Sustainable Process for Tortilla Production Using Ohmic Heating with Minimal Impact on the Nutritional Value, Protein, and Calcium Performance
Abstract
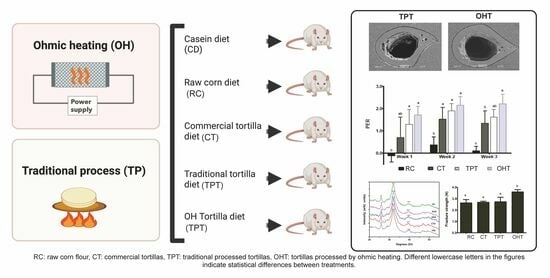
:1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Flour and Tortilla Preparation
2.2.1. Flour Obtained using the Traditional Nixtamalization Process
2.2.2. Flour Obtained using Ohmic Heating Nixtamalization
2.2.3. Tortilla Elaboration
2.3. Nutritional Composition
2.4. Sequential Extraction of Protein Fractions
2.5. SDS-PAGE
2.6. Biological Assay
2.6.1. Study Design
2.6.2. Diet Preparation
2.6.3. Food Intake and Efficiency
2.6.4. Protein Efficiency Ratio, Apparent Protein Digestibility, and Serum Albumin
2.6.5. Physical Characteristics of Bones
2.6.6. Resistance Test
2.6.7. X-ray Diffraction Pattern
2.6.8. Scanning Electron Microscope (SEM)
2.7. Statistical Analysis
3. Results
3.1. Nutritional Composition
3.2. Protein Profile of Tortillas
3.3. Biological Assay
3.3.1. Food Intake, Food Efficiency, and Body Weight Changes
3.3.2. Protein Performance
3.4. Bone Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lyddon, C. New Products Drive Income Gain at Gruma USA|Baking Business|Baking Industry News and Opinions. Available online: http://www.bakingbusiness.com/articles/news_home/Financial-Performance/2017/07/New_products_drive_income_gain.aspx?ID=%7BD9EB2AC4-6027-427B-AFAB-DD961E8DA704%7D&cck=1 (accessed on 28 November 2017).
- Serna-Saldivar, S.O.; Amaya Guerra, C.A.; Herrera Macias, P.; Melesio Cuellar, J.L.; Preciado Ortiz, R.E.; Terron Ibarra, A.D.; Vazquez Carrillo, G. Evaluation of the Lime-Cooking and Tortilla Making Properties of Quality Protein Maize Hybrids Grown in Mexico. Plant Foods Hum. Nutr. 2008, 63, 119–125. [Google Scholar] [CrossRef]
- Bressani, R.; Scrimshaw, N.S. Lime-Heat Effects on Corn Nutrients, Effect of Lime Treatment on in Vitro Availability of Essential Amino Acids and Solubility of Protein Fractions in Corn. J. Agric. Food Chem. 1958, 6, 774–778. [Google Scholar] [CrossRef]
- Martínez-Flores, H.E.; Martínez-Bustos, F.; Figueroa, J.D.C.; González-Hernández, J. Studies and Biological Assays in Corn Tortillas Made from Fresh Masa Prepared by Extrusion and Nixtamalization Processes. J. Food Sci. 2002, 67, 1196–1199. [Google Scholar] [CrossRef]
- Maya-Cortés, D.C.; Figueroa Cárdenas, J.D.D.; Garnica-Romo, M.G.; Cuevas-Villanueva, R.A.; Cortés-Martínez, R.; Véles-Medina, J.J.; Martínez-Flores, H.E. Whole-Grain Corn Tortilla Prepared Using an Ecological Nixtamalisation Process and Its Impact on the Nutritional Value. Int. J. Food Sci. Technol. 2010, 45, 23–28. [Google Scholar] [CrossRef]
- Fernández-Muñoz, J.L.; Rojas-Molina, I.; González-Dávalos, M.L.; Leal, M.; Valtierra, M.E.; San Martín-Martinez, E.; Rodríguez, M.E. Study of Calcium Ion Diffusion in Components of Maize Kernels during Traditional Nixtamalization Process. Cereal Chem. 2004, 81, 65–69. [Google Scholar] [CrossRef]
- Valderrama-Bravo, C.; Rojas-Molina, A.; Gutiérrez-Cortez, E.; Rojas-Molina, I.; Oaxaca-Luna, A.; De la Rosa-Rincón, E.; Rodríguez-García, M.E. Mechanism of Calcium Uptake in Corn Kernels during the Traditional Nixtamalization Process: Diffusion, Accumulation and Percolation. J. Food Eng. 2010, 98, 126–132. [Google Scholar] [CrossRef]
- Willows, N. Food Sources of Calcium Vary by Ethnicity and Geography. In Food and Nutritional Components in Focus; Royal Society of Chemistry: Burlington, UK, 2016. [Google Scholar]
- Rosado, J.L.; Díaz, M.; Rosas, A.; Griffit, I.; García, O.P. Calcium Absorption from Corn Tortilla Is Relatively High and Is Dependent upon Calcium Content and Liming in Mexican Women. J. Nutr. 2005, 135, 2578–2581. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.K.; Castro-Muñoz, R. Emerging Techniques Assisting Nixtamalization Products and By-Products Processing: An Overview. Crit. Rev. Food Sci. Nutr. 2021, 61, 3407–3420. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V.; Durán-Páramo, E. A Review of the Primary By-Product (Nejayote) of the Nixtamalization During Maize Processing: Potential Reuses. Waste Biomass Valorization 2017, 10, 13–22. [Google Scholar] [CrossRef]
- Méndez-Albores, A.; Zamora-Rodríguez, D.; Arámbula-Villa, G.; Vázquez-Duran, A.; Moreno-Martínez, E. Impact of Different Alkaline-Heating Processes on Technological and Nutritional Properties of Maize Tortillas. J. Food Nutr. Res. 2014, 53, 60–70. [Google Scholar]
- Flores-Farías, R.; Martínez-Bustos, F.; Salinas-Moreno, Y.; Chang, Y.K.; Hernández, J.G.; Ríos, E. Physicochemical and Rheological Characteristics of Commercial Nixtamalised Mexican Maize Flours for Tortillas. J. Sci. Food Agric. 2000, 80, 657–664. [Google Scholar] [CrossRef]
- Contreras-Jiménez, B.; Morales-Sánchez, E.; Reyes-Vega, M.L.; Gaytán-Martínez, M. Propiedades Funcionales de Harinas de Maíz Nixtamalizado Obtenidas Por Extrusión a Baja Temperatura. CYTA J. Food 2014, 12, 263–270. [Google Scholar] [CrossRef]
- Varghese, K.S.; Pandey, M.C.; Radhakrishna, K.; Bawa, A.S. Technology, Applications and Modelling of Ohmic Heating: A Review. J. Food Sci. Technol. 2014, 51, 2304–2317. [Google Scholar] [CrossRef] [PubMed]
- Bobinaite, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of Pulsed Electric Field in the Production of Juice and Extraction of Bioactive Compounds from Blueberry Fruits and Their By-Products. J. Food Sci. Technol. 2014, 52, 5898–5905. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, O.P.; Sayanfar, S.; Toepfl, S. Effect of Pulsed Electric Field on Texture and Drying Time of Apple Slices. J. Food Sci. Technol. 2018, 55, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Sastry, S. Ohmic Heating and Moderate Electric Field Processing. Food Sci. Technol. Int. 2008, 14, 419–422. [Google Scholar] [CrossRef]
- Vicente, A. Food Industry. Available online: https://www.newfoodmagazine.com/article/610/ohmic-heating-in-the-food-industry/ (accessed on 12 February 2018).
- Sakr, M.; Liu, S. A Comprehensive Review on Applications of Ohmic Heating (OH). Renew. Sustain. Energy Rev. 2014, 39, 262–269. [Google Scholar] [CrossRef]
- Sastry, S.K.; Shynkaryk, M.; Somavat, R. Ohmic and Moderate Electric Field Processing: Developments and New Applications. In Proceedings of the 11th International Congress on Engineering and Food, Athens, Greece, 22–26 May 2011. [Google Scholar]
- Gaytán-Martínez, M.; Figueroa, J.D.C.; Vázquez-Landaverde, P.A.; Morales-Sánchez, E.; Martínez-Flores, H.E.; Reyes-Vega, M.L. Physicochemical, Functional, and Chemical Characterization of Nixtamalized Corn Flour Obtained by Ohmic Heating and Traditional Process. CYTA J. Food 2012, 10, 182–195. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.K.; Rangel-Hernández, J.; Morales-Sánchez, E.; Loarca-Piña, G.; Gaytán-Martínez, M. Changes on the Phytochemicals Profile of Instant Corn Flours Obtained by Traditional Nixtamalization and Ohmic Heating Process. Food Chem. 2019, 276, 128–135. [Google Scholar] [CrossRef]
- Reznik, D. Electroheating Methods. US Patent US005863580A, 26 January 1999. [Google Scholar]
- Kolbe, E.R.; Park, J.W.; Wells, J.H.; Flugstad, B.A.; Zhao, Y. Capacative Dielectric Heating System. US Patent US006303166B1, 16 October 2001. [Google Scholar]
- Ménera-López, I.; Gaytán-Martínez, M.; Reyes-Vega, M.L.; Morales-Sánchez, E.; Figueroa, J.D.C. Physico-Chemical Properties and Quality Assessment of Corn Flour Processed by a Continuous Ohmic Heating System and Traditional Nixtamalization. CYTA J. Food 2013, 11, 8–14. [Google Scholar] [CrossRef]
- Montiel-Ojeda, D.; Cerdas, S.; Clark, P.; Caló, M.; Wullich, S.; da Llibre, R.S.; Levin, J. Assessment of Calcium Intake in Costa Rica and Panama with the International Osteoporosis Foundation Calcium Calculator. Nutr. Hosp. 2023, 40, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R.; Mithal, A.; Rizzoli, R.; Zerbini, C.A.F.; Pierroz, D.D.; et al. Global Dietary Calcium Intake among Adults: A Systematic Review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef] [PubMed]
- FAO Regional Office for Latin America and the Caribbean. Panorama Regional de La Seguridad Alimentaria y Nutricional; FAO: Rome, Italy, 2023. [Google Scholar]
- Morales-Sánchez, E.; Figueroa, J.D.C.; Gaytán-Martínez, M. Proceso y Aparato Cocedor de Calentamiento Ohmico Continuo Para Obtener Masa y Harina de Maiz Nixtamalizada y Productos Derivados. MX Patent MX/a/2010/004151, 15 April 2010. [Google Scholar]
- AOAC. AOAC Official Methods of Analysis; Association of Official Agricultural Chemists: Washington, DC, USA, 2002; Volume 15, pp. 136–138. [Google Scholar]
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; DeVries, J.W.; Furda, I. Determination of Insoluble, Soluble, and Total Dietary Fiber in Foods and Food Products: Interlaboratory Study. J. Assoc. Off. Anal. Chem. 1988, 71, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Dlaby, A.; Jones, R.A. Lysine and Tryptophan Increases during Germination of Maize Seed. Cereal Chem. 1975, 52, 356–360. [Google Scholar]
- Gallicia, L.; Nurit, E.; Rosales, A.; Palacios Rojas, N. Laboratory Protocols 2008: Maize Nutrition Quality and Plant Tissue Analysis Laboratory; CIMMYT: Texcoco, Mexico, 2009. [Google Scholar]
- Landry, J.; Delhaye, S.; Damerval, C. Improved Method for Isolating and Quantitating α-Amino Nitrogen as Nonprotein, True Protein, Salt-Soluble Proteins, Zeins, and True Glutelins in Maize Endosperm. Cereal Chem. 2000, 77, 620–626. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Rivera-Muñoz, E.M.; Gutiérrez-Cortez, E.; del López, A.R.; Rodríguez-García, M.E. Characterization of Crystalline Structures in Opuntia Ficus-Indica. J. Biol. Phys. 2015, 41, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh, C.; Bhat, Z.R.; Aaliya, B.; Sunooj, K.V. Chapter 2—Cereal Proteins. In Nutraceuticals and Health Care; Kour, J., Nayik, G.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 29–60. ISBN 978-0-323-89779-2. [Google Scholar]
- Fuhrman, M.P.; Charney, P.; Mueller, C.M. Hepatic Proteins and Nutrition Assessment. J. Am. Diet. Assoc. 2004, 104, 1258–1264. [Google Scholar] [CrossRef]
- Campechano Carrera, E.M.; de Dios Figueroa Cárdenas, J.; Arámbula Villa, G.; Martínez Flores, H.E.; Jiménez Sandoval, S.J.; Luna Bárcenas, J.G. New Ecological Nixtamalisation Process for Tortilla Production and Its Impact on the Chemical Properties of Whole Corn Flour and Wastewater Effluents. Int. J. Food Sci. Technol. 2012, 47, 564–571. [Google Scholar] [CrossRef]
- Bello-Perez, L.A.; Flores-Silva, P.C.; Agama-Acevedo, E.; de Dios Figueroa-Cardenas, J.; Lopez-Valenzuela, J.A.; Campanella, O.H. Effect of the Nixtamalization with Calcium Carbonate on the Indigestible Carbohydrate Content and Starch Digestibility of Corn Tortilla. J. Cereal Sci. 2014, 60, 421–425. [Google Scholar] [CrossRef]
- Gutiérrez Hernández, G.F.G.; Arellano Vázquez, J.L.; Váquez Ramos, J.M.; García Ramírez, E.; Vázquez Lozano, P.; Flores Gómez, E. Formación de Híbridos de Maíz Con Calidad Proteica: Lisina, Triptófano e Índice de Calidad Formation of Quality Protein Maize Hybrids: Lysine, Tryptophan and Quality Index Resumen. Rev. De La Fac. De Agron. 2014, 31, 171–189. [Google Scholar]
- Vázquez Carrillo, G.; Mejía Andrade, H.; Salinas Moreno, Y.; Santiago Ramos, D. Effect of Plant Density on Kernel, Nixtamal and Tortilla Qualities of High Quality Protein Maize Hybrids. Rev. Fitotec. Mex. 2013, 36, 225–232. [Google Scholar]
- Martinez-Flores, H.E.; Garnica-Romo, M.G.; Romero, V.J.U.; Yahuaca, J.B. Evaluating the Quality of Lipids during Alkaline Cooking of Corn. J. Food Lipids 2006, 13, 177–185. [Google Scholar] [CrossRef]
- Thachil, M.T.; Chouksey, M.K.; Gudipati, V. Amylose-Lipid Complex Formation during Extrusion Cooking: Effect of Added Lipid Type and Amylose Level on Corn-Based Puffed Snacks. Int. J. Food Sci. Technol. 2014, 49, 309–316. [Google Scholar] [CrossRef]
- Santiago-Ramos, D.; De Dios Figueroa-Cárdenas, J.; Véles-Medina, J.J.; Mariscal-Moreno, R.M.; Reynoso-Camacho, R.; Ramos-Gómez, M.; Gaytán-Martínez, M.; Morales-Sánchez, E. Resistant Starch Formation in Tortillas from an Ecological Nixtamalization Process. Cereal Chem. 2015, 92, 185–192. [Google Scholar] [CrossRef]
- Cervantes-Ramírez, J.E.; Cabrera-Ramirez, A.H.; Morales-Sánchez, E.; Rodriguez-García, M.E.; de la Reyes-Vega, M.L.; Ramírez-Jiménez, A.K.; Contreras-Jiménez, B.L.; Gaytán-Martínez, M. Amylose-Lipid Complex Formation from Extruded Maize Starch Mixed with Fatty Acids. Carbohydr. Polym. 2020, 246, 116555. [Google Scholar] [CrossRef]
- Overby, H.B.; Ferguson, J.F. Gut Microbiota-Derived Short-Chain Fatty Acids Facilitate Microbiota: Host Cross Talk and Modulate Obesity and Hypertension. Curr. Hypertens. Rep. 2021, 23, 8. [Google Scholar] [CrossRef]
- Santos, E.M.; Quintanar-Guzman, A.; Solorza-Feria, J.; Sanchez-Ortega, I.; Rodriguez, J.A.; Wang, Y.-J. Thermal and Rheological Properties of Masa from Nixtamalized Corn Subjected to a Sequential Protein Extraction. J. Cereal Sci. 2014, 60, 490–496. [Google Scholar] [CrossRef]
- Rojas-Molina, I.; Gutiérrez, E.; Cortés-Acevedo, M.E.; Falcón, A.; Bressani, R.; Rojas, A.; Ibarra, C.; Pons-Hernández, J.L.; Guzmán-Maldonado, S.H.; Cornejo-Villegas, A.; et al. Analysis of Quality Protein Changes in Nixtamalized QPM Flours as a Function of the Steeping Time. Cereal Chem. 2008, 85, 409–416. [Google Scholar] [CrossRef]
- Chaidez-Laguna, L.D.; Torres-Chavez, P.; Ramírez-Wong, B.; Marquez-Ríos, E.; Islas-Rubio, A.R.; Carvajal-Millan, E. Corn Proteins Solubility Changes during Extrusion and Traditional Nixtamalization for Tortilla Processing: A Study Using Size Exclusion Chromatography. J. Cereal Sci. 2016, 69, 351–357. [Google Scholar] [CrossRef]
- Laleg, K.; Salles, J.; Berry, A.; Giraudet, C.; Patrac, V.; Guillet, C.; Denis, P.; Tessier, F.J.; Guilbaud, A.; Howsam, M.; et al. Nutritional Evaluation of Mixed Wheat-Faba Bean Pasta in Growing Rats: Impact of Protein Source and Drying Temperature on Protein Digestibility and Retention. Br. J. Nutr. 2019, 121, 496–507. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Suen, P.K.; Zhang, C.-Q.; Mak, W.S.; Gu, M.-H.; Liu, Q.-Q.; Sai, S.; Sun, M. Improved Growth Performance, Food Efficiency, and Lysine Availability in Growing Rats Fed with Lysine-Biofortified Rice. Sci. Rep. 2017, 7, 1389. [Google Scholar] [CrossRef]
- Martínez-Velasco, A.; Alvarez-Ramirez, J.; Rodríguez-Huezo, E.; Meraz-Rodríguez, M.; Vernon-Carter, E.J.; Lobato-Calleros, C. Effect of the Preparation Method and Storage Time on the in Vitro Protein Digestibility of Maize Tortillas. J. Cereal Sci. 2018, 84, 7–12. [Google Scholar] [CrossRef]
- Acevedo-Pacheco, L.; Serna-Saldívar, S.O. In Vivo Protein Quality of Selected Cereal-Based Staple Foods Enriched with Soybean Proteins. Food Nutr. Res. 2016, 60, 31382. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.N.; Teixeira, J.A.; Vicente, A.A. Exploring the Denaturation of Whey Proteins upon Application of Moderate Electric Fields: A Kinetic and Thermodynamic Study. J. Agric. Food Chem. 2011, 59, 11589–11597. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.F.; Morton, J.D.; El-Din Bekhit, A.A.; Kumar, S.; Bhat, H.F. Processing Technologies for Improved Digestibility of Milk Proteins. Trends Food Sci. Technol. 2021, 118, 1–16. [Google Scholar] [CrossRef]
- Alizadeh, O.; Aliakbarlu, J. Effects of Ultrasound and Ohmic Heating Pretreatments on Hydrolysis, Antioxidant and Antibacterial Activities of Whey Protein Concentrate and Its Fractions. LWT 2020, 131, 109913. [Google Scholar] [CrossRef]
- Zazueta, C.; Ramos, G.; Fernández-Muñoz, J.L.; Rodríguez, M.E.; Acevedo-Hernández, G.; Pless, R.C. A Radioisotopic Study of the Entry of Calcium Ion into the Maize Kernel during Nixtamalization. Cereal Chem. 2002, 79, 500–503. [Google Scholar] [CrossRef]





| Component (g/100 g) | CD | RC | CT | TPT | OHT |
|---|---|---|---|---|---|
| Moisture | 7.21 ± 0.07 b | 9.22 ± 0.07 a | 6.61 ± 0.07 c | 6.37 ± 0.03 d | 6.23 ± 0.06 d |
| Ash | 2.55 ± 0.06 a | 2.50 ± 0.01 a | 2.51 ± 0.07 a | 2.62 ± 0.03 a | 2.65 ± 0.04 a |
| Fat | 7.50 ± 0.06 a | 5.07 ± 0.14 b | 4.85 ± 0.23 b | 4.70 ± 0.03 b | 4.87 ± 0.20 b |
| Protein | 20.61 ± 0.30 a | 6.92 ± 0.05 d | 7.08 ± 0.07 c,d | 7.62 ± 0.01 b | 7.41 ± 0.07 b,c |
| Total dietary fiber | 5.69 ± 0.17 d | 8.16 ± 0.33 b | 6.95 ± 0.21 c | 6.72 ± 0.39 c | 10.83 ± 0.48 a |
| Insoluble dietary fiber | 5.03 ± 0.14 d | 7.82 ± 0.32 b | 6.46 ± 0.11 c | 6.21 ± 0.32 c | 10.03 ± 0.30 a |
| Soluble dietary fiber | 0.66 ± 0.03 a,b | 0.34 ± 0.00 b | 0.49 ± 0.10 b | 0.51 ± 0.07 a,b | 0.80 ± 0.18 a |
| Carbohydrates | 64.30 ± 0.28 d | 77.68 ± 0.13 b | 79.08 ± 0.29 a | 78.72 ± 0.39 a | 75.01 ± 29 c |
| Component (g/100 g) | RC | CTs | TPTs | OHTs |
|---|---|---|---|---|
| Moisture | 9.29 ± 0.31 a | 6.66 ± 0.07 c | 4.21 ± 0.31 b | 6.25 ± 0.01 c |
| Ash | 1.20 ± 0.05 c | 1.32 ± 0.01 b | 1.50 ± 0.10 a | 1.48 ± 0.07 a |
| Fat | 4.43 ± 0.18 a | 2.45 ± 0.23 c | 3.09 ± 0.05 b | 2.73 ± 0.24 b,c |
| Protein | 8.68 ± 0.45 a | 8.30 ± 0.14 a | 7.62 ± 0.10 b | 7.46 ± 0.05 b,c |
| Lysine | 0.214 ± 0.01 a | 0.221 ± 0.01 a | 0.18 ± 0.01 b | 0.15 ± 0.01 c |
| Tryptophan | 0.041 ± 0.01 a | 0.034 ± 0.01 a | 0.02 ± 0.01 b | 0.02 ± 0.01 b |
| Calcium | 0.56 ± 2.00 c | 0.96 ± 2.00 b | 2.55 ± 2.00 a | 2.56 ± 1.00 a |
| Total dietary fiber | 10.04 ± 0.13 a | 7.65 ± 0.34 b | 7.53 ± 0.22 b | 10.72 ± 0.42 a |
| Insoluble dietary fiber | 9.49 ± 0.01 a | 7.60 ± 0.32 b | 6.95 ± 0.39 b | 9.52 ± 0.20 a |
| Soluble dietary fiber | 0.543 ± 0.12 b,c | 0.05 ± 0.07 c | 0.58 ± 0.28 b | 1.20 ± 0.22 a |
| Carbohydrates | 76.35 ± 0.39 c | 81.27 ± 0.42 b | 83.58 ± 0.10 a | 82.08 ± 0.12 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Jiménez, A.K.; Cota-López, R.; Morales-Sánchez, E.; Gaytán-Martínez, M.; Martinez-Flores, H.E.; Reyes-Vega, M.d.l.L.; Figueroa-Cárdenas, J.d.D. Sustainable Process for Tortilla Production Using Ohmic Heating with Minimal Impact on the Nutritional Value, Protein, and Calcium Performance. Foods 2023, 12, 3327. https://doi.org/10.3390/foods12183327
Ramírez-Jiménez AK, Cota-López R, Morales-Sánchez E, Gaytán-Martínez M, Martinez-Flores HE, Reyes-Vega MdlL, Figueroa-Cárdenas JdD. Sustainable Process for Tortilla Production Using Ohmic Heating with Minimal Impact on the Nutritional Value, Protein, and Calcium Performance. Foods. 2023; 12(18):3327. https://doi.org/10.3390/foods12183327
Chicago/Turabian StyleRamírez-Jiménez, Aurea K., Rubén Cota-López, Eduardo Morales-Sánchez, Marcela Gaytán-Martínez, Héctor Eduardo Martinez-Flores, María de la Luz Reyes-Vega, and Juan de Dios Figueroa-Cárdenas. 2023. "Sustainable Process for Tortilla Production Using Ohmic Heating with Minimal Impact on the Nutritional Value, Protein, and Calcium Performance" Foods 12, no. 18: 3327. https://doi.org/10.3390/foods12183327
APA StyleRamírez-Jiménez, A. K., Cota-López, R., Morales-Sánchez, E., Gaytán-Martínez, M., Martinez-Flores, H. E., Reyes-Vega, M. d. l. L., & Figueroa-Cárdenas, J. d. D. (2023). Sustainable Process for Tortilla Production Using Ohmic Heating with Minimal Impact on the Nutritional Value, Protein, and Calcium Performance. Foods, 12(18), 3327. https://doi.org/10.3390/foods12183327