioxide Extraction of Antioxidant Fractions from Selected Lamiaceae Herbs and Their Antioxidant Capacity. Innov. Food Sci. Emerg. Technol. 2010, 11, 98–107. [Google Scholar] [CrossRef]
- Radaelli, M.; da Silva, B.P.; Weidlich, L.; Hoehne, L.; Flach, A.; da Costa, L.A.M.A.; Ethur, E.M. Antimicrobial Activities of Six Essential Oils Commonly Used as Condiments in Brazil against Clostridium Perfringens. Braz. J. Microbiol. 2016, 47, 424–430. [Google Scholar] [CrossRef]
- Ksouri, S.; Djebir, S.; Bentorki, A.A.; Gouri, A.; Hadef, Y.; Benakhla, A. Antifungal Activity of Essential Oils Extract from Origanum floribundum Munby, Rosmarinus officinalis L. and Thymus ciliatus Desf. against Candida albicans Isolated from Bovine Clinical Mastitis. J. Mycol. Med. 2017, 27, 245–249. [Google Scholar] [CrossRef]
- Boskovic, M.; Djordjevic, J.; Glisic, M.; Ciric, J.; Janjic, J.; Zdravkovic, N.; Krnjaic, D.; Baltic, M.Z. The Effect of Oregano (Origanum vulgare) Essential Oil on Four Salmonella Serovars and Shelf Life of Refrigerated Pork Meat Packaged under Vacuum and Modified Atmosphere. J. Food Process. Preserv. 2020, 44, e14311. [Google Scholar] [CrossRef]
- Naghdibadi, H.; Abdollahi, M.; Mehrafarin, A.; Ghorbanpour, M.; Tolyat, S.; Qaderi, A.; Ghiaci Yekta, M. An Overview on Two Valuable Natural and Bioactive Compounds, Thymol and Carvacrol, in Medicinal Plants. J. Med. Plants 2017, 16, 1–32. [Google Scholar]
- Javidanpour, S.; Dianat, M.; Badavi, M.; Mard, S.A. The Cardioprotective Effect of Rosmarinic Acid on Acute Myocardial Infarction and Genes Involved in Ca2+homeostasis. Free Radic. Res. 2017, 51, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Aghdam, M.A.; Kafil, H.S. Carvacrol and Thymol: Strong Antimicrobial Agents against Resistant Isolates. Rev. Med. Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Wei, Y.; Pu, X.; Zhao, L. Preclinical Studies for the Combination of Paclitaxel and Curcumin in Cancer Therapy. Oncol. Rep. 2017, 37, 3159–3166. [Google Scholar] [CrossRef]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevão-Silva, C.F.; Carvalho, M.D.D.B.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of Thymol and Carvacrol, Constituents of Thymus vulgaris L. Essential Oil, on the Inflammatory Response. Evid.-Based Complement. Altern. Med. 2012, 2012, 657026. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The Antibacterial Mechanism of Carvacrol and Thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
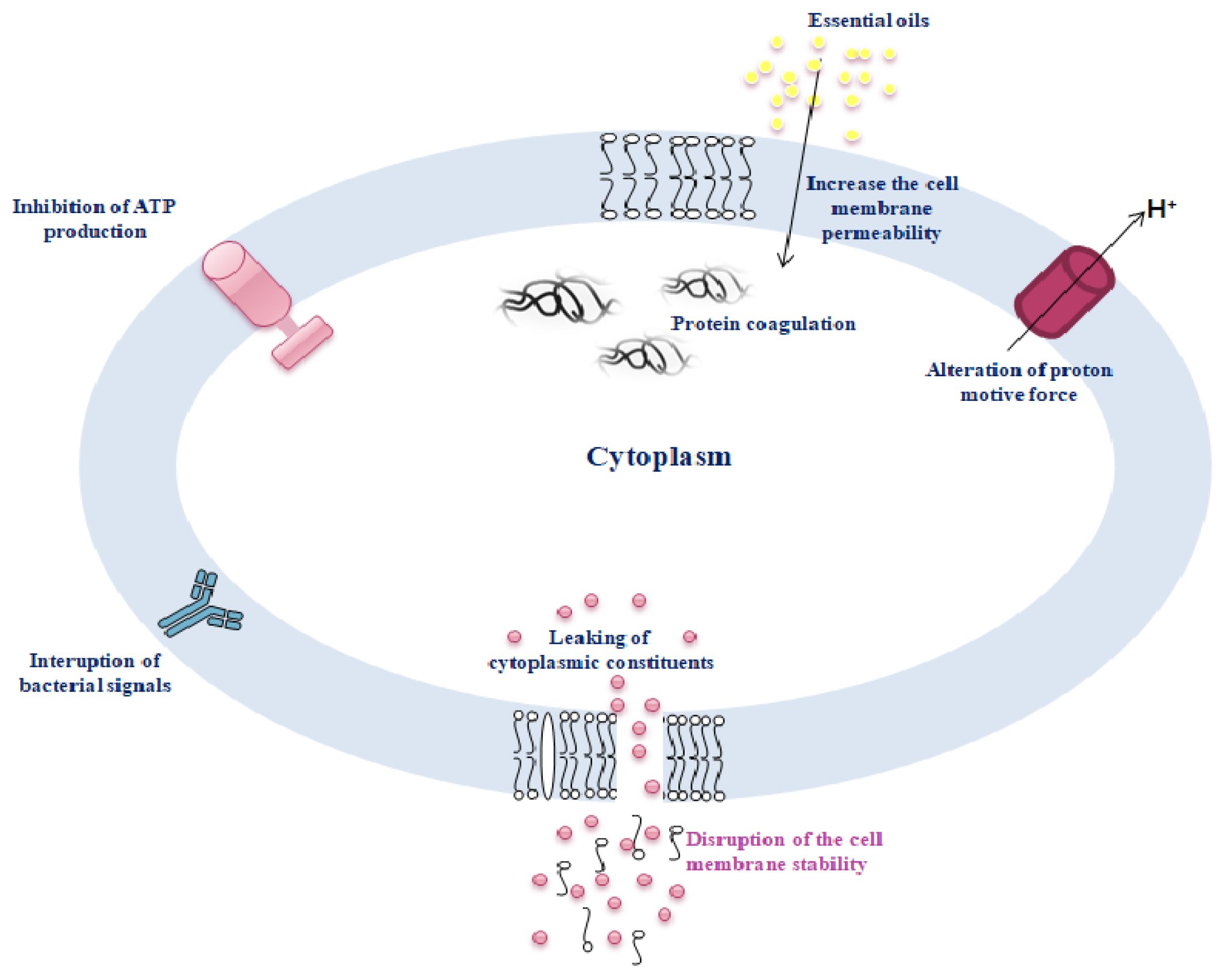
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Sokolik, C.G.; Ben-Shabat-Binyamini, R.; Gedanken, A.; Lellouche, J.P. Proteinaceous Microspheres as a Delivery System for Carvacrol and Thymol in Antibacterial Applications. Ultrason. Sonochem. 2018, 41, 288–296. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; Abd El-Hack, M.E. Using Essential Oils to Overcome Bacterial Biofilm Formation and Their Antimicrobial Resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156. [Google Scholar] [CrossRef]
- Bouyahya, A.; Abrini, J.; Dakka, N.; Bakri, Y. Essential Oils of Origanum Compactum Increase Membrane Permeability, Disturb Cell Membrane Integrity, and Suppress Quorum-Sensing Phenotype in Bacteria. J. Pharm. Anal. 2019, 9, 301–311. [Google Scholar] [CrossRef]
- Bouyahya, A.; Dakka, N.; Et-Touys, A.; Abrini, J.; Bakri, Y. Medicinal Plant Products Targeting Quorum Sensing for Combating Bacterial Infections. Asian Pac. J. Trop. Med. 2017, 10, 729–743. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, L.; Lu, H.; Zhu, J.; Kumar, V.; Liu, X. Transcriptomic Analysis of the Food Spoilers Pseudomonas Fluorescens Reveals the Antibiofilm of Carvacrol by Interference with Intracellular Signaling Processes. Food Control 2021, 127, 108115. [Google Scholar] [CrossRef]
- Rathod, N.B.; Kulawik, P.; Ozogul, F.; Regenstein, J.M.; Ozogul, Y. Biological Activity of Plant-Based Carvacrol and Thymol and Their Impact on Human Health and Food Quality. Trends Food Sci. Technol. 2021, 116, 733–748. [Google Scholar] [CrossRef]
- Martínez, A.; Manrique-Moreno, M.; Klaiss-Luna, M.C.; Stashenko, E.; Zafra, G.; Ortiz, C. Effect of Essential Oils on Growth Inhibition, Biofilm Formation and Membrane Integrity of Escherichia coli and Staphylococcus Aureus. Antibiotics 2021, 10, 1474. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical Composition, Antibacterial Activity and Mechanism of Action of Essential Oil from Seeds of Fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene against Listeria monocytogenes. Molecules 2020, 25, 33. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.N.; Rall, V.L.M.; Fernandes, A.A.H.; Ushimaru, P.I.; da Silva Probst, I.; Fernandes, A., Jr. Essential Oils Against Foodborne Pathogens and Spoilage Bacteria in Minced Meat. Foodborne Pathog. Dis. 2009, 6, 725. [Google Scholar] [CrossRef]
- Bukvicki, D.; Giweli, A.; Stojkovic, D.; Vujisic, L.; Tesevic, V.; Nikolic, M.; Sokovic, M.; Marin, P.D. Short Communication: Cheese Supplemented with Thymus algeriensis Oil, a Potential Natural Food Preservative. J. Dairy Sci. 2018, 101, 3859–3865. [Google Scholar] [CrossRef]
- Sessou, P.; Farougou, S.; Ahounou, S.; Hounnankpon, Y.; Azokpota, P.; Youssao, I.; Sohounhloue, D. Comparative Study of Antifungal Activities of Six Selected Essential Oils against Fungal Isolates from Cheese Wagashi in Benin. Pak. J. Biol. Sci. 2013, 16, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Kulabas, S.S.; Ipek, H.; Tufekci, A.R.; Arslan, S.; Demirtas, I.; Ekren, R.; Sezerman, U.; Tumer, T.B. Ameliorative Potential of Lavandula Stoechas in Metabolic Syndrome via Multitarget Interactions. J. Ethnopharmacol. 2018, 223, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ekpenyong, C.E.; Akpan, E.E. Use of Cymbopogon Citratus Essential Oil in Food Preservation: Recent Advances and Future Perspectives. Crit. Rev. Food Sci. Nutr. 2017, 57, 2541–2559. [Google Scholar] [CrossRef]
- Nardoni, S.; D’Ascenzi, C.; Caracciolo, I.; Mannaioni, G.; Amerigo Papini, R.; Pistelli, L.; Najar, B.; Mancianti, F. Activity of Selected Essential Oils on Spoiling Fungi Cultured from Marzolino Cheese. Ann. Agric. Environ. Med. 2018, 25, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Cefola, M.; Bonifacio, M.A.; Cometa, S.; Bocchino, C.; Pace, B.; De Giglio, E.; Palumbo, M.; Sada, A.; Logrieco, A.F.; et al. Effect of Red Thyme Oil (Thymus vulgaris L.) Vapours on Fungal Decay, Quality Parameters and Shelf-Life of Oranges during Cold Storage. Food Chem. 2021, 336, 127590. [Google Scholar] [CrossRef]
- Juneja, V.K.; Dwivedi, H.P.; Yan, X. Novel Natural Food Antimicrobials. Annu. Rev. Food Sci. Technol. 2012, 3, 381–403. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential Oils and Their Application in Food Safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Soliva-Fortuny, R.; Martín-Belloso, O. Control of Pathogenic and Spoilage Microorganisms in Fresh-Cut Fruits and Fruit Juices by Traditional and Alternative Natural Antimicrobials. Compr. Rev. Food Sci. Food Saf. 2009, 8, 157–180. [Google Scholar] [CrossRef]
- Fischer, S.W.; Titgemeyer, F. Protective Cultures in Food Products: From Science to Market. Foods 2023, 12, 1541. [Google Scholar] [CrossRef]
- Ito, A.; Sato, Y.; Kudo, S.; Sato, S.; Nakajima, H.; Toba, T. The Screening of Hydrogen Peroxide-Producing Lactic Acid Bacteria and Their Application to Inactivating Psychrotrophic Food-Borne Pathogens. Curr. Microbiol. 2003, 47, 231–236. [Google Scholar] [CrossRef]
- Lanciotti, R.; Patrignani, F.; Bagnolini, F.; Guerzoni, M.E.; Gardini, F. Evaluation of Diacetyl Antimicrobial Activity against Escherichia coli, Listeria monocytogenes and Staphylococcus Aureus. Food Microbiol. 2003, 20, 537–543. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative Strategies Based on the Use of Bio-Control Agents to Improve the Safety, Shelf-Life and Quality of Minimally Processed Fruits and Vegetables. Trends Food Sci. Technol. 2015, 46, 302–310. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins-a Viable Alternative to Antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Castellano, P.; Ibarreche, M.P.; Massani, M.B.; Fontana, C.; Vignolo, G.M. Strategies for Pathogen Biocontrol Using Lactic Acid Bacteria and Their Metabolites: A Focus on Meat Ecosystems and Industrial Environments. Microorganisms 2017, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Beyond Biopreservatives, Bacteriocins Biotechnological Applications: History, Current Status, and Promising Potentials. Biocatal. Agric. Biotechnol. 2022, 39, 102248. [Google Scholar] [CrossRef]
- Šimat, V.; Čagalj, M.; Skroza, D.; Gardini, F.; Tabanelli, G.; Montanari, C.; Hassoun, A.; Ozogul, F. Sustainable Sources for Antioxidant and Antimicrobial Compounds Used in Meat and Seafood Products. Adv. Food Nutr. Res. 2021, 97, 55–118. [Google Scholar] [CrossRef]
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from Lactic Acid Bacteria and Their Applications in Meat and Meat Products. Meat Sci. 2016, 120, 118–132. [Google Scholar] [CrossRef]
- Iseppi, R.; Camellini, S.; Sabia, C.; Messi, P. Combined Antimicrobial Use of Essential Oils and Bacteriocin BacLP17 as Seafood Biopreservative to Control Listeria monocytogenes Both in Planktonic and in Sessile Forms. Res. Microbiol. 2020, 171, 351–356. [Google Scholar] [CrossRef]
- Turgis, M.; Vu, K.D.; Dupont, C.; Lacroix, M. Combined Antimicrobial Effect of Essential Oils and Bacteriocins against Foodborne Pathogens and Food Spoilage Bacteria. Food Res. Int. 2012, 48, 696–702. [Google Scholar] [CrossRef]
- Gaamouche, S.; Arakrak, A.; Bakkali, M.; Laglaoui, A. Combined Antimicrobial Effect of Bacteriocins of LAB Isolated from a Traditional Brine Table Olives and Essential Oils against Foodborne Pathogens. Moroccan J. Chem. 2021, 9, 464–473. [Google Scholar]
- Sharafati-chaleshtori, R.; Rokni, N.; Rafieian-kopaei, M.; Drees, F.; Sharafati-Chaleshtori, A.; Salehi, E. Use of Tarragon (Artemisia dracunculus) Essential Oil as a Natural Preservative in Beef Burger. Ital. J. Food Sci. 2014, 26, 427–432. [Google Scholar]
- Yadav, A.; Singh, R. Natural Preservatives in Poultry Meat Products. Nat. Prod. Rad. 2004, 3, 300–303. [Google Scholar]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Diallyl Sulfide Content and Antimicrobial Activity against Food-Borne Pathogenic Bacteria of Chives (Allium schoenoprasum). Biosci. Biotechnol. Biochem. 2008, 72, 2987–2991. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, T.; Mojaddar Langroodi, A.; Shakouri, R.; Khorshidi, S. Physicochemical, Microbiological, and Sensory Characteristics of Probiotic Yogurt Enhanced with Anethum Graveolens Essential Oil. J. Food Saf. 2019, 39, e12683. [Google Scholar] [CrossRef]
- Radha Krishnan, K.; Babuskin, S.; Azhagu Saravana Babu, P.; Sasikala, M.; Sabina, K.; Archana, G.; Sivarajan, M.; Sukumar, M. Antimicrobial and Antioxidant Effects of Spice Extracts on the Shelf Life Extension of Raw Chicken Meat. Int. J. Food Microbiol. 2014, 171, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Tomović, V.; Šojić, B.; Savanović, J.; Kocić-Tanackov, S.; Pavlić, B.; Jokanović, M.; Đorđević, V.; Parunović, N.; Martinović, A.; Vujadinović, D. Caraway (Carum carvi L.) Essential Oil Improves Quality of Dry-Fermented Sausages Produced with Different Levels of Sodium Nitrite. J. Food Process. Preserv. 2021, 46, e15786. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, B.; Abbasi, A.; Mazloomi, S.M. The Effect of Saffron on Microbial, Physicochemical and Texture Profile of Chicken (Breast) Meat Stored in Refrigerator. Int. J. Nutr. Sci. 2018, 3, 164–170. [Google Scholar]
- Bojorges, H.; Ríos-Corripio, M.A.; Hernández-Cázares, A.S.; Hidalgo-Contreras, J.V.; Contreras-Oliva, A. Effect of the Application of an Edible Film with Turmeric (Curcuma longa L.) on the Oxidative Stability of Meat. Food Sci. Nutr. 2020, 8, 4308. [Google Scholar] [CrossRef]
- Mancini, S.; Paci, G.; Pisseri, F.; Preziuso, G. Effect of Turmeric (Curcuma longa L.) Powder as Dietary Antioxidant Supplementation on Pig Meat Quality. J. Food Process. Preserv. 2017, 41, e12878. [Google Scholar] [CrossRef]
- Hernández-Ochoa, L.; Aguirre-Prieto, Y.B.; Nevárez-Moorillón, G.V.; Gutierrez-Mendez, N.; Salas-Muñoz, E. Use of Essential Oils and Extracts from Spices in Meat Protection. J. Food Sci. Technol. 2014, 51, 957. [Google Scholar] [CrossRef]
- Hartanti, D.; Haqqi, M.Z.U.; Hamad, A. Potency of Combination of Essential Oils of Ginger and Lemongrass as Fresh Chicken Meat Natural Preservative. Adv. Sci. Lett. 2018, 24, 91–94. [Google Scholar] [CrossRef]
- Sayyari, Z.; Rabani, M.; Farahmandfar, R.; Kenari, R.E.; Nadoshan, R.M. The Effect of Nanocomposite Edible Coating Enriched with Foeniculum vulgare Essential Oil on the Shelf Life of Oncorhynchus Mykiss Fish Fillets during the Storage. J. Aquat. Food Prod. Technol. 2021, 30, 579–595. [Google Scholar] [CrossRef]
- Michalczyk, M.; Macura, R.; Tesarowicz, I.; Banaś, J. Effect of Adding Essential Oils of Coriander (Coriandrum sativum L.) and Hyssop (Hyssopus officinalis L.) on the Shelf Life of Ground Beef. Meat Sci. 2012, 90, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wu, X.; Xie, J.; Zhang, H.; Yang, H.; Zeng, Q.; Yang, X.; Xie, W. Kaempferia Galanga Linn. Extract—A Potential Antibacterial Agent for Preservation of Poultry Products. LWT 2021, 147, 111553. [Google Scholar] [CrossRef]
- Mahmoud, S.H.; Wafa, M.M. The Antibacterial Activity of Laurus Nobilis Leaf Extract and Its Potential Use as a Preservative for Fresh Lamb Meat. Afr. J. Microbiol. Res. 2020, 14, 617–624. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Castillo-Hernández, G.; González-Gutiérrez, C.J.; Silva-Dávila, A.J.; Gracida-Rodríguez, J.N.; García-Almendárez, B.E.; Pierro, P.D.; Vázquez-Landaverde, P.; Regalado-González, C. Microbiological and Physicochemical Properties of Meat Coated with Microencapsulated Mexican Oregano (Lippia graveolens Kunth) and Basil (Ocimum basilicum L.) Essential Oils Mixture. Coatings 2019, 9, 414. [Google Scholar] [CrossRef]
- Smaoui, S.; Hsouna, A.B.; Lahmar, A.; Ennouri, K.; Mtibaa-Chakchouk, A.; Sellem, I.; Najah, S.; Bouaziz, M.; Mellouli, L. Bio-Preservative Effect of the Essential Oil of the Endemic Mentha piperita Used Alone and in Combination with BacTN635 in Stored Minced Beef Meat. Meat Sci. 2016, 117, 196–204. [Google Scholar] [CrossRef]
- Raeisi, M.; Hashemi, M.; Ansarian, E.; Hejazi, J.; Hassanzad Azar, H.; Daneshamooz, S.; Jannat, B.; Aminzare, M. Antibacterial Effect of Mentha Piperita Essential Oil Against Foodborne Pathogens in Minced Meat During Storage at Abuse Refrigeration Temperature. Adv. Anim. Vet. Sci. 2019, 7, 720–726. [Google Scholar] [CrossRef]
- Fratianni, F.; De Martino, L.; Melone, A.; De Feo, V.; Coppola, R.; Nazzaro, F. Preservation of Chicken Breast Meat Treated with Thyme and Balm Essential Oils. J. Food Sci. 2010, 75, M528–M535. [Google Scholar] [CrossRef]
- Mohammadi, L.; Tanaka, F.; Tanaka, F. Preservation of Strawberry Fruit with an Aloe Vera Gel and Basil (Ocimum basilicum) Essential Oil Coating at Ambient Temperature. J. Food Process. Preserv. 2021, 45, e15836. [Google Scholar] [CrossRef]
- Anthony, S.; Abeywickrama, K.; Wijeratnam, S.W. The Effect of Spraying Essential Oils of Cymbopogon nardus, Cymbopogon flexuosus and Ocimum basilicum on Postharvest Diseases and Storage Life of Embul Banana. J. Hortic. Sci. Biotechnol. 2003, 78, 780–785. [Google Scholar] [CrossRef]
- Das, A.K.; Rajkumar, V.; Dwivedi, D.K. Antioxidant Effect of Curry Leaf (Murraya koenigii) Powder on Quality of Ground and Cooked Goat Meat. Int. Food Res. J. 2011, 18, 563–569. [Google Scholar]
- Kiarsi, Z.; Hojjati, M.; Behbahani, B.A.; Noshad, M. In Vitro Antimicrobial Effects of Myristica Fragrans Essential Oil on Foodborne Pathogens and Its Influence on Beef Quality during Refrigerated Storage. J. Food Saf. 2020, 40, e12782. [Google Scholar] [CrossRef]
- Ozpolat, E.; Duman, M. Effect of Black Cumin Oil (Nigella sativa L.) on Fresh Fish (Barbus grypus) Fillets during Storage at 2 ± 1 °C. Food Sci. Technol. 2016, 37, 148–152. [Google Scholar] [CrossRef]
- Bhargava, K.; Conti, D.S.; da Rocha, S.R.P.; Zhang, Y. Application of an Oregano Oil Nanoemulsion to the Control of Foodborne Bacteria on Fresh Lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef]
- Hulankova, R.; Borilova, G.; Steinhauserova, I. Combined Antimicrobial Effect of Oregano Essential Oil and Caprylic Acid in Minced Beef. Meat Sci. 2013, 95, 190–194. [Google Scholar] [CrossRef]
- Fathi-Achachlouei, B.; Babolanimogadam, N.; Zahedi, Y. Influence of Anise (Pimpinella anisum L.) Essential Oil on the Microbial, Chemical, and Sensory Properties of Chicken Fillets Wrapped with Gelatin Film. Food Sci. Technol. Int. 2021, 27, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Gulmez, M.; Oral, N.; Vatansever, L. The Effect of Water Extract of Sumac (Rhus coriaria L.) and Lactic Acid on Decontamination and Shelf Life of Raw Broiler Wings. Poult. Sci. 2006, 85, 1466–1471. [Google Scholar] [CrossRef]
- Sirocchi, V.; Devlieghere, F.; Peelman, N.; Sagratini, G.; Maggi, F.; Vittori, S.; Ragaert, P. Effect of Rosmarinus officinalis L. Essential Oil Combined with Different Packaging Conditions to Extend the Shelf Life of Refrigerated Beef Meat. Food Chem. 2017, 221, 1069–1076. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Srour, T.M.; Abdallah, A.E. The Effect of Sage Essential Oil on the Compositional Quality of Anchovy Fish Burger During Freeze Storage. J. Adv. Agric. Res. 2019, 24, 534–557. [Google Scholar] [CrossRef]
- Hassan, A.A.; Rasmy, N.M.; Foda, I.; El-Moghazy, M.M. Assessment of the Antioxidant Activity of Sage (Salvia officinalis L.) Extracts on the Shelf Life of Mayonnaise. World J. Dairy Food Sci. 2012, 7, 28–40. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Jafarie, S.; Kaboudari, A. Effect of Salvia Officinalis L. Extract on Chemical, Microbial, Sensory and Shelf Life of Rainbow Trout Fillet. Food Sci. Biotechnol. 2019, 28, 1499. [Google Scholar] [CrossRef]
- Cegiełka, A.; Hać-Szymańczuk, E.; Piwowarek, K.; Dasiewicz, K.; Słowiński, M.; Wrońska, K. The Use of Bioactive Properties of Sage Preparations to Improve the Storage Stability of Low-Pressure Mechanically Separated Meat from Chickens. Poult. Sci. 2019, 98, 5045–5053. [Google Scholar] [CrossRef] [PubMed]
- Jokanović, M.; Ivić, M.; Škaljac, S.; Tomović, V.; Pavlić, B.; Šojić, B.; Zeković, Z.; Peulić, T.; Ikonić, P. Essential Oil and Supercritical Extracts of Winter Savory (Satureja montana L.) as Antioxidants in Precooked Pork Chops during Chilled Storage. LWT 2020, 134, 110260. [Google Scholar] [CrossRef]
- Hernández, A.; García García, B.; Jordán, M.J.; Hernández, M.D. Study of the Dose of Thyme Essential Oil in Feed to Prolong the Shelf Life of Gilthead Seabream (Sparus aurata). Aquac. Nutr. 2015, 21, 740–749. [Google Scholar] [CrossRef]
- Jayari, A.; Jouini, A.; Boukhris, H.; Hamrouni, S.; Damergi, C.; Ben Hadj Ahmed, S.; Maaroufi, A. Essential Oils from Thymus Capitatus and Thymus algeriensis as Antimicrobial Agents to Control Pathogenic and Spoilage Bacteria in Ground Meat. J. Food Qual. 2021, 2021, 5599374. [Google Scholar] [CrossRef]
- Karimnezhad, F.; Razavilar, V.; Anvar, A.A.; Eskandari, S. Study the Antimicrobial Effects of Chitosan-Based Edible Film Containing the Trachyspermum Ammi Essential Oil on Shelf-Life of Chicken Meat. Microbiol. Res. 2017, 8, 7226. [Google Scholar] [CrossRef]
- Al-Fadhly, N.K.Z. Extraction, Characterization of Essential Oils of Ginger (Zingiber officinale Rosco) and Study the Effectiveness of Antimicrobial on Shelf Life Extension of Minced Beef Meat. Basrah J. Agric. Sci. 2016, 29, 523–535. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Tappi, S.; Rocculi, P.; Gardini, F.; Lanciotti, R. Efficacy of Natural Antimicrobials to Prolong the Shelf-Life of Minimally Processed Apples Packaged in Modified Atmosphere. Food Control 2014, 46, 403–411. [Google Scholar] [CrossRef]
- De Azeredo, G.A.; Stamford, T.L.M.; Nunes, P.C.; Gomes Neto, N.J.; De Oliveira, M.E.G.; De Souza, E.L. Combined Application of Essential Oils from Origanum vulgare L. and Rosmarinus officinalis L. to Inhibit Bacteria and Autochthonous Microflora Associated with Minimally Processed Vegetables. Food Res. Int. 2011, 44, 1541–1548. [Google Scholar] [CrossRef]
- Abadias, M.; Alegre, I.; Usall, J.; Torres, R.; Viñas, I. Evaluation of Alternative Sanitizers to Chlorine Disinfection for Reducing Foodborne Pathogens in Fresh-Cut Apple. Postharvest Biol. Technol. 2011, 3, 289–297. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple Puree-Alginate Edible Coating as Carrier of Antimicrobial Agents to Prolong Shelf-Life of Fresh-Cut Apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Singh, N.; Singh, R.K.; Bhunia, A.K.; Stroshine, R.L. Efficacy of Chlorine Dioxide, Ozone, and Thyme Essential Oil or a Sequential Washing in Killing Escherichia coli O157:H7 on Lettuce and Baby Carrots. LWT Food Sci. Technol. 2002, 35, 720–729. [Google Scholar] [CrossRef]
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Jadhav, S.R. Encapsulation of Essential Oils and Their Application in Antimicrobial Active Packaging. Food Control 2022, 136, 108883. [Google Scholar] [CrossRef]
- Valero, D.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Castillo, S.; Serrano, M. The Combination of Modified Atmosphere Packaging with Eugenol or Thymol to Maintain Quality, Safety and Functional Properties of Table Grapes. Postharvest Biol. Technol. 2006, 41, 317–327. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Lopez-Carballo, G.; Catala, R.; Gavara, R.; Hernandez-Munoz, P. Antifungal Properties of Gliadin Films Incorporating Cinnamaldehyde and Application in Active Food Packaging of Bread and Cheese Spread Foodstuffs. Int. J. Food Microbiol. 2013, 166, 369–377. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Rusu, A.G.; Nita, L.E.; Chiriac, V.M.; Neamtu, I.; Sandu, A. Polymeric Carriers Designed for Encapsulation of Essential Oils with Biological Activity. Pharmaceutics 2021, 13, 631. [Google Scholar] [CrossRef]
- Belletti, N.; Lanciotti, R.; Patrignani, F.; Gardini, F. Antimicrobial Efficacy of Citron Essential Oil on Spoilage and Pathogenic Microorganisms in Fruit-Based Salads. J. Food Sci. 2008, 73, M331–M338. [Google Scholar] [CrossRef]
- Lanciotti, R.; Gianotti, A.; Patrignani, F.; Belletti, N.; Guerzoni, M.E.; Gardini, F. Use of Natural Aroma Compounds to Improve Shelf-Life and Safety of Minimally Processed Fruits. Trends Food Sci. Technol. 2004, 15, 201–208. [Google Scholar] [CrossRef]
- Serrano, M.; Martínez-Romero, D.; Castillo, S.; Guillén, F.; Valero, D. The Use of Natural Antifungal Compounds Improves the Beneficial Effect of MAP in Sweet Cherry Storage. Innov. Food Sci. Emerg. Technol. 2005, 6, 115–123. [Google Scholar] [CrossRef]
- Roller, S.; Seedhar, P. Carvacrol and Cinnamic Acid Inhibit Microbial Growth in Fresh-Cut Melon and Kiwifruit at 4° and 8 °C. Lett. Appl. Microbiol. 2002, 35, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Muriel-Galet, V.; Cerisuelo, J.P.; López-Carballo, G.; Aucejo, S.; Gavara, R.; Hernández-Muñoz, P. Evaluation of EVOH-Coated PP Films with Oregano Essential Oil and Citral to Improve the Shelf-Life of Packaged Salad. Food Control 2013, 30, 137–143. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Mafune, M.; Sivakumar, D.; Soundy, P. Thyme Oil Vapour and Modified Atmosphere Packaging Reduce Anthracnose Incidence and Maintain Fruit Quality in Avocado. J. Sci. Food Agric. 2013, 93, 3024–3031. [Google Scholar] [CrossRef]
- Lanciotti, R.; Belletti, N.; Patrignani, F.; Gianotti, A.; Gardini, F.; Guerzoni, M.E. Application of Hexanal, (E)-2-Hexenal, and Hexyl Acetate To Improve the Safety of Fresh-Sliced Apples. J. Agric. Food Chem. 2003, 51, 2958–2963. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Vernocchi, P.; Del Chierico, F.; Russo, A.; Torriani, S.; Putignani, L.; Gardini, F.; Lanciotti, R. Effect of Thyme Essential Oil and Lactococcus Lactis CBM21 on the Microbiota Composition and Quality of Minimally Processed Lamb’s Lettuce. Food Microbiol. 2017, 68, 61–70. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tappi, S.; Rocculi, P.; Gardini, F.; Lanciotti, R. Natural Antimicrobials to Prolong the Shelf-Life of Minimally Processed Lamb’s Lettuce. Postharvest Biol. Technol. 2015, 103, 35–44. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F.; Lanciotti, R. Lactic Acid Bacteria and Natural Antimicrobials to Improve the Safety and Shelf-Life of Minimally Processed Sliced Apples and Lamb’s Lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Nychas, G.J.E. Development and Evaluation of a Model Predicting the Survival of Escherichia coli O157:H7 NCTC 12900 in Homemade Eggplant Salad at Various Temperatures, PHs, and Oregano Essential Oil Concentrations. Appl. Environ. Microbiol. 2000, 66, 1646–1653. [Google Scholar] [CrossRef]
- Gündüz, G.T.; Gönül, Ş.A.; Karapinar, M. Efficacy of Oregano Oil in the Inactivation of Salmonella Typhimurium on Lettuce. Food Control 2010, 21, 513–517. [Google Scholar] [CrossRef]
- Charfi, S.; Boujida, N.; El Moussaoui, N.; Abrini, J.; Senhaji, N.S. Thymbra Capitata Essential Oil Use to Preserve Physicochemical and Microbiological Qualities of Pomegranate Juice. Food Sci. Technol. Res. 2019, 25, 257–263. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E. Antibacterial Activities of Plant Essential Oils and Their Components against Escherichia coli O157:H7 and Salmonella Enterica in Apple Juice. J. Agric. Food Chem. 2004, 52, 6042–6048. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rubio, M.; Taboada-Rodríguez, A.; Cava-Roda, R.; López-Gómez, A.; Marín-Iniesta, F. Combined Use of Thermo-Ultrasound and Cinnamon Leaf Essential Oil to Inactivate Saccharomyces Cerevisiae in Natural Orange and Pomegranate Juices. Lwt 2016, 73, 140–146. [Google Scholar] [CrossRef]
- Krisch, J.; Tserennadmid, R.; Vágvölgyi, C. Essential Oils against Yeasts and Moulds Causing Food Spoilage. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 1135–1142. [Google Scholar]
- Gutierrez, J.; Bourke, P.; Lonchamp, J.; Barry-Ryan, C. Impact of Plant Essential Oils on Microbiological, Organoleptic and Quality Markers of Minimally Processed Vegetables. Innov. Food Sci. Emerg. Technol. 2009, 10, 195–202. [Google Scholar] [CrossRef]
- Hadian, M.; Rajaei, A.; Mohsenifar, A.; Tabatabaei, M. Encapsulation of Rosmarinus officinalis Essential Oils in Chitosan-Benzoic Acid Nanogel with Enhanced Antibacterial Activity in Beef Cutlet against Salmonella Typhimurium during Refrigerated Storage. LWT 2017, 84, 394–401. [Google Scholar] [CrossRef]
- Pálmai, M.; Buchanan, R.L. The Effect of Lactococcus Lactis on the Growth Characteristics of Listeria monocytogenes in Alfalfa Sprout Broth. Acta Aliment. 2002, 31, 379–392. [Google Scholar] [CrossRef]
- Mostafavi, H.A.; Mirmajlessi, S.M.; Mirjalili, S.M.; Fathollahi, H.; Askari, H. Gamma Radiation Effects on Physico-Chemical Parameters of Apple Fruit during Commercial Post-Harvest Preservation. Radiat. Phys. Chem. 2012, 81, 666–671. [Google Scholar] [CrossRef]
- Alegre, I.; Viñas, I.; Usall, J.; Teixidó, N.; Figge, M.J.; Abadias, M. Control of Foodborne Pathogens on Fresh-Cut Fruit by a Novel Strain of Pseudomonas Graminis. Food Microbiol. 2013, 34, 390–399. [Google Scholar] [CrossRef]
- Leverentz, B.; Conway, W.S.; Janisiewicz, W.J.; Saftner, R.A.; Camp, M.J. Effect of Combining MCP Treatment, Heat Treatment, and Biocontrol on the Reduction of Postharvest Decay of ‘Golden Delicious’ Apples. Postharvest Biol. Technol. 2003, 27, 221–233. [Google Scholar] [CrossRef]
- Trias, R.; Badosa, E.; Montesinos, E.; Bañeras, L. Bioprotective Leuconostoc Strains against Listeria monocytogenes in Fresh Fruits and Vegetables. Int. J. Food Microbiol. 2008, 127, 91–98. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Vannini, L.; Salvetti, E.; Torriani, S.; Gardini, F.; Lanciotti, R. Use of a Nisin-Producing Lactococcus Lactis Strain, Combined with Natural Antimicrobials, to Improve the Safety and Shelf-Life of Minimally Processed Sliced Apples. Food Microbiol. 2016, 54, 11–19. [Google Scholar] [CrossRef]
- Devatkal, S.K.; Thorat, P.; Manjunatha, M. Effect of Vacuum Packaging and Pomegranate Peel Extract on Quality Aspects of Ground Goat Meat and Nuggets. J. Food Sci. Technol. 2014, 51, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical Constituents, Advanced Extraction Technologies and Techno-Functional Properties of Selected Mediterranean Plants for Use in Meat Products. A Comprehensive Review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Aminzare, M.; Hashemi, M.; Ansarian, E.; Bimkar, M.; Azar, H.H.; Mehrasbi, M.R.; Daneshamooz, S.; Raeisi, M.; Jannat, B.; Afshari, A. Using Natural Antioxidants in Meat and Meat Products as Preservatives: A Review. Adv. Anim. Vet. Sci. 2019, 7, 417–426. [Google Scholar] [CrossRef]
- Bukvički, D.; Stojković, D.; Soković, M.; Vannini, L.; Montanari, C.; Pejin, B.; Savić, A.; Veljić, M.; Grujić, S.; Marin, P.D. Satureja Horvatii Essential Oil: In Vitro Antimicrobial and Antiradical Properties and in Situ Control of Listeria monocytogenes in Pork Meat. Meat Sci. 2014, 96, 1355–1360. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus, G. Antimicrobial Activity of Whey Protein Based Edible Films Incorporated with Oregano, Rosemary and Garlic Essential Oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant Activity in Meat Treated with Oregano and Sage Essential Oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Estévez, M.; Ramírez, R.; Ventanas, S.; Cava, R. Sage and Rosemary Essential Oils versus BHT for the Inhibition of Lipid Oxidative Reactions in Liver Pâté. LWT Food Sci. Technol. 2007, 40, 58–65. [Google Scholar] [CrossRef]
- Chivandi, E.; Dangarembizi, R.; Nyakudya, T.T.; Erlwanger, K.H. Chapter 8—Use of Essential Oils as a Preservative of Meat. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 85–91. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial Populations and the Volatilome Associated to Meat Spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.J.E. Spoilage Microbiota Associated to the Storage of Raw Meat in Different Conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Pennacchia, C.; Ercolini, D.; Villani, F. Spoilage-Related Microbiota Associated with Chilled Beef Stored in Air or Vacuum Pack. Food Microbiol. 2011, 28, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Heredia, N.; García, S. Animals as Sources of Food-Borne Pathogens: A Review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef]
- Hu, J.; Li, Z.; Xu, L.T.; Sun, A.J.; Fu, X.Y.; Zhang, L.; Jing, L.L.; Lu, A.D.; Dong, Y.F.; Jia, Z.P. Protective Effect of Apigenin on Ischemia/Reperfusion Injury of the Isolated Rat Heart. Cardiovasc. Toxicol. 2015, 15, 241–249. [Google Scholar] [CrossRef]
- Shiningeni, D.; Chimwamurombe, P.; Shilangale, R.; Misihairabgwi, J. Prevalence of Pathogenic Bacteria in Street Vended Ready-to-Eat Meats in Windhoek, Namibia. Meat Sci. 2019, 148, 223–228. [Google Scholar] [CrossRef]
- Bertoli, A.; Cüneyt Çõrak, Ç.; Teixeira Da Silva, J.A. Hypericum Species as Sources of Valuable Essential Oils. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 29–47. [Google Scholar]
- Dussault, D.; Vu, K.D.; Lacroix, M. In Vitro Evaluation of Antimicrobial Activities of Various Commercial Essential Oils, Oleoresin and Pure Compounds against Food Pathogens and Application in Ham. Meat Sci. 2014, 96, 514–520. [Google Scholar] [CrossRef]
- Hussain, Z.; Li, X.; Zhang, D.; Hou, C.; Ijaz, M.; Bai, Y.; Xiao, X.; Zheng, X. Influence of Adding Cinnamon Bark Oil on Meat Quality of Ground Lamb during Storage at 4 °C. Meat Sci. 2021, 171, 108269. [Google Scholar] [CrossRef]
- Amariei, S.; Poroch- Serițan, M.; Gutt, G.; Oroian, M.; Ciornei, E. Rosemary, thyme and oregano essential oils influence on physicochemical properties and microbiological stability of minced meat. J. Microbiol. Biotechnol. Food Sci. 2016, 2017, 670–676. [Google Scholar] [CrossRef]
- Sariçoban, C.; Yilmaz, M.T. Effect of Thyme/Cumin Essential Oils and Butylated Hydroxyl Anisole/Butylated Hydroxyl Toluene on Physicochemical Properties and Oxidative/Microbial Stability of Chicken Patties. Poult. Sci. 2014, 93, 456–463. [Google Scholar] [CrossRef]
- Sultanbawa, Y. Plant Antimicrobials in Food Applications: Minireview. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; Volume 2, pp. 1084–1093. [Google Scholar]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active Packaging Films with Natural Antioxidants to Be Used in Meat Industry: A Review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, M.; Caillet, S.; Salmiéri, S.; Saucier, L.; Lacroix, M. Antimicrobial and Antioxidant Effects of Milk Protein-Based Film Containing Essential Oils for the Preservation of Whole Beef Muscle. J. Agric. Food Chem. 2004, 52, 5598–5605. [Google Scholar] [CrossRef]
- Catarino, M.D.; Alves-Silva, J.M.; Fernandes, R.P.; Gonçalves, M.J.; Salgueiro, L.R.; Henriques, M.F.; Cardoso, S.M. Development and Performance of Whey Protein Active Coatings with Origanum Virens Essential Oils in the Quality and Shelf Life Improvement of Processed Meat Products. Food Control 2017, 80, 273–280. [Google Scholar] [CrossRef]
- Krkić, N.; Šojić, B.; Lazić, V.; Petrović, L.; Mandić, A.; Sedej, I.; Tomović, V. Lipid Oxidative Changes in Chitosan-Oregano Coated Traditional Dry Fermented Sausage Petrovská Klobása. Meat Sci. 2013, 93, 767–770. [Google Scholar] [CrossRef]
- Siroli, L.; Baldi, G.; Soglia, F.; Bukvicki, D.; Patrignani, F.; Petracci, M.; Lanciotti, R. Use of Essential Oils to Increase the Safety and the Quality of Marinated Pork Loin. Foods 2020, 9, 987. [Google Scholar] [CrossRef]
- Van Haute, S.; Raes, K.; Van der Meeren, P.; Sampers, I. The Effect of Cinnamon, Oregano and Thyme Essential Oils in Marinade on the Microbial Shelf Life of Fish and Meat Products. Food Control 2016, 68, 30–39. [Google Scholar] [CrossRef]
- Oliveira, M.; Ferreira, V.; Magalhães, R.; Teixeira, P. Biocontrol Strategies for Mediterranean-Style Fermented Sausages. Food Res. Int. 2018, 103, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, L.; Gaudreau, H.; Dallaire, L.; Tessier, M.; Fliss, I. Bioprotective Culture: A New Generation of Food Additives for the Preservation of Food Quality and Safety. Ind. Biotechnol. 2019, 15, 138–147. [Google Scholar] [CrossRef]
- Favaro, L.; Todorov, S.D. Bacteriocinogenic LAB Strains for Fermented Meat Preservation: Perspectives, Challenges, and Limitations. Probiotics Antimicrob. Proteins 2017, 9, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Quintavalla, S.; Vicini, L. Antimicrobial Food Packaging in Meat Industry. Meat Sci. 2002, 62, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.L.; Sporns, P.; Stiles, M.E.; McMullen, L.M. Inactivation of Nisin by Glutathione in Fresh Meat. J. Food Sci. 1999, 64, 759–762. [Google Scholar] [CrossRef]
- Pattanayaiying, R.; H-Kittikun, A.; Cutter, C.N. Incorporation of Nisin Z and Lauric Arginate into Pullulan Films to Inhibit Foodborne Pathogens Associated with Fresh and Ready-to-Eat Muscle Foods. Int. J. Food Microbiol. 2015, 207, 77–82. [Google Scholar] [CrossRef]
- Millette, M.; Le Tien, C.; Smoragiewicz, W.; Lacroix, M. Inhibition of Staphylococcus Aureus on Beef by Nisin-Containing Modified Alginate Films and Beads. Food Control 2007, 18, 878–884. [Google Scholar] [CrossRef]
- Luchansky, J.B.; Call, J.E. Evaluation of Nisin-Coated Cellulose Casings for the Control of Listeria monocytogenes Inoculated onto the Surface of Commercially Prepared Frankfurters. J. Food Prot. 2004, 67, 1017–1021. [Google Scholar] [CrossRef]
- Franklin, N.B.; Cooksey, K.D.; Getty, K.J.K. Inhibition of Listeria monocytogenes on the Surface of Individually Packaged Hot Dogs with a Packaging Film Coating Containing Nisin. J. Food Prot. 2004, 67, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Scannell, A.G.M.; Hill, C.; Ross, R.P.; Marx, S.; Hartmeier, W.; Arendt, E.K. Development of Bioactive Food Packaging Materials Using Immobilised Bacteriocins Lacticin 3147 and Nisaplin. Int. J. Food Microbiol. 2000, 60, 241–249. [Google Scholar] [CrossRef]
- Ghalfi, H.; Benkerroum, N.; Doguiet, D.D.K.; Bensaid, M.; Thonart, P. Effectiveness of Cell-Adsorbed Bacteriocin Produced by Lactobacillus Curvatus CWBI-B28 and Selected Essential Oils to Control Listeria monocytogenes in Pork Meat during Cold Storage. Lett. Appl. Microbiol. 2007, 44, 268–273. [Google Scholar] [CrossRef]
- Abdollahzadeh, E.; Rezaei, M.; Hosseini, H. Antibacterial Activity of Plant Essential Oils and Extracts: The Role of Thyme Essential Oil, Nisin, and Their Combination to Control Listeria monocytogenes Inoculated in Minced Fish Meat. Food Control 2014, 35, 177–183. [Google Scholar] [CrossRef]
- Iseppi, R.; Camellini, S.; Zurlini, C.; Cigognini, I.M.; Cannavacciuolo, M.; Messi, P. Essential Oils and Bacteriocin-Based Active Edible Coating: An Innovative, Natural and Sustainable Approach for the Control of Listeria monocytogenes in Seafoods. Appl. Sci. 2023, 13, 2562. [Google Scholar] [CrossRef]
- Bukvicki, D.; Stojkovic, D.; Sokovic, M.; Nikolic, M.; Vannini, L.; Montanari, C.; Marin, P.D. Potential Application of Micromeria Dalmatica Essential Oil as a Protective Agent in a Food System. LWT Food Sci. Technol. 2015, 63, 262–267. [Google Scholar] [CrossRef]
- Karam, L.; Chehab, R.; Osaili, T.M.; Savvaidis, I.N. Antimicrobial Effect of Thymol and Carvacrol Added to a Vinegar-Based Marinade for Controlling Spoilage of Marinated Beef (Shawarma) Stored in Air or Vacuum Packaging. Int. J. Food Microbiol. 2020, 332, 108769. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Kim, N.H.; Kim, S.H.; Kim, Y.; Ryu, J.H.; Rhee, M.S. Teriyaki Sauce with Carvacrol or Thymol Effectively Controls Escherichia coli O157:H7, Listeria monocytogenes, Salmonella Typhimurium, and Indigenous Flora in Marinated Beef and Marinade. Meat Sci. 2017, 129, 147–152. [Google Scholar] [CrossRef]
- Thanissery, R.; Smith, D.P. Marinade with Thyme and Orange Oils Reduces Salmonella Enteritidis and Campylobacter Coli on Inoculated Broiler Breast Fillets and Whole Wings. Poult. Sci. 2014, 93, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Ritota, M.; Manzi, P. Natural Preservatives from Plant in Cheese Making. Animals 2020, 10, 749. [Google Scholar] [CrossRef]
- Gad, A.S.; Sayd, A.F.; Gad, A.S.; Sayd, A.F. Antioxidant Properties of Rosemary and Its Potential Uses as Natural Antioxidant in Dairy Products—A Review. Food Nutr. Sci. 2015, 6, 179–193. [Google Scholar] [CrossRef]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Melo Coutinho, H.D.; et al. Combination of Essential Oils in Dairy Products: A Review of Their Functions and Potential Benefits. LWT 2020, 133, 110116. [Google Scholar] [CrossRef]
- Hassanien, M.F.R.; Mahgoub, S.A.; El-Zahar, K.M. Soft Cheese Supplemented with Black Cumin Oil: Impact on Food Borne Pathogens and Quality during Storage. Saudi J. Biol. Sci. 2014, 21, 280. [Google Scholar] [CrossRef] [PubMed]
- Wahba, N.M.; Ahmed, A.S.; Ebraheim, Z.Z. Antimicrobial Effects of Pepper, Parsley, and Dill and Their Roles in the Microbiological Quality Enhancement of Traditional Egyptian Kareish Cheese. Foodborne Pathog. Dis. 2010, 7, 411–418. [Google Scholar] [CrossRef]
- Tayel, A.A.; Hussein, H.; Sorour, N.M.; El-Tras, W.F. Foodborne Pathogens Prevention and Sensory Attributes Enhancement in Processed Cheese via Flavoring with Plant Extracts. J. Food Sci. 2015, 80, M2886–M2891. [Google Scholar] [CrossRef]
- Kholy, W.E.; Aamer, R.A.; Mailam, M.A. Effect of Some Essential Oils on the Quality of UF-Soft Cheese During Storage. Alex. J. Food Sci. Technol. 2017, 14, 13–28. [Google Scholar] [CrossRef]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. The Potential Application of Plant Essential Oils as Natural Food Preservatives in Soft Cheese. Food Microbiol. 2001, 18, 463–470. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Ciric, A.; Soković, M.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Foeniculum vulgare Mill. as Natural Conservation Enhancer and Health Promoter by Incorporation in Cottage Cheese. J. Funct. Foods 2015, 12, 428–438. [Google Scholar] [CrossRef]
- Solhi, P.; Azadmard-Damirchi, S.; Hesari, J.; Hamishehkar, H. Production of the Processed Cheese Containing Tomato Powder and Evaluation of Its Rheological, Chemical and Sensory Characteristics. J. Food Sci. Technol. 2020, 57, 2198–2205. [Google Scholar] [CrossRef]
- Alexa, E.; Danciu, C.; Cocan, I.; Negrea, M.; Morar, A.; Obistioiu, D.; Dogaru, D.; Berbecea, A.; Radulov, I. Chemical Composition and Antimicrobial Potential of Satureja hortensis L. in Fresh Cow Cheese. J. Food Qual. 2018, 2018, 8424035. [Google Scholar] [CrossRef]
- Ben Jemaa, M.; Falleh, H.; Saada, M.; Oueslati, M.; Snoussi, M.; Ksouri, R. Thymus Capitatus Essential Oil Ameliorates Pasteurization Efficiency. J. Food Sci. Technol. 2018, 55, 3446–3452. [Google Scholar] [CrossRef]
- Azizkhani, M.; Tooryan, F. Antimicrobial Activities of Probiotic Yogurts Flavored with Peppermint, Basil, and Zataria against Escherichia coli and Listeria monocytogenes. J. Food Qual. Hazards Control 2016, 3, 79–86. [Google Scholar]
- Ehsani, A.; Hashemi, M.; Jazani, N.H.; Aliakbarlu, J.; Shokri, S.; Naghibi, S.S.; Dvm, M.H. Effect of Echinophora Platyloba DC. Essential Oil and Lycopene on the Stability of Pasteurized Cream Obtained from Cow Milk. Vet. Res. Forum 2016, 7, 139. [Google Scholar]
- Farag, R.S.; Ali, M.N.; Taha, S.H. Use of Some Essential Oils as Natural Preservatives for Butter. J. Am. Oil Chem. Soc. 1990, 67, 188–191. [Google Scholar] [CrossRef]
- Ozkan, G.; Simsek, B.; Kuleasan, H. Antioxidant Activities of Satureja Cilicica Essential Oil in Butter and in Vitro. J. Food Eng. 2007, 79, 1391–1396. [Google Scholar] [CrossRef]
- Yilmaztekin, M.; Lević, S.; Kalušević, A.; Cam, M.; Bugarski, B.; Rakić, V.; Pavlović, V.; Nedović, V. Characterisation of Peppermint (Mentha piperita L.) Essential Oil Encapsulates. J. Microencapsul. 2019, 36, 109–119. [Google Scholar] [CrossRef]
- Tomar, O.; Akarca, G. Effects of Ice Cream Produced with Lemon, Mandarin, and Orange Peel Essential Oils on Some Physicochemical, Microbiological and Sensorial Properties. Kocatepe Vet. J. 2019, 12, 62–70. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Yoon, J.I.; Bhardwaj, M.; Kang, S.C. Anti-Listerial Synergism of Leaf Essential Oil of Metasequoia glyptostroboides with Nisin in Whole, Low and Skim Milks. Asian Pac. J. Trop. Med. 2014, 7, 602–608. [Google Scholar] [CrossRef]
- Babashahi, M.; Mirlohi, M.; Torki-Baghbadorani, S.; Ghiasvand, R.; Azadbakht, L.; Mosharaf, L. Effects of Probiotic Soy Milk Fermented by Lactobacillus Plantarum A7 (KC 355240) Added with Cuminum Cyminum Essential Oil on Fasting Blood Glucose Levels, Serum Lipid Profile and Body Weight in Diabetic Wistar Rats. Int. J. Prev. Med. 2020, 11, 8. [Google Scholar] [CrossRef]
- Tornambé, G.; Cornu, A.; Verdier-Metz, I.; Pradel, P.; Kondjoyan, N.; Figueredo, G.; Hulin, S.; Martin, B. Addition of Pasture Plant Essential Oil in Milk: Influence on Chemical and Sensory Properties of Milk and Cheese. J. Dairy Sci. 2008, 91, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Davidson, P.M.; Zhong, Q. Inhibition of Escherichia coli O157:H7 and Listeria Monocytognes Growth in Milk and Cantaloupe Juice by Thymol Nanoemulsions Prepared with Gelatin and Lecithin. Food Control 2017, 73, 1499–1506. [Google Scholar] [CrossRef]
- Singh, G.; Kapoor, I.P.S.; Singh, P. Effect of volatile oil and oleoresin of anise on the shelf life of yogurt. J. Food Process Preserv. 2011, 35, 778–783. [Google Scholar] [CrossRef]
- Azizkhani, M.; Parsaeimehr, M. Probiotics Survival, Antioxidant Activity and Sensory Properties of Yogurt Flavored with Herbal Essential Oils. Int. Food Res. J. 2018, 25, 921–927. [Google Scholar]
- Sobrino-López, A.; Martín-Belloso, O. Use of Nisin and Other Bacteriocins for Preservation of Dairy Products. Int. Dairy J. 2008, 18, 329–343. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Crit. Rev. Food Sci. Nutr. 2015, 56, 1262–1274. [Google Scholar] [CrossRef]
- Arqués, J.L.; Rodríguez, E.; Nuñez, M.; Medina, M. Combined Effect of Reuterin and Lactic Acid Bacteria Bacteriocins on the Inactivation of Food-Borne Pathogens in Milk. Food Control 2011, 22, 457–461. [Google Scholar] [CrossRef]
- Chen, H.; Hoover, D.G. Bacteriocins and Their Food Applications. Compr. Rev. Food Sci. Food Saf. 2003, 2, 82–100. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Q. Lactobionic Acid Enhances the Synergistic Effect of Nisin and Thymol against Listeria monocytogenes Scott A in Tryptic Soy Broth and Milk. Int. J. Food Microbiol. 2017, 260, 36–41. [Google Scholar] [CrossRef]
- Cao-Hoang, L.; Grégoire, L.; Chaine, A.; Waché, Y. Importance and Efficiency of In-Depth Antimicrobial Activity for the Control of Listeria Development with Nisin-Incorporated Sodium Caseinate Films. Food Control 2010, 21, 1227–1233. [Google Scholar] [CrossRef]
- Malheiros, P.d.S.; Sant’Anna, V.; Barbosa, M.d.S.; Brandelli, A.; Franco, B.D.G.d.M. Effect of Liposome-Encapsulated Nisin and Bacteriocin-like Substance P34 on Listeria monocytogenes Growth in Minas Frescal Cheese. Int. J. Food Microbiol. 2012, 156, 272–277. [Google Scholar] [CrossRef]
- Ercolini, D.; Ferrocino, I.; La Storia, A.; Mauriello, G.; Gigli, S.; Masi, P.; Villani, F. Development of Spoilage Microbiota in Beef Stored in Nisin Activated Packaging. Food Microbiol. 2010, 27, 137–143. [Google Scholar] [CrossRef] [PubMed]
- López Aguayo, M.d.C.; Grande Burgos, M.J.; Pérez Pulido, R.; Gálvez, A.; López, R.L. Effect of Different Activated Coatings Containing Enterocin AS-48 against Listeria monocytogenes on Apple Cubes. Innov. Food Sci. Emerg. Technol. 2016, 35, 177–183. [Google Scholar] [CrossRef]
- Malheiros, P.S.; Cuccovia, I.M.; Franco, B.D.G.M. Inhibition of Listeria monocytogenes in Vitro and in Goat Milk by Liposomal Nanovesicles Containing Bacteriocins Produced by Lactobacillus Sakei Subsp. Sakei 2a. Food Control 2016, 63, 158–164. [Google Scholar] [CrossRef]
- Concha-Meyer, A.; Schöbitz, R.; Brito, C.; Fuentes, R. Lactic Acid Bacteria in an Alginate Film Inhibit Listeria monocytogenes Growth on Smoked Salmon. Food Control 2011, 22, 485–489. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Todorov, S.D.; Jurkiewicz, C.H.; Franco, B.D.G.M. Bacteriocin Production by Lactobacillus Curvatus MBSa2 Entrapped in Calcium Alginate during Ripening of Salami for Control of Listeria monocytogenes. Food Control 2015, 47, 147–153. [Google Scholar] [CrossRef]
- Mirhosseini, M.; Afzali, M. Investigation into the Antibacterial Behavior of Suspensions of Magnesium Oxide Nanoparticles in Combination with Nisin and Heat against Escherichia coli and Staphylococcus Aureus in Milk. Food Control 2016, 68, 208–215. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, C.; Meng, R.; Liu, Z.; Huang, Y.; Zhao, Z.; Guo, N. Effect of Nisin and Perilla Oil Combination against Listeria monocytogenes and Staphylococcus Aureus in Milk. J. Food Sci. Technol. 2016, 53, 2644. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, X.; Zhao, X.; Meng, R.; Liu, Z.; Chen, X.; Guo, N. Synergistic Interactions of Nisin in Combination with Cinnamaldehyde against Staphylococcus Aureus in Pasteurized Milk. Food Control 2017, 71, 10–16. [Google Scholar] [CrossRef]
- García, P.; Martínez, B.; Rodríguez, L.; Rodríguez, A. Synergy between the Phage Endolysin LysH5 and Nisin to Kill Staphylococcus Aureus in Pasteurized Milk. Int. J. Food Microbiol. 2010, 141, 151–155. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, X.; Meng, R.; Liu, Z.; Zhang, G.; Guo, N. Synergistic Antimicrobial Effects of Nisin and P-Anisaldehyde on Staphylococcus aureus in Pasteurized Milk. LWT 2017, 84, 222–230. [Google Scholar] [CrossRef]
- Martínez, B.; Obeso, J.M.; Rodríguez, A.; García, P. Nisin-Bacteriophage Crossresistance in Staphylococcus Aureus. Int. J. Food Microbiol. 2008, 122, 253–258. [Google Scholar] [CrossRef] [PubMed]
- De, N.; Pimentel-Filho, J.; Mantovani, H.C.; Diez-Gonzalez, F.; Dantas Vanetti, M.C.; Cristina, M.; Vanetti, D. Inhibition of Listeria and Staphylococcus aureus by Bovicin HC5 and Nisin Combination in Milk. J. Agric. Sci. 2013, 5, p188. [Google Scholar] [CrossRef]
- Alves, F.C.B.; Barbosa, L.N.; Andrade, B.F.M.T.; Albano, M.; Furtado, F.B.; Marques Pereira, A.F.; Rall, V.L.M.; Júnior, A.F. Short Communication: Inhibitory Activities of the Lantibiotic Nisin Combined with Phenolic Compounds against Staphylococcus aureus and Listeria monocytogenes in Cow Milk. J. Dairy Sci. 2016, 99, 1831–1836. [Google Scholar] [CrossRef]
- Yoon, J.I.; Bajpai, V.K.; Kang, S.C. Synergistic Effect of Nisin and Cone Essential Oil of Metasequoia glyptostroboides Miki Ex Hu against Listeria monocytogenes in Milk Samples. Food Chem. Toxicol. 2011, 49, 109–114. [Google Scholar] [CrossRef]
- Kim, E.L.; Choi, N.H.; Bajpai, V.K.; Kang, S.C. Synergistic Effect of Nisin and Garlic Shoot Juice against Listeria monocytogenes in Milk. Food Chem. 2008, 110, 375–382. [Google Scholar] [CrossRef]
- Field, D.; Daly, K.; O’Connor, P.M.; Cotter, P.D.; Hill, C.; Paul Ross, R. Efficacies of Nisin a and Nisin V Semipurified Preparations Alone and in Combination with Plant Essential Oils for Controlling Listeria monocytogenes. Appl. Environ. Microbiol. 2015, 81, 2762–2769. [Google Scholar] [CrossRef]
- Campion, A.; Morrissey, R.; Field, D.; Cotter, P.D.; Hill, C.; Ross, R.P. Use of Enhanced Nisin Derivatives in Combination with Food-Grade Oils or Citric Acid to Control Cronobacter Sakazakii and Escherichia coli O157:H7. Food Microbiol. 2017, 65, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Sobrino-Lopez, A.; Viedma-Martínez, P.; Abriouel, H.; Valdivia, E.; Gálvez, A.; Martin-Belloso, O. The Effect of Adding Antimicrobial Peptides to Milk Inoculated with Staphylococcus aureus and Processed by High-Intensity Pulsed-Electric Field. J. Dairy Sci. 2009, 92, 2514–2523. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, Y. Ziziphora clinopodioides Essential Oil and Nisin as Potential Antimicrobial Agents against Escherichia coli O157:H7 in Doogh (Iranian Yoghurt Drink). J. Pathog. 2015, 2015, 176024. [Google Scholar] [CrossRef] [PubMed]
| Microbial Target | Food and Beverages | EO or Component | Concentration Used | Reference(s) |
|---|---|---|---|---|
| Total aerobic bacteria | Lettuce and carrots Four season salad Table grape Avocado Sweet cherries Kiwifruit and melon Honeydew melon | Oregano and thyme EOs Oregano EO and citral in packaging Eugenol thymol Thyme EO in MAP Eugenol, thymol, and menthol Eucalyptol Carvacrol and cinnamic acid Carvacrol and cinnamic acid | Alone 250 mg/L; combination 125 mg/L 7.5% w/w 75–150 mL in the gas used for MAP 75 mL in the filter 1000 mL in the gas used for MAP 5–15 mM in the dipping solution 1 mM in the dipping solution | [2,108,113,114,115,116,117] |
| Total aerobic bacteria and inoculated L. innocua | Fresh sliced apples | Oregano, lemongrass, and vanillin used encapsulated | 0.5–2.0% (w/w) | [105] |
| Total aerobic bacteria, Escherichia coli, Listeria monocytogenes, Salmonella enteritidis | Lamb’s lettuce Fresh cut apples in MAP | oregano and thyme EOs Citron EO, hexanal, E(2)hexenal, citral, and carvacrol | 250 mg/L Alone 250 mg/L; combination 125 mg/L | [102,118,119,120] |
| Salmonella enteritidis, Escherichia coli, Listeria monocytogenes | Fruit salads Fresh sliced apples | citral Citron EO Hexanal, hexyl acetate, E(2)hexenal | 25–125 ppm 300–600 ppm 50–250 ppm | [111,117] |
| Listeria monocytogenes, Yersinia enterocolitica and Aeromonas hydrophilla | Iceberg lettuce | Oregano and rosemary | 0.003–80 mL/m | [103] |
| Listeria spp., E. coli O157:H7 | Fresh cut apples | Vanillin Oregano EO | 12 g/L 0.7–2.1% v/w | [104,121] |
| E. coli O157:H7 | Eggplant salad Carrots | Oregano EO Thyme EO | 0.7–2.1% v/w 0.1–10 mL/L | [106,121] |
| Salmonella tiphymurium | Lettuce | Oregano EO | 25–75 mg/L | [122] |
| Streptococcus thermophilus, | Pomegranate juice | Thymbra capitata EO | 0.06 and 0.125% v/v | [123] |
| aerobic mesophilic bacteria | ||||
| Escherichia coli O157:H7 | Apple Juice | Melissa oil, carvacrol, and oregano oil, | 0.067 and 0.67% v/w | [124] |
| Salmonella enterica | Terpeineol, geraniol, lemon oil, and citral | |||
| S. enteritidis, E. coli, L. innocua | Apple, pear, and melon juice | Palmarosa, clove, and lemongrass | 5–10 μL/mL | [105] |
| Saccharomyces cerevisiae | Orange and pomegranate juices | Cinnamon leaf EO | 0.02–0.65 mg/mL | [125] |
| S. cerevisiae, S. pombe, Pichia anomala | Apple juice | Lemon EO | 0.25 μL/mL | [126] |
| Plant Name | Essential Oils/ | Activity Reported | Dairy Product | References |
|---|---|---|---|---|
| Components Used | ||||
| Mentha piperita | Peppermint oil | Added functional properties without negative effects on rheological and sensorial properties | Ice-cream | [195] |
| Citrus limon, Citrus reticulata, Citrus aurantium | α- and β-Pinene, Limonene, trans- β-Ocimene, Linalool, α-Terpineol | Increased physiochemical, sensorial, and antimicrobial properties | [196] | |
| Echinophora platyloba | Trans-b-ocimene, 2-Furanone, Myrcene, Linalool, Cis-b-ocimene | Increase antimicrobial properties and stability | Cream | [192] |
| Metasequoia glyptostroboides | α-Pinene, Totarol, α-Thujene, Bornylene, β-Caryophyllene, δ-3-Carene, and 2-β-Pinene | Antibacterial and anti-listerial effect | Milk/milk samples | [197] |
| Cuminum cyminum | Cumin oil | Reduce cholesterol, LDL, and increases HDL | [198] | |
| Thymus capitatus | Thyme oil | Enhancement of oxidative and fermentative activity, increases physico-chemical, microbiological, and sensory properties | [190,199] | |
| Thymus vulgaris | Thymol | Antimicrobial properties | [200] | |
| Pimpinella anisum | Anise oil | Increased antimicrobial properties | Yogurt | [201] |
| Syzygium aromaticum, Salvia rosmarinus, Cinnamomum verum | Eugenol, Acetyl-eugenol, Linalool, β-Caryophyllene, Cineole, Camphor, Camphene, Limonene, α-Pinene, β-Pinene, α-Terpineol, Borneol, and Cinnamaldehyde | Increases shelf life and antioxidant properties | [202] | |
| Zataria multiflora, Ocimum basilicum, Mentha piperita | Zataria, Basil, and Peppermint oil | Antimicrobial and antioxidant properties | ||
| Satureja cilicica | Thymol, Carvacrol, p-Cymene, and c-Terpinene | Increases antioxidant properties and aroma | Butter | [194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukvicki, D.; D’Alessandro, M.; Rossi, S.; Siroli, L.; Gottardi, D.; Braschi, G.; Patrignani, F.; Lanciotti, R. Essential Oils and Their Combination with Lactic Acid Bacteria and Bacteriocins to Improve the Safety and Shelf Life of Foods: A Review. Foods 2023, 12, 3288. https://doi.org/10.3390/foods12173288
Bukvicki D, D’Alessandro M, Rossi S, Siroli L, Gottardi D, Braschi G, Patrignani F, Lanciotti R. Essential Oils and Their Combination with Lactic Acid Bacteria and Bacteriocins to Improve the Safety and Shelf Life of Foods: A Review. Foods. 2023; 12(17):3288. https://doi.org/10.3390/foods12173288
Chicago/Turabian StyleBukvicki, Danka, Margherita D’Alessandro, Samantha Rossi, Lorenzo Siroli, Davide Gottardi, Giacomo Braschi, Francesca Patrignani, and Rosalba Lanciotti. 2023. "Essential Oils and Their Combination with Lactic Acid Bacteria and Bacteriocins to Improve the Safety and Shelf Life of Foods: A Review" Foods 12, no. 17: 3288. https://doi.org/10.3390/foods12173288
APA StyleBukvicki, D., D’Alessandro, M., Rossi, S., Siroli, L., Gottardi, D., Braschi, G., Patrignani, F., & Lanciotti, R. (2023). Essential Oils and Their Combination with Lactic Acid Bacteria and Bacteriocins to Improve the Safety and Shelf Life of Foods: A Review. Foods, 12(17), 3288. https://doi.org/10.3390/foods12173288








