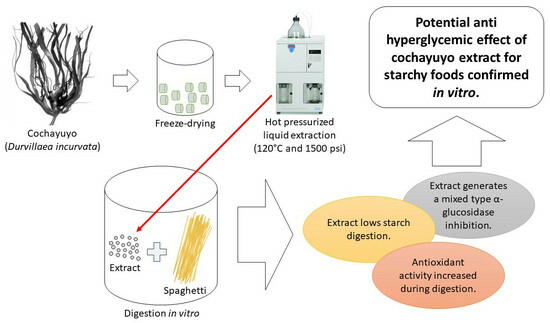
Cochayuyo (Durvillaea incurvata) Extracts: Their Impact on Starch Breakdown and Antioxidant Activity in Pasta during In Vitro Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seaweed Collection
2.2. Phenolic Compounds Extraction
2.3. Type of Inhibition for α-Glucosidase by Extracts
2.4. Polyphenols Profile in the Ethanol Extract by UHPLC
2.5. Impact of Phenolic Extracts on Starchy Food Digestion
2.5.1. Starch Digestion
2.5.2. Changes in Total Phenolic Content and Antioxidant Capacity during Food Digestion
- Total phenolic content
- Antioxidant capacity
2.6. Statistics
3. Results and Discussion
3.1. Type of α-Glucosidase Inhibition
3.2. Profile of Polyphenols in the Ethanol Extract
3.3. In Vitro Digestion of Pasta Affected by Phenolic Compounds
3.4. Bioaccessibility of Antioxidant Compounds from Durvillaea Antarctica Extracts during In Vitro Paste Digestion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dillehay, T.D.; Rami, C.; Pino, M.; Collins, M.B.; Rossen, J.; Pino-Navarro, J.D. Monte Verde: Seaweed, Food, Medicine, and the Peopling of South America. Science 2008, 320, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-based natural ingredients: Stability of phlorotannins during extraction, storage, passage through the gastrointestinal tract and potential incorporation into functional foods. Food Res. Int. 2020, 137, 109676. [Google Scholar] [CrossRef] [PubMed]
- Erpel, F.; Camilo, C.; Mateos, R.; Pérez-Correa, J.R. A macroporous resin purification process to obtain food-grade phlorotannin-rich extracts with α-glucosidase inhibitory activity from Chilean brown seaweeds: An UHPLC-MS profiling. Food Chem. 2023, 402, 134472. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.W.; Chen, J.-H. Seaweeds as Ingredients to Lower Glycemic Potency of Cereal Foods Synergistically—A Perspective. Foods 2022, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Bellesia, A.; Verzelloni, E.; Tagliazucchi, D. Pomegranate ellagitannins inhibit α-glucosidase activity in vitro and reduce starch digestibility under simulated gastro-intestinal conditions. Int. J. Food Sci. Nutr. 2015, 66, 85–92. [Google Scholar] [CrossRef]
- Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Erpel, F.; Zambrano, A.; Palacios, M. Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Mar. Drugs 2020, 18, 353. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. Measurement of Oxygen Radical Absorbance Capacity in BIological Samples. Methods Enzymol. 1999, 299, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Zaharudin, N.; Staerk, D.; Dragsted, L.O. Inhibition of α-glucosidase activity by selected edible seaweeds and fucoxanthin. Food Chem. 2019, 270, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Stryer, L.; Tymoczko, J.L.; Gatto, G.J. Biochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Barrett, A.H.; Farhadi, N.F.; Smith, T.J. Slowing starch digestion and inhibiting digestive enzyme activity using plant flavanols/tannins— A review of efficacy and mechanisms. LWT 2018, 87, 394–399. [Google Scholar] [CrossRef]
- Shai, L.; Masoko, P.; Mokgotho, M.; Magano, S.; Mogale, A.; Boaduo, N.; Eloff, J. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. S. Afr. J. Bot. 2010, 76, 465–470. [Google Scholar] [CrossRef]
- Olate-Gallegos, C.; Barriga, A.; Vergara, C.; Fredes, C.; García, P.; Giménez, B.; Robert, P. Identification of Polyphenols from Chilean Brown Seaweeds Extracts by LC-DAD-ESI-MS/MS. J. Aquat. Food Prod. Technol. 2019, 28, 375–391. [Google Scholar] [CrossRef]
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, ellagic acid and vascular health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Review: Starch Matrices and the Glycemic Response. Food Sci. Technol. Int. 2011, 17, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Sobota, A.; Zarzycki, P. Effect of Pasta Cooking Time on the Content and Fractional Composition of Dietary Fiber. J. Food Qual. 2013, 36, 127–132. [Google Scholar] [CrossRef]
- Giacco, R.; Vitale, M.; Riccardi, G. Pasta: Role in Diet. In Encyclopedia of Food and Health; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 242–245. [Google Scholar]
- Miao, M.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Slowly Digestible Starch—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1642–1657. [Google Scholar] [CrossRef]
- Eelderink, C.; Schepers, M.; Preston, T.; Vonk, R.J.; Oudhuis, L.; Priebe, M.G. Slowly and rapidly digestible starchy foods can elicit a similar glycemic response because of differential tissue glucose uptake in healthy men. Am. J. Clin. Nutr. 2012, 96, 1017–1024. [Google Scholar] [CrossRef]
- Gabbia, D.; De Martin, S. Brown Seaweeds for the Management of Metabolic Syndrome and Associated Diseases. Molecules 2020, 25, 4182. [Google Scholar] [CrossRef]
- Sharifuddin, Y.; Chin, Y.-X.; Lim, P.-E.; Phang, S.-M. Potential Bioactive Compounds from Seaweed for Diabetes Management. Mar. Drugs 2015, 13, 5447–5491. [Google Scholar] [CrossRef]
- Wilcox, M.D.; Cherry, P.; Chater, P.I.; Yang, X.; Zulali, M.; Okello, E.J.; Seal, C.J.; Pearson, J.P. The effect of seaweed enriched bread on carbohydrate digestion and the release of glucose from food. J. Funct. Foods 2021, 87, 104747. [Google Scholar] [CrossRef]
- Attjioui, M.; Ryan, S.; Ristic, A.K.; Higgins, T.; Goñi, O.; Gibney, E.R.; Tierney, J.; O’Connell, S. Comparison of edible brown algae extracts for the inhibition of intestinal carbohydrate digestive enzymes involved in glucose release from the diet. J. Nutr. Sci. 2021, 10, e5. [Google Scholar] [CrossRef]
- Petitot, M.; Abecassis, J.; Micard, V. Structuring of pasta components during processing: Impact on starch and protein digestibility and allergenicity. Trends Food Sci. Technol. 2009, 20, 521–532. [Google Scholar] [CrossRef]
- Galanakis, C. What is the Difference Between Bioavailability Bioaccessibility and Bioactivity of Food Components? Elsevier SciTech Connect: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Catarino, M.D.; Circuncisão, A.R.; Neves, B.; Marçal, C.; Silva, A.M.S.; Cruz, M.T.; Cardoso, S.M. Impact of Gastrointestinal Digestion on the Anti-Inflammatory Properties of Phlorotannins from Himanthalia elongata. Antioxidants 2022, 11, 1518. [Google Scholar] [CrossRef]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Q.; Hu, K.; Zhang, R.; Yuan, Y.; He, S.; Zeng, Q.; Su, D. Effects of in vitro simulated digestion on the free and bound phenolic content and antioxidant activity of seven species of seaweeds. Int. J. Food Sci. Technol. 2021, 56, 2365–2374. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Wu, Y.; Wang, D.; Wei, Y.; Wu, J.; Ji, B. Stability and absorption of anthocyanins from blueberries subjected to a simulated digestion process. Int. J. Food Sci. Nutr. 2014, 65, 440–448. [Google Scholar] [CrossRef]
- Catarino, M.; Silva, A.; Cardoso, S. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Arnold, T.M.; Targett, N.M. To grow and defend: Lack of tradeoffs for brown algal phlorotannins. Oikos 2003, 100, 406–408. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Gisbert, M.; Aleixandre, A.; Sineiro, J.; Rosell, C.M.; Moreira, R. Interactions between Ascophyllum nodosum Seaweeds Polyphenols and Native and Gelled Corn Starches. Foods 2022, 11, 1165. [Google Scholar] [CrossRef]




| Kinetic Parameters | W/I | D. incurvata Extracts | |
|---|---|---|---|
| Ethanol | Acetone | ||
| Vmax (μmol/min) | 71.55 | 3.84 × 10−12 | 1.09 × 10−11 |
| Km | 0.24 | 4.76 | 5.15 |
| Km/Vmax | 0.003 | 1.24 × 1012 | 4.73 × 1011 |
| Type of inhibition | - | Mixed | Mixed |
| Peak # | Retention Time (min.) | Tentative Identification | Elemental Composition [M-H]− | Theoretical Mass (m/z) | Measured Mass (m/z) | Accuracy (δppm) |
|---|---|---|---|---|---|---|
| 1 | 2.75 | Sorbitol | C6H13O6− | 181.07066 | 181.07167 | 5.55 |
| 2 | 3.70 | 2,6,10,14-Tetraoxapentadecane-4,12-diol | C28H45O11− | 557.27564 | 557.27961 | 3.53 |
| 3 | 4,20 | Leucodelphinidin | C15H13O8− | 321.06049 | 321.06188 | 4.30 |
| 4 | 5.73 | Triplhoroethol | C18H13O9− | 373.05685 | 373.05685 | 2.51 |
| 5 | 6.64 | Unknown | C13H27O8− | 311.17004 | 311.16901 | −3.3 |
| 6 | 11.44 | Hexaphloroethol or—bis-trifucophloroethol | C36H25O8− | 745.10464 | 745.10657 | 4.45 |
| 7 | 12.03 | Tetraphloroethol | C24H17O12− | 497.07413 | 497.07364 | |
| 8 | 13.58 | Unknown | C14H29O8− | 325.18472 | 325.18569 | −2.98 |
| 9 | 20.30 | Hexaphloroethol or—bis-trifucophloroethol | C36H25O8− | 745.10464 | 745.10693 | 5.44 |
| 10 | 21.75 | Hexaphloroethol or—bis-trifucophloroethol | C36H25O8− | 745.10464 | 745.10675 | 4.26 |
| 11 | 24.32 | Pentaphloroethol or trifucophloroethol | C30H21O15− | 621.08859 | 621.09015 | |
| 12 | 24.65 | Stigmatellin | C29H39O6− | 483.27412 | 483.27341 | −1.46 |
| 13 | 25.25 | Pentaphloroethol or trifucophloroethol | C30H21O15− | 621.08750 | 621.08978 | 3.68 |
| 14 | 25.63 | Unknown | C28H51O11− | 563.34259 | 563.34460 | 3.57 |
| Parameters | Pasta | Ethanol | Acetone |
|---|---|---|---|
| D0 (g/100 g dry starch) | −51.14 | −23.21 | −123.20 |
| D∞ (g/100 g dry starch) | 80.48 | 77.43 | 62.12 |
| k (1/min) | 0.02606 | 0.01577 | 0.03751 |
| R2 | 0.984 | 0.973 | 0.922 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Simirgiotis, M. Cochayuyo (Durvillaea incurvata) Extracts: Their Impact on Starch Breakdown and Antioxidant Activity in Pasta during In Vitro Digestion. Foods 2023, 12, 3326. https://doi.org/10.3390/foods12183326
Pacheco LV, Parada J, Pérez-Correa JR, Mariotti-Celis MS, Simirgiotis M. Cochayuyo (Durvillaea incurvata) Extracts: Their Impact on Starch Breakdown and Antioxidant Activity in Pasta during In Vitro Digestion. Foods. 2023; 12(18):3326. https://doi.org/10.3390/foods12183326
Chicago/Turabian StylePacheco, Luz Verónica, Javier Parada, José R. Pérez-Correa, María Salomé Mariotti-Celis, and Mario Simirgiotis. 2023. "Cochayuyo (Durvillaea incurvata) Extracts: Their Impact on Starch Breakdown and Antioxidant Activity in Pasta during In Vitro Digestion" Foods 12, no. 18: 3326. https://doi.org/10.3390/foods12183326
APA StylePacheco, L. V., Parada, J., Pérez-Correa, J. R., Mariotti-Celis, M. S., & Simirgiotis, M. (2023). Cochayuyo (Durvillaea incurvata) Extracts: Their Impact on Starch Breakdown and Antioxidant Activity in Pasta during In Vitro Digestion. Foods, 12(18), 3326. https://doi.org/10.3390/foods12183326