Application of Tannic Acid and Fe3+ Crosslinking-Enhanced Pectin Films for Passion Fruit Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
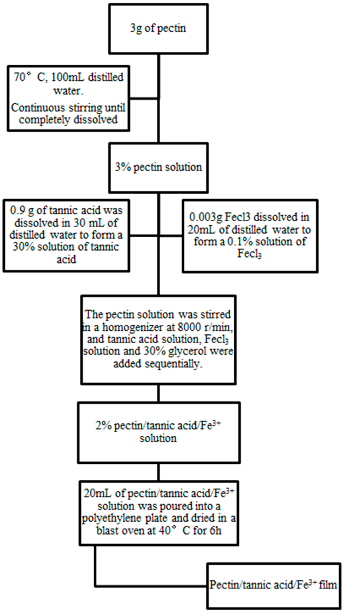
2.2. Preparation of PE/TA/Fe3+ Composite Film
2.3. Color Change and Ultraviolet Barrier Properties
2.4. ATR-FTIR and XRD
2.5. Microscopic Morphology
2.6. Moisture Content (MC), Water Vapor Permeability (WVP) and Water Contact Angle (WCA)
2.7. Mechanical Properties
2.8. Antioxidant Properties and Biodegradability
2.9. Application of PE/TA/Fe3+ Coating in Passion Fruit
2.10. Statistical Analysis
3. Results and Discussion
3.1. Thickness, Color and Optical Properties of PE Composite Films
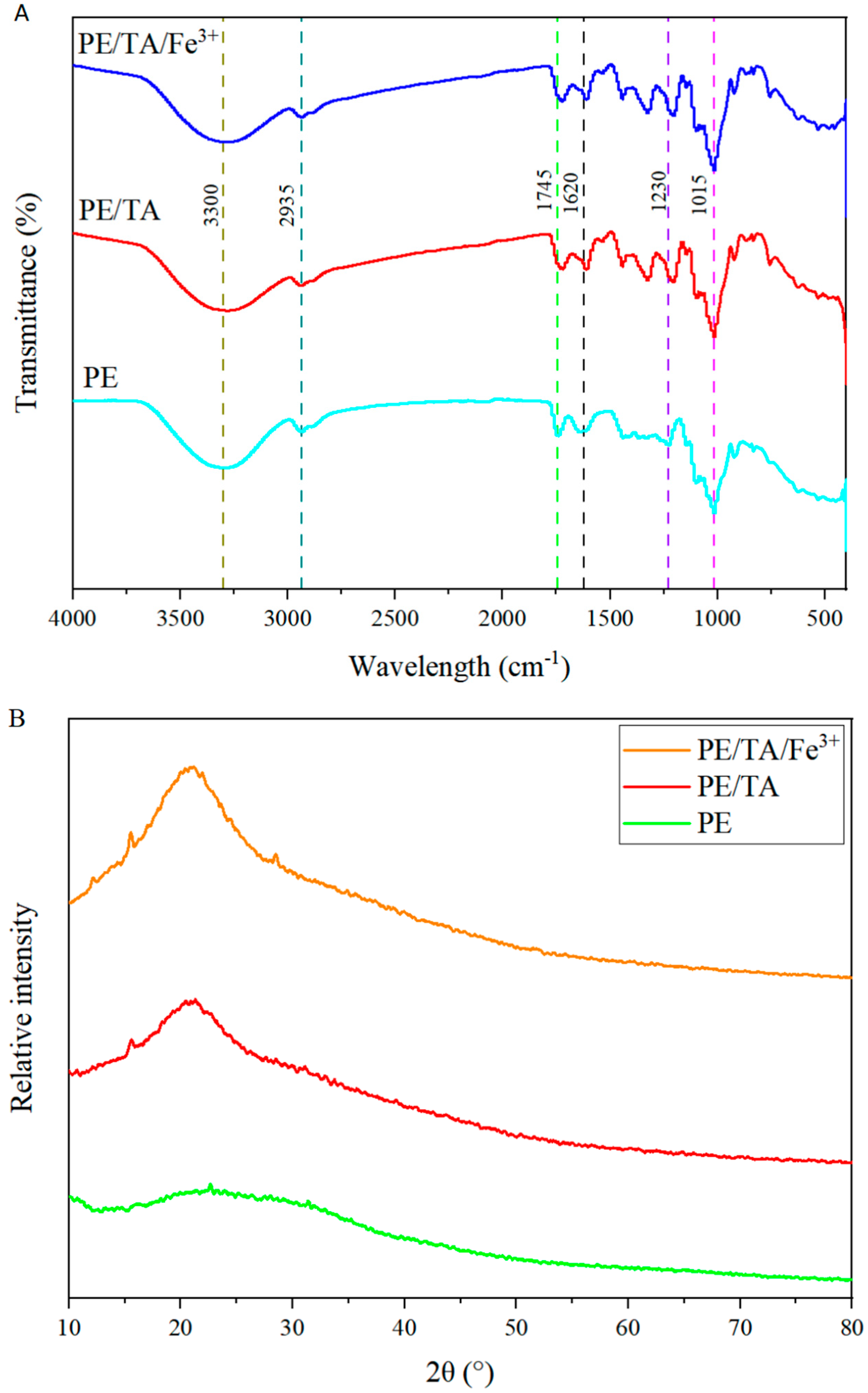
3.2. FTIR and XRD Analysis
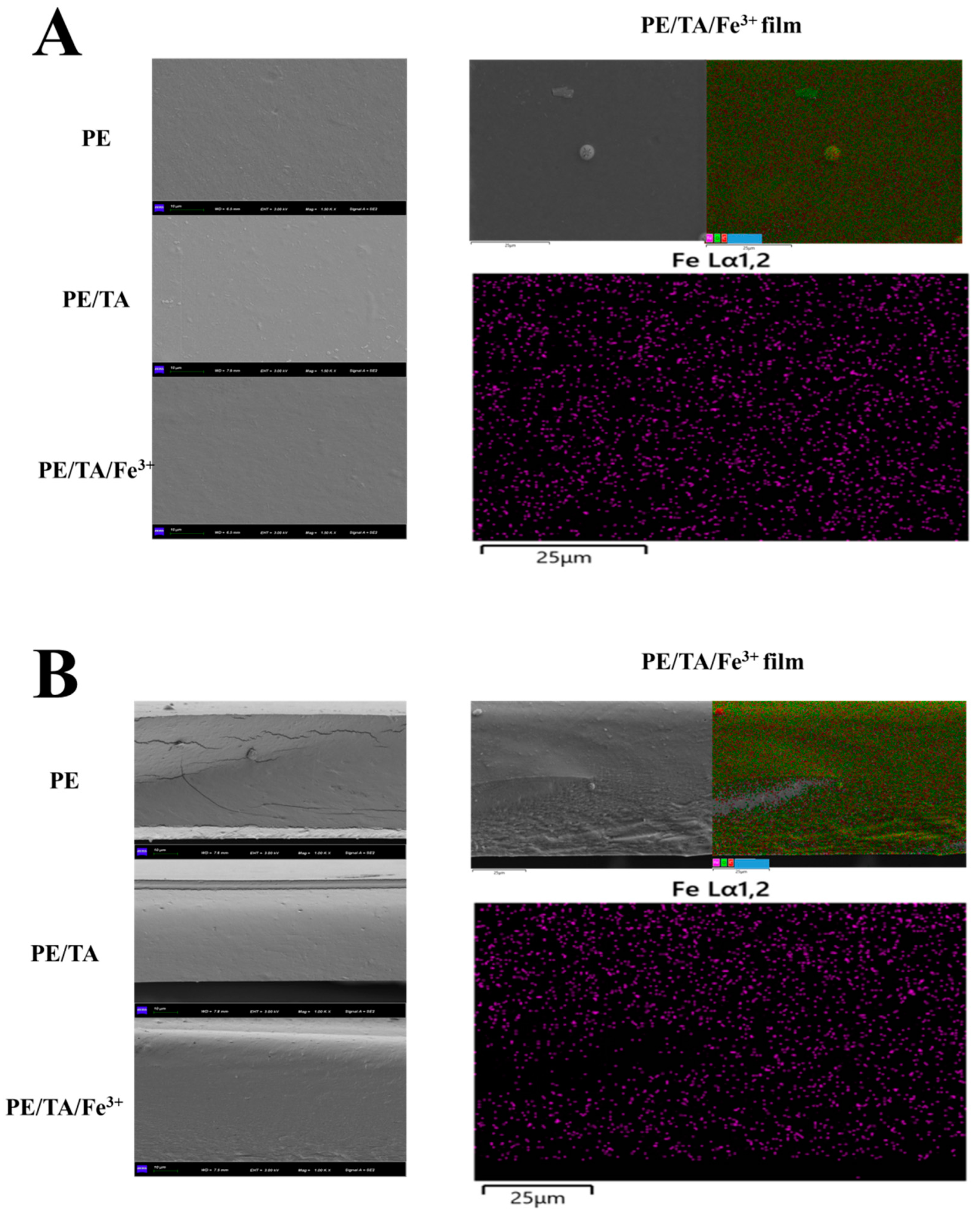
3.3. Micromorphology of PE Composite Films
3.4. MC, WVP and WCA of Pectin Composite Films
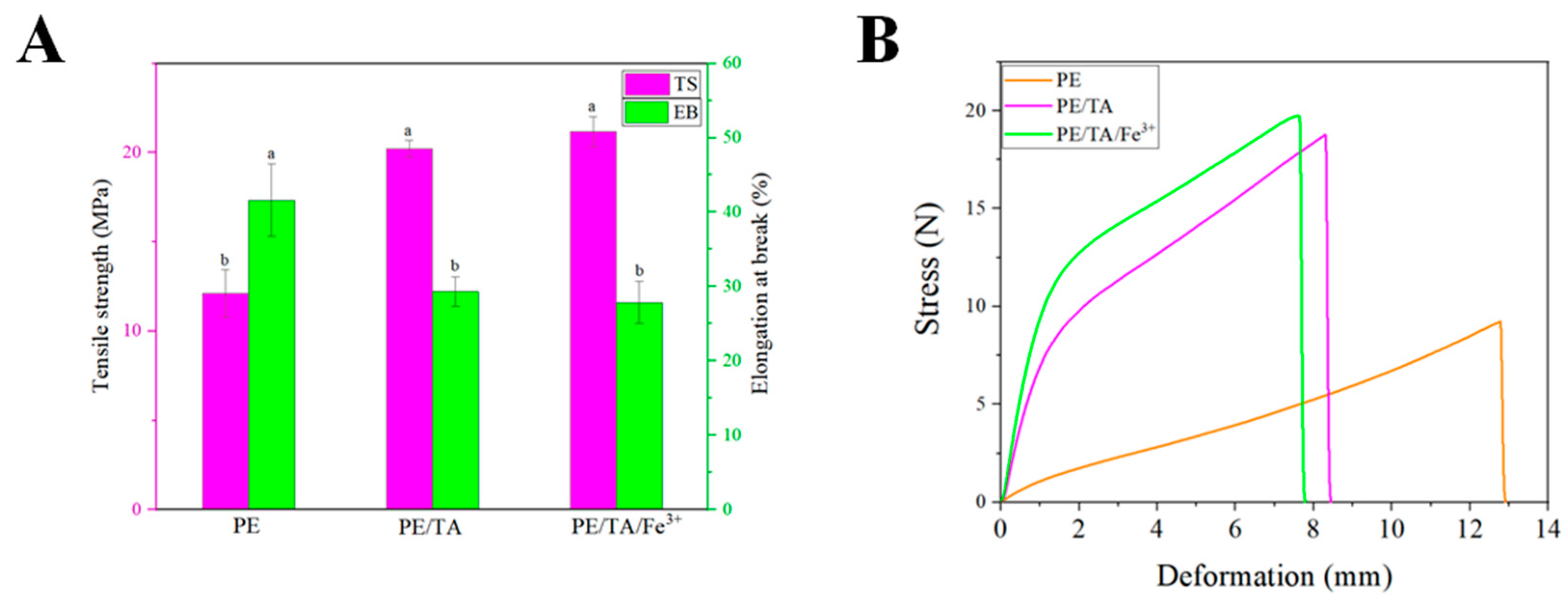
3.5. Mechanical Properties
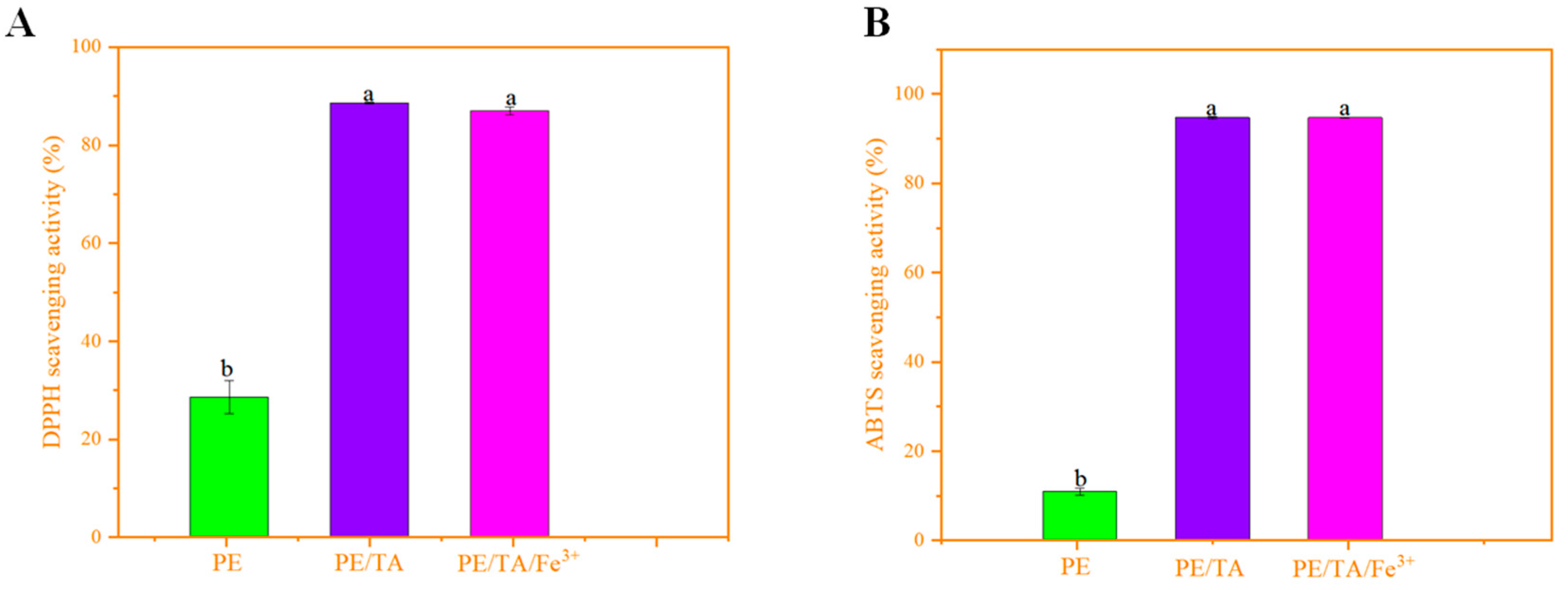
3.6. Antioxidant Properties
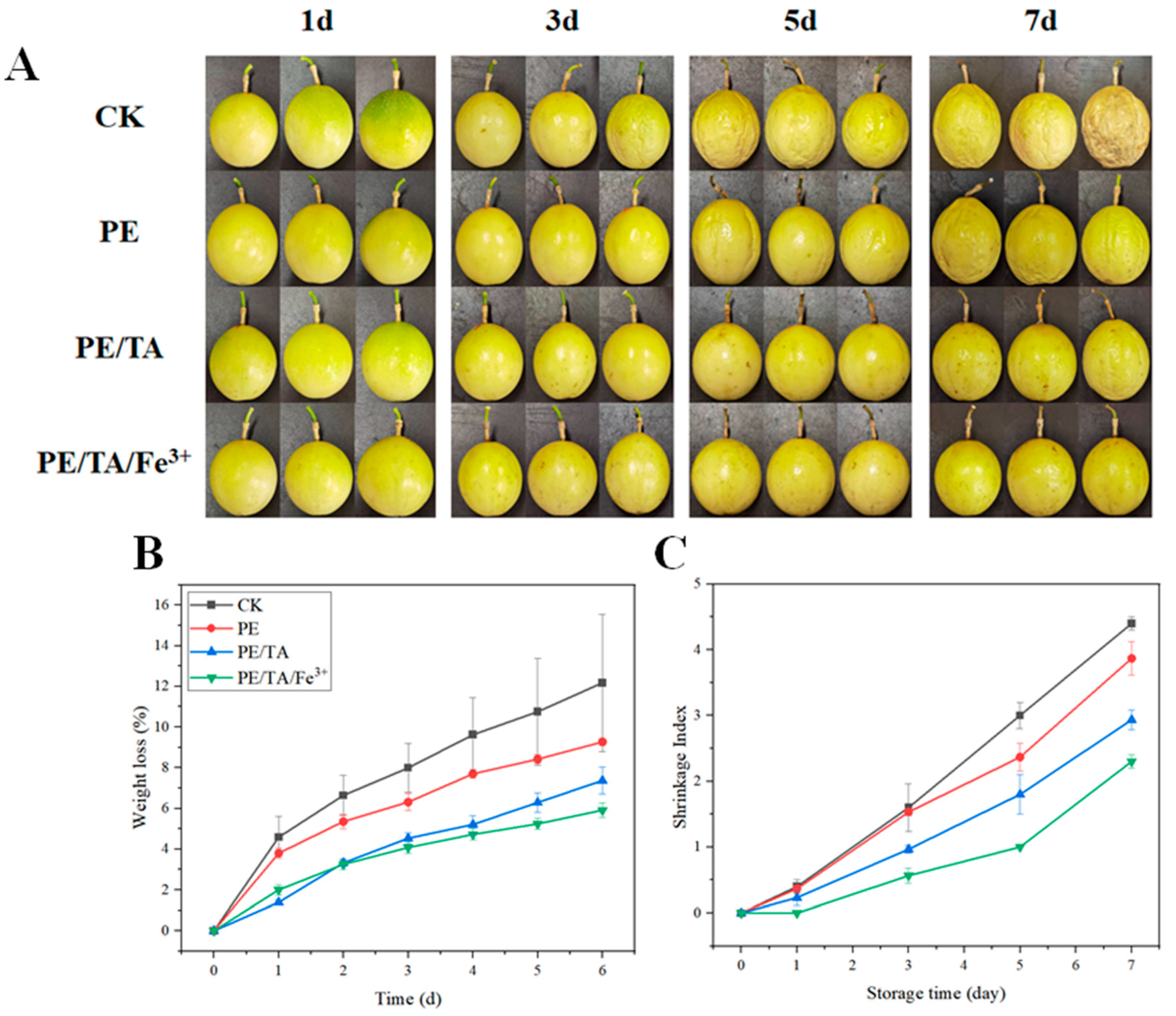
3.7. Application of PE/TA/Fe3+ Coating in Passion Fruit
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Espitia, P.J.P.; Du, W.-X.; de Jesús Avena-Bustillos, R.; Soares, N.d.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties-A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Rhim, J.-W.; Cao, J.; Jiang, W. Tea polyphenols (TP): A promising natural additive for the manufacture of multifunctional active food packaging films. Crit. Rev. Food Sci. Nutr. 2022, 63, 288–301. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J.-W. Functional edible films/coatings integrated with lactoperoxidase and lysozyme and their application in food preservation. Food Control. 2022, 133, 108670. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Frone, A.N.; Berlioz, S.; Chailan, J.-F.; Panaitescu, D.M. Morphology and thermal properties of PLA–cellulose nanofibers composites. Carbohydr. Polym. 2013, 91, 377–384. [Google Scholar] [CrossRef]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Łopusiewicz, Ł.; Biswas, D.; Chandel, V.; Rhim, J.-W. Recent progress in pectin extraction, characterization, and pectin-based films for active food packaging applications: A review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef]
- Chaichi, M.; Hashemi, M.; Badii, F.; Mohammadi, A. Preparation and characterization of a novel bionanocomposite edible film based on pectin and crystalline nanocellulose. Carbohydr. Poly. 2017, 157, 167–175. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Jiang, W. Effect of different cation in situ cross-linking on the properties of pectin-thymol active film. Food Hydrocoll. 2022, 128, 107594. [Google Scholar] [CrossRef]
- Yan, W.; Shi, M.; Dong, C.; Liu, L.; Gao, C. Applications of tannic acid in membrane technologies: A review. Adv. Colloid Interface Sci. 2020, 284, 102267. [Google Scholar] [CrossRef]
- Zhang, W.; Roy, S.; Ezati, P.; Yang, D.-P.; Rhim, J.-W. Tannic acid: A green crosslinker for biopolymer-based food packaging films. Trends Food Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Igarzabal, C.I.A. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic acid: A crosslinker leading to versatile functional polymeric networks: A review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef]
- Yang, J.; Li, M.; Wang, Y.; Wu, H.; Zhen, T.; Xiong, L.; Sun, Q. Double cross-linked chitosan composite films developed with oxidized tannic acid and ferric ions exhibit high strength and excellent water resistance. Biomacromolecules 2019, 20, 801–812. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, Y.; Li, L.; Jiang, K.; Gao, J.; Zhong, K.; Pan, M.; Yan, B. A multifunctional chitosan-derived conformal coating for the preservation of passion fruit. LWT 2022, 163, 113584. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, H.; Zhang, J.; Sheng, Z.; Cao, J.; Jiang, W. Different molecular weights chitosan coatings delay the senescence of postharvest nectarine fruit in relation to changes of redox state and respiratory pathway metabolism. Food Chem. 2019, 289, 160–168. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Cao, J.; Jiang, W. Effect of purple sugarcane peel extracts on properties of films based on lemon peel waste pectin and the application in the visible detection of food freshness. Food Hydrocoll. 2022, 133, 107982. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the performance of edible food packaging films by using nanocellulose as an additive. Int. J. Biol. Macromol. 2021, 166, 288–296. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.; Sun, J.; Lv, S. Bioactive Edible Sodium Alginate Films Incorporated with Tannic Acid as Antimicrobial and Antioxidative Food Packaging. Foods 2022, 11, 3044. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, Y.; Li, Y.; Xu, J.; Fu, X.; Gao, X.; Mao, X.; Li, Z. UV-shielding alginate films crosslinked with Fe3+ containing EDTA. Carbohydr. Polym. 2020, 239, 115480. [Google Scholar] [CrossRef]
- Hoang-Minh, T.; Le, T.; Kasbohm, J.; Gieré, R. UV-protection characteristics of some clays. Appl. Clay Sci. 2010, 48, 349–357. [Google Scholar] [CrossRef]
- Othman, S.H.; Nordin, N.; Azman, N.A.A.; Tawakkal, I.S.M.A.; Basha, R.K. Effects of nanocellulose fiber and thymol on mechanical, thermal, and barrier properties of corn starch films. Int. J. Biol. Macromol. 2021, 183, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Lorevice, M.V.; Otoni, C.G.; de Moura, M.R.; Mattoso, L.H.C. Chitosan nanoparticles on the improvement of thermal, barrier, and mechanical properties of high-and low-methyl pectin films. Food Hydrocoll. 2016, 52, 732–740. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Wu, D.; Zhu, K.; Ye, X. Manosonication assisted extraction and characterization of pectin from different citrus peel wastes. Food Hydrocoll. 2021, 121, 106952. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, J.; Zhou, H.; Zhou, S.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Wang, H. Biodegradable intelligent film for food preservation and real-time visual detection of food freshness. Food Hydrocoll. 2022, 129, 107665. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Zhang, X. Gelatin and high methyl pectin coacervates crosslinked with tannic acid: The characterization, rheological properties, and application for peppermint oil microencapsulation. Food Hydrocoll. 2019, 97, 105174. [Google Scholar] [CrossRef]
- Shankar, S.; Tanomrod, N.; Rawdkuen, S.; Rhim, J.-W. Preparation of pectin/silver nanoparticles composite films with UV-light barrier and properties. Int. J. Biol. Macromol. 2016, 92, 842–849. [Google Scholar] [CrossRef]
- Tian, B.; Li, W.; Wang, J.; Liu, Y. Functional polysaccharide-based film prepared from chitosan and β-acids: Structural, physicochemical, and bioactive properties. Int. J. Biol. Macromol. 2021, 181, 966–977. [Google Scholar] [CrossRef]
- Xu, H.; Huang, W.-P.; Ren, K.-F.; Tang, Y.-M. Spraying layer-by-layer assembly of tannin-Fe3+ and polyethyleneimine for antibacterial coating. Colloid Interface Sci. Commun. 2021, 42, 100422. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef] [PubMed]
- Isenburg, J.C.; Karamchandani, N.V.; Simionescu, D.T.; Vyavahare, N.R. Structural requirements for stabilization of vascular elastin by polyphenolic tannins. Biomaterials 2006, 27, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sousa, A.M.; Fan, X.; Jin, T.; Li, X.; Tomasula, P.M.; Liu, L. Electrospun ultra-fine cellulose acetate fibrous mats containing tannic acid-Fe3+ complexes. Carbohydr. Polym. 2017, 157, 1173–1179. [Google Scholar] [CrossRef]
- Xu, K.; Li, H.; Huang, X.; Qin, Z. Multi-crosslinked network chitosan films containing caffeic acid and Fe3+ with high anti-oxidation and anti-UV abilities. Int. J. Biol. Macromol. 2022, 223, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, S.; Salamanca, M.C.; Sierra, C.A.; Palomeque, L.A.; Castellanos, D.A. Determination of changes in physicochemical and sensory characteristics of purple passion fruit with the application of different packaging systems, including an ethylene scavenger additive. J. Food Sci. 2021, 86, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- Nor, S.M.; Ding, P. Trends and advances in edible biopolymer coating for tropical fruit: A review. Food Res. Int. 2020, 134, 109208. [Google Scholar] [CrossRef]
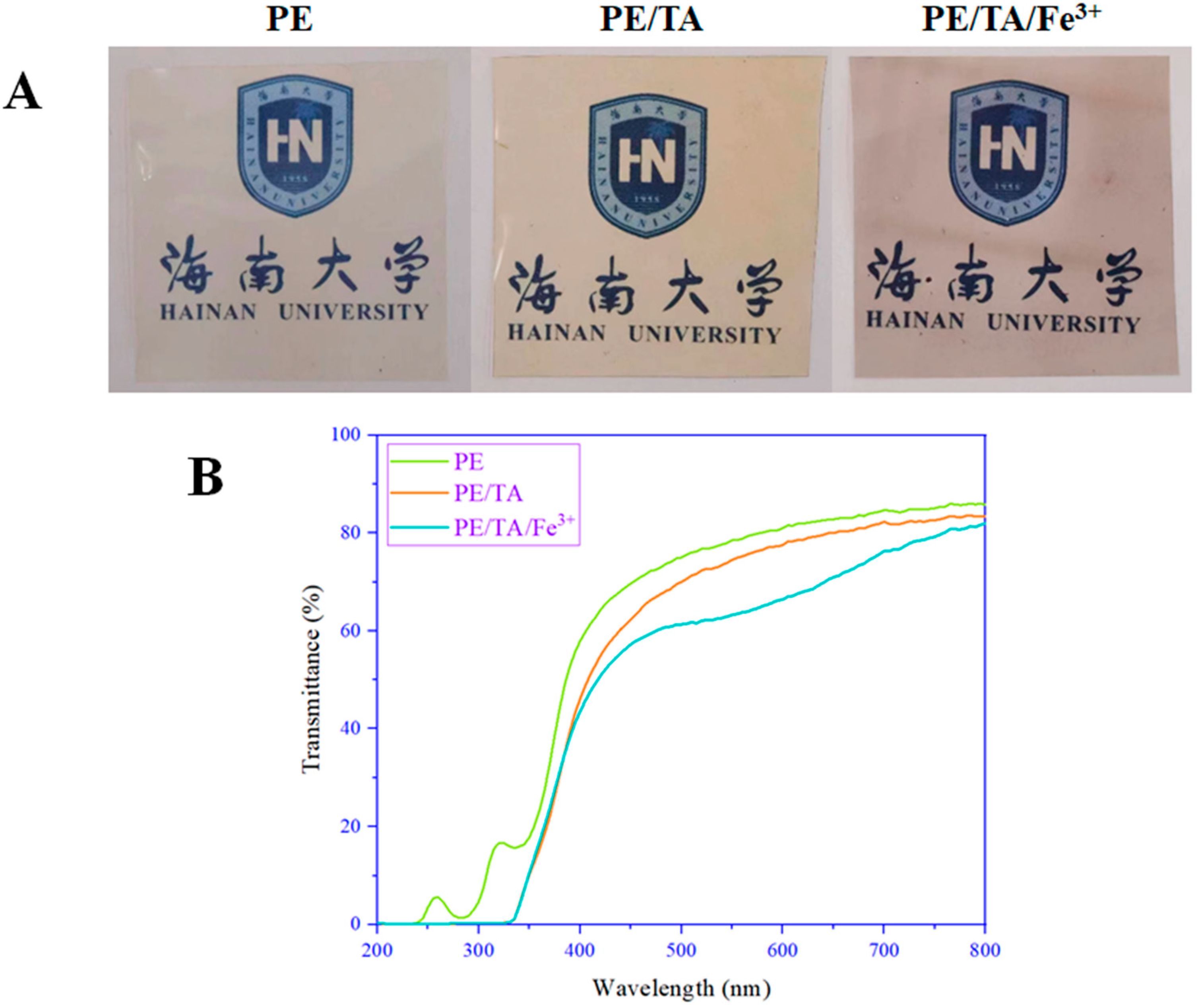
| Film | Thickness (μm) | L* | a* | b* | Opacity |
|---|---|---|---|---|---|
| PE | 86 ± 0.37 c | 86.99 ± 3.91 a | 0.32 ± 0.17 b | 0.63 ± 1.52 b | 1.049 ± 0.027 b |
| PE/TA | 92 ± 0.17 b | 74.33 ± 5.67 b | 0.15 ± 0.28 b | 6.45 ± 1.89 a | 1.129 ± 0.066 b |
| PE/TA/Fe3+ | 97 ± 0.78 a | 68.80 ± 1.97 c | 3.63 ± 0.74 a | 1.02 ± 1.07 b | 1.749 ± 0.077 a |
| Film | WC (%) | WVP (×10−10 gm−1s−1Pa−1) | WCA (°) |
|---|---|---|---|
| PE | 18.57 ± 0.26 a | 1.61 ± 0.06 a | 52.73 ± 3.23 a |
| PE/TA | 13.78 ± 0.32 b | 1.36 ± 0.08 b | 30.36 ± 2.48 b |
| PE/TA/Fe3+ | 14.42 ± 0.44 b | 1.23 ± 0.14 b | 17.92 ± 1.66 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Cai, W.; Rizwan Khan, M.; Ahmad, N.; Zhang, Z.; Meng, L.; Zhang, W. Application of Tannic Acid and Fe3+ Crosslinking-Enhanced Pectin Films for Passion Fruit Preservation. Foods 2023, 12, 3336. https://doi.org/10.3390/foods12183336
Yang J, Cai W, Rizwan Khan M, Ahmad N, Zhang Z, Meng L, Zhang W. Application of Tannic Acid and Fe3+ Crosslinking-Enhanced Pectin Films for Passion Fruit Preservation. Foods. 2023; 12(18):3336. https://doi.org/10.3390/foods12183336
Chicago/Turabian StyleYang, Jun, Wenjin Cai, Mohammad Rizwan Khan, Naushad Ahmad, Zhengke Zhang, Lanhuan Meng, and Wanli Zhang. 2023. "Application of Tannic Acid and Fe3+ Crosslinking-Enhanced Pectin Films for Passion Fruit Preservation" Foods 12, no. 18: 3336. https://doi.org/10.3390/foods12183336
APA StyleYang, J., Cai, W., Rizwan Khan, M., Ahmad, N., Zhang, Z., Meng, L., & Zhang, W. (2023). Application of Tannic Acid and Fe3+ Crosslinking-Enhanced Pectin Films for Passion Fruit Preservation. Foods, 12(18), 3336. https://doi.org/10.3390/foods12183336





