UHPLC-ESI-QTOF-MS/MS Profiling of Phytochemicals from Araticum Fruit (Annona crassiflora Mart.) and Its Antioxidant Activity
Abstract
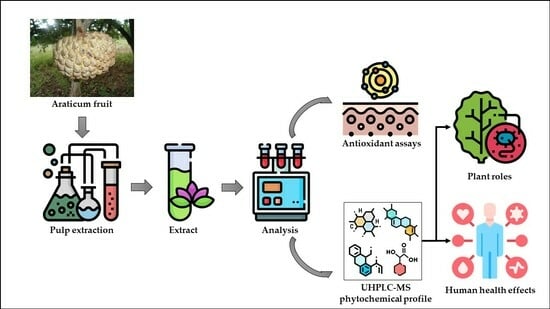
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Sample Preparation
2.3. Extraction Procedure
2.4. Determination of Total Phenolic Compounds (TPC)
2.5. Trolox Equivalent Antioxidant Capacity (TEAC) Assay Using ABTS•+ radical
2.6. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.7. Phytochemical Profile Analysis by UHPLC-ESI-QTOF-MS/MS
2.8. Data Analysis
3. Results and Discussion
3.1. Total Phenolic Content and Antioxidant Activities
3.2. Phytochemical Profile by UHPLC-ESI-QTOF-MS/MS
3.2.1. Organic Acids
3.2.2. Jasmonates
3.2.3. Iridoids
3.2.4. Phenolic Compounds
3.2.5. Alkaloids
3.2.6. Annonaceous Acetogenins
3.2.7. Fatty Acid Derivatives
3.2.8. Other Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, P.M.P.; Arcanjo, D.D.R.; Peron, A.P. Drug development, Brazilian biodiversity and political choices: Where are we heading? J. Toxicol. Environ. Health Part B 2023, 26, 257–274. [Google Scholar] [CrossRef]
- Convention on Biological Diversity Brazil—Main Details. Available online: https://www.cbd.int/countries/profile/?country=br (accessed on 18 May 2023).
- Arruda, H.S.; Botrel, D.A.; Fernandes, R.V.d.B.; Ferreira de Almeida, M.E. Development and sensory evaluation of products containing the Brazilian Savannah fruits araticum (Annona crassiflora Mart.) and cagaita (Eugenia dysenterica Mart.). Braz. J. Food Technol. 2016, 19, e2015105. [Google Scholar] [CrossRef]
- Arruda, H.S.; Araújo, M.V.L.; Marostica Junior, M.R. Underexploited Brazilian Cerrado fruits as sources of phenolic compounds for diseases management: A review. Food Chem. Mol. Sci. 2022, 5, 100148. [Google Scholar] [CrossRef]
- Embrapa Bioma Cerrado. Available online: https://www.embrapa.br/contando-ciencia/bioma-cerrado (accessed on 8 June 2023).
- Arruda, H.S.; Borsoi, F.T.; Andrade, A.C.; Pastore, G.M.; Marostica Junior, M.R. Scientific Advances in the Last Decade on the Recovery, Characterization, and Functionality of Bioactive Compounds from the Araticum Fruit (Annona crassiflora Mart.). Plants 2023, 12, 1536. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Pereira, G.A.; Pastore, G.M. Optimization of Extraction Parameters of Total Phenolics from Annona crassiflora Mart. (Araticum) Fruits Using Response Surface Methodology. Food Anal. Methods 2017, 10, 100–110. [Google Scholar] [CrossRef]
- Arruda, H.S.; Pereira, G.A.; de Morais, D.R.; Eberlin, M.N.; Pastore, G.M. Determination of free, esterified, glycosylated and insoluble-bound phenolics composition in the edible part of araticum fruit (Annona crassiflora Mart.) and its by-products by HPLC-ESI-MS/MS. Food Chem. 2018, 245, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.C.G.; Gomes, M.d.S.; Lima, L.M.Z.; Sales, P.F.; da Cunha, M.C.; Rodrigues, L.J.; de Barros, H.E.A.; Pires, C.R.F.; dos Santos, V.F.; Natarelli, C.V.L.; et al. Application of Chemometric Techniques In The Evaluation of Bioactive Compounds and Antioxidant Activity of Fruit From Brazilian Cerrado. J. Food Meas. Charact. 2023, 17, 2095–2106. [Google Scholar] [CrossRef]
- Seixas, F.R.F.; Bassoli, B.K.; Virgolin, L.B.; Garcia, L.C.; Janzantti, N.S. Physicochemical Properties and Effects of Fruit Pulps from the Amazon Biome on Physiological Parameters in Rats. Nutrients 2021, 13, 1484. [Google Scholar] [CrossRef] [PubMed]
- Stafussa, A.P.; Maciel, G.M.; Bortolini, D.G.; Maroldi, W.V.; Ribeiro, V.R.; Fachi, M.M.; Pontarolo, R.; Bach, F.; Pedro, A.C.; Haminiuk, C.W.I. Bioactivity and bioaccessibility of phenolic compounds from Brazilian fruit purees. Futur. Foods 2021, 4, 100066. [Google Scholar] [CrossRef]
- da Silva, J.J.; Cerdeira, C.D.; Chavasco, J.M.; Cintra, A.B.P.; da Silva, C.B.P.; de Mendonça, A.N.; Ishikawa, T.; Boriollo, M.F.G.; Chavasco, J.K. In vitro screening antibacterial activity of Bidens pilosa Linné and Annona crassiflora Mart. against oxacillin resistant Staphylococcus aureus (ORSA) from the aerial environment at the dental clinic. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 333–340. [Google Scholar] [CrossRef]
- Lucas dos Santos, E.; Leite, N.; Alves de Araújo, L.C.; Giffoni de Carvalho, J.T.; de Picoli Souza, K. Protective effect of Annona crassiflora on oxidative stress and Alzheimer’s models in Caenorhabditis elegans. Free Radic. Biol. Med. 2018, 128, S125. [Google Scholar] [CrossRef]
- Carvalho, N.C.C.; Monteiro, O.S.; da Rocha, C.Q.; Longato, G.B.; Smith, R.E.; da Silva, J.K.R.; Maia, J.G.S. Phytochemical Analysis of the Fruit Pulp Extracts from Annona crassiflora Mart. and Evaluation of Their Antioxidant and Antiproliferative Activities. Foods 2022, 11, 2079. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Silva, E.K.; Pereira, G.A.; Angolini, C.F.F.; Eberlin, M.N.; Meireles, M.A.A.; Pastore, G.M. Effects of high-intensity ultrasound process parameters on the phenolic compounds recovery from araticum peel. Ultrason. Sonochem. 2019, 50, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; de Camargo, A.C.; Shahidi, F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC−Fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Abeywickrama, G.; Debnath, S.C.; Ambigaipalan, P.; Shahidi, F. Phenolics of selected cranberry genotypes (Vaccinium macrocarpon Ait.) and their antioxidant efficacy. J. Agric. Food Chem. 2016, 64, 9342–9351. [Google Scholar] [CrossRef]
- Arruda, H.S.; Pereira, G.A.; Pastore, G.M. Brazilian Cerrado fruit araticum (Annona crassiflora Mart.) as a potential source of natural antioxidant compounds. Int. Food Res. J. 2018, 25, 2005–2012. [Google Scholar]
- Villela, P.; Batista, Â.G.; Dessimoni-Pinto, N.A.V. Nutritional composition of Annona crassiflora pulp and acceptability of bakery products prepared with its flour. Food Sci. Technol. 2013, 33, 417–423. [Google Scholar] [CrossRef]
- Siqueira, E.M.d.A.; Rosa, F.R.; Fustinoni, A.M.; de Sant’Ana, L.P.; Arruda, S.F. Brazilian Savanna Fruits Contain Higher Bioactive Compounds Content and Higher Antioxidant Activity Relative to the Conventional Red Delicious Apple. PLoS ONE 2013, 8, e72826. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.L.C.C.; Minighin, E.C.; Soares, I.I.C.; Ferreira, R.M.d.S.B.; de Sousa, I.M.N.; Augusti, R.; Labanca, R.A.; de Araújo, R.L.B.; Melo, J.O.F. Evaluation of the total phenolic content, antioxidative capacity, and chemical fingerprint of Annona crassiflora Mart. bioaccessible molecules. Food Res. Int. 2023, 165, 112514. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.R.; Pereira, P.A.P.; Queiroz, F.; Borges, S.V.; de Deus Souza Carneiro, J. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 2012, 134, 381–386. [Google Scholar] [CrossRef]
- Schiassi, M.C.E.V.; de Souza, V.R.; Lago, A.M.T.; Campos, L.G.; Queiroz, F. Fruits from the Brazilian Cerrado region: Physico-chemical characterization, bioactive compounds, antioxidant activities, and sensory evaluation. Food Chem. 2018, 245, 305–311. [Google Scholar] [CrossRef]
- Nascimento, A.L.A.A.; Brandi, I.V.; Durães, C.A.F.; De Lima, J.P.; Soares, S.B.; Mesquita, B.M.A.D.C. Chemical characterization and antioxidant potential of native fruits of the Cerrado of northern Minas Gerais. Braz. J. Food Technol. 2020, 23, e2019296. [Google Scholar] [CrossRef]
- Morais, E.C.D.; Patias, S.G.D.O.; Ferreira, N.S.D.S.; Picanço, N.F.M.; Rodrigues, E.C.; Nascimento, E.; Faria, R.A.P.G.D. Bioactive compounds and physicochemical characteristics of in natura and pasteurized araticum pulp. Braz. J. Food Technol. 2017, 20, e2016142. [Google Scholar] [CrossRef]
- Damiani, C.; Vilas Boas, E.V.d.B.; Asquieri, E.R.; Lage, M.E.; de Oliveira, R.A.; da Silva, F.A.; Pinto, D.M.; Rodrigues, L.J.; da Silva, E.P.; de Paula, N.R.F. Characterization of fruits from the savanna: Araça (Psidium guinnensis Sw.) and Marolo (Annona crassiflora Mart.). Ciência Tecnol. Aliment. 2011, 31, 723–729. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Chen, G.-L.; Zhang, X.; Chen, S.-G.; Han, M.-D.; Gao, Y.-Q. Antioxidant activities and contents of free, esterified and insoluble-bound phenolics in 14 subtropical fruit leaves collected from the south of China. J. Funct. Foods 2017, 30, 290–302. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Moo-Huchin, V.M.; Estrada-Mota, I.; Estrada-León, R.; Cuevas-Glory, L.; Ortiz-Vázquez, E.; de Lourdes Vargas y Vargas, M.; Betancur-Ancona, D.; Sauri-Duch, E. Determination of some physicochemical characteristics, bioactive compounds and antioxidant activity of tropical fruits from Yucatan, Mexico. Food Chem. 2014, 152, 508–515. [Google Scholar] [CrossRef] [PubMed]
- de la Luz Cádiz-Gurrea, M.; Fernández-Arroyo, S.; Joven, J.; Segura-Carretero, A. Comprehensive characterization by UHPLC-ESI-Q-TOF-MS from an Eryngium bourgatii extract and their antioxidant and anti-inflammatory activities. Food Res. Int. 2013, 50, 197–204. [Google Scholar] [CrossRef]
- Zhu, G.-L.; Wang, B.; Feng, G.; Huang, A.-X.; Yin, G.; Wang, S.-H.; Su, H.-M.; Wang, W.-J.; Wang, P.; Yu, X.-A. Exploring the processing-related components from asparagi radix via diversified spectrum-effect relationship. Chin. J. Anal. Chem. 2023, 51, 100214. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Yang, Z.; Zhang, W.; Hua, J. Simultaneous determination of furfural and its degradation products, furoic acid and maleic acid, in transformer oil by the reversed-phase vortex-assisted liquid-liquid microextraction followed by high-performance liquid chromatography. J. Sep. Sci. 2017, 40, 4805–4812. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; del Mar Contreras, M.; Arráez-Román, D.; Fernández-Gutiérrez, A.; Segura-Carretero, A. UHPLC-ESI-QTOF-MS-based metabolic profiling of Vicia faba L. (Fabaceae) seeds as a key strategy for characterization in foodomics. Electrophoresis 2014, 35, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Melguizo, D.; Diaz-de-Cerio, E.; Quirantes-Piné, R.; Švarc-Gajić, J.; Segura-Carretero, A. The potential of Artemisia vulgaris leaves as a source of antioxidant phenolic compounds. J. Funct. Foods 2014, 10, 192–200. [Google Scholar] [CrossRef]
- Elsadig Karar, M.G.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS Characterization of Phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) Leaves, Fruits and their Herbal Derived Drops (Crataegutt Tropfen). J. Chem. Biol. Ther. 2016, 1, 1000102. [Google Scholar] [CrossRef]
- HMDB Human Metabolome Database: Browsing Metabolites. Available online: https://hmdb.ca/metabolites (accessed on 6 June 2023).
- Zhang, Y.-D.; Huang, X.; Zhao, F.-L.; Tang, Y.-L.; Yin, L. Study on the chemical markers of Caulis Lonicerae japonicae for quality control by HPLC-QTOF/MS/MS and chromatographic fingerprints combined with chemometrics methods. Anal. Methods 2015, 7, 2064–2076. [Google Scholar] [CrossRef]
- da Silva, G.S.; Canuto, K.M.; Ribeiro, P.R.V.; de Brito, E.S.; Nascimento, M.M.; Zocolo, G.J.; Coutinho, J.P.; de Jesus, R.M. Chemical profiling of guarana seeds (Paullinia cupana) from different geographical origins using UPLC-QTOF-MS combined with chemometrics. Food Res. Int. 2017, 102, 700–709. [Google Scholar] [CrossRef]
- Kurt-Celep, I.; Zheleva-Dimitrova, D.; Gevrenova, R.; Uba, A.I.; Zengin, G.; Yıldıztugay, E.; Picot-Allain, C.M.N.; Lorenzo, J.M.; Mahomoodally, M.F.; Montesano, D. An In-Depth Study on the Metabolite Profile and Biological Properties of Primula auriculata Extracts: A Fascinating Sparkle on the Way from Nature to Functional Applications. Antioxidants 2022, 11, 1377. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef]
- Mekky, R.H.; Contreras, M.D.M.; El-Gindi, M.R.; Abdel-Monem, A.R.; Abdel-Sattar, E.; Segura-Carretero, A. Profiling of phenolic and other compounds from Egyptian cultivars of chickpea (Cicer arietinum L.) and antioxidant activity: A comparative study. RSC Adv. 2015, 5, 17751–17767. [Google Scholar] [CrossRef]
- Wallis, C.; Eyles, A.; Chorbadjian, R.A.; Riedl, K.; Schwartz, S.; Hansen, R.; Cipollini, D.; Herms, D.A.; Bonello, P. Differential effects of nutrient availability on the secondary metabolism of Austrian pine (Pinus nigra) phloem and resistance to Diplodia pinea. For. Pathol. 2011, 41, 52–58. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, X.; Liu, J.; Kang, L.; Chen, S.; Ma, B.; Guo, B. Quantitative and Qualitative Analysis of Flavonoids and Phenolic Acids in Snow Chrysanthemum (Coreopsis tinctoria Nutt.) by HPLC-DAD and UPLC-ESI-QTOF-MS. Molecules 2016, 21, 1307. [Google Scholar] [CrossRef] [PubMed]
- Engels, C.; Gräter, D.; Esquivel, P.; Jiménez, V.M.; Gänzle, M.G.; Schieber, A. Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- Gevrenova, R.; Zengin, G.; Sinan, K.I.; Zheleva-Dimitrova, D.; Balabanova, V.; Kolmayer, M.; Voynikov, Y.; Joubert, O. An In-Depth Study of Metabolite Profile and Biological Potential of Tanacetum balsamita L. (Costmary). Plants 2022, 12, 22. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.; Xie, X.; Wang, H.; Wang, H.; Wang, Z.; Sha, X.; Lu, Y. Jackfruit (Artocarpus heterophyllus Lam.) peel: A better source of antioxidants and α-glucosidase inhibitors than pulp, flake and seed, and phytochemical profile by HPLC-QTOF-MS/MS. Food Chem. 2017, 234, 303–313. [Google Scholar] [CrossRef]
- Zeng, X.; Su, W.; Bai, Y.; Chen, T.; Yan, Z.; Wang, J.; Su, M.; Zheng, Y.; Peng, W.; Yao, H. Urinary metabolite profiling of flavonoids in Chinese volunteers after consumption of orange juice by UFLC-Q-TOF-MS/MS. J. Chromatogr. B 2017, 1061–1062, 79–88. [Google Scholar] [CrossRef]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Nardin, T.; Serra, M.; Larcher, R. Non-targeted glycosidic profiling of international wines using neutral loss-high resolution mass spectrometry. J. Chromatogr. A 2018, 1557, 75–89. [Google Scholar] [CrossRef]
- FooDB Spectra Search Tandom Mass Spectrum. Available online: https://foodb.ca/spectra/ms_ms/search (accessed on 7 June 2023).
- Contreras, M.d.M.; Algieri, F.; Rodriguez-Nogales, A.; Gálvez, J.; Segura-Carretero, A. Phytochemical profiling of anti-inflammatory Lavandula extracts via RP-HPLC-DAD-QTOF-MS and -MS/MS: Assessment of their qualitative and quantitative differences. Electrophoresis 2018, 39, 1284–1293. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Spínola, V.; Castilho, P.C. Phenolic profiles of Lauraceae plant species endemic to Laurisilva forest: A chemotaxonomic survey. Ind. Crops Prod. 2017, 107, 1–12. [Google Scholar] [CrossRef]
- Wang, T.-M.; Fu, Y.; Yu, W.-J.; Chen, C.; Di, X.; Zhang, H.; Zhai, Y.-J.; Chu, Z.-Y.; Kang, T.-G.; Chen, H.-B. Identification of Polar Constituents in the Decoction of Juglans mandshurica and in the Medicated Egg Prepared with the Decoction by HPLC-Q-TOF MS2. Molecules 2017, 22, 1452. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Rodríguez-Nogales, A.; Algieri, F.; Romero, M.; Verardo, V.; Segura-Carretero, A.; Duarte, J.; Galvez, J. The hypoglycemic effects of guava leaf (Psidium guajava L.) extract are associated with improving endothelial dysfunction in mice with diet-induced obesity. Food Res. Int. 2017, 96, 64–71. [Google Scholar] [CrossRef]
- Baky, M.H.; Badawy, M.T.; Bakr, A.F.; Hegazi, N.M.; Abdellatif, A.; Farag, M.A. Metabolome-based profiling of African baobab fruit (Adansonia digitata L.) using a multiplex approach of MS and NMR techniques in relation to its biological activity. RSC Adv. 2021, 11, 39680–39695. [Google Scholar] [CrossRef]
- Nguyen, T.-K.-O.; Jamali, A.; Grand, E.; Morreel, K.; Marcelo, P.; Gontier, E.; Dauwe, R. Phenylpropanoid profiling reveals a class of hydroxycinnamoyl glucaric acid conjugates in Isatis tinctoria leaves. Phytochemistry 2017, 144, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Zeng, X.; Yang, L.; Deng, Y. Chemical profiling of bioactive constituents in Sarcandra glabra and its preparations using ultra-high-pressure liquid chromatography coupled with LTQ Orbitrap mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 2439–2447. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yang, Q.; Zhao, K.; Zhao, M. Identification of the free phenolic profile of Adlay bran by UPLC-QTOF-MS/MS and inhibitory mechanisms of phenolic acids against xanthine oxidase. Food Chem. 2018, 253, 108–118. [Google Scholar] [CrossRef]
- Zhu, Y.; Sang, S. Phytochemicals in whole grain wheat and their health-promoting effects. Mol. Nutr. Food Res. 2017, 61, 1600852. [Google Scholar] [CrossRef]
- Michalak, B.; Filipek, A.; Chomicki, P.; Pyza, M.; Woźniak, M.; Żyżyńska-Granica, B.; Piwowarski, J.P.; Kicel, A.; Olszewska, M.A.; Kiss, A.K. Lignans From Forsythia x Intermedia Leaves and Flowers Attenuate the Pro-inflammatory Function of Leukocytes and Their Interaction With Endothelial Cells. Front. Pharmacol. 2018, 9, 325286. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Gómez-Caravaca, A.M.; Guerra-Hernández, E.; Cerretani, L.; García-Villanova, B.; Verardo, V. Comprehensive metabolite profiling of Solanum tuberosum L. (potato) leaves by HPLC-ESI-QTOF-MS. Food Res. Int. 2018, 112, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Ciulu, M.; Quirantes-Piné, R.; Spano, N.; Sanna, G.; Borrás-Linares, I.; Segura-Carretero, A. Evaluation of new extraction approaches to obtain phenolic compound-rich extracts from Stevia rebaudiana Bertoni leaves. Ind. Crops Prod. 2017, 108, 106–112. [Google Scholar] [CrossRef]
- Karioti, A.; Kukić-Marković, J.; Bilia, A.R.; Niketić, M.; Petrović, S. Chemical profiling of six Stachys taxa from Balkan Peninsula. Biochem. Syst. Ecol. 2022, 104, 104482. [Google Scholar] [CrossRef]
- Rasheed, D.M.; El Zalabani, S.M.; Koheil, M.A.; El-Hefnawy, H.M.; Farag, M.A. Metabolite profiling driven analysis of Salsola species and their anti-acetylcholinesterase potential. Nat. Prod. Res. 2013, 27, 2320–2327. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Y.; Mi, S.; Zhang, J.; Sun, Y. Rapid screening and characterization of caffeic acid metabolites in rats by UHPLC-Q-TOF mass spectrometry. Trop. J. Pharm. Res. 2022, 20, 389–401. [Google Scholar] [CrossRef]
- Baskaran, R.; Pullencheri, D.; Somasundaram, R. Characterization of free, esterified and bound phenolics in custard apple (Annona squamosa L.) fruit pulp by UPLC-ESI-MS/MS. Food Res. Int. 2016, 82, 121–127. [Google Scholar] [CrossRef]
- MassBank. MassBank Database. Available online: https://massbank.eu/MassBank/Search (accessed on 7 June 2023).
- Avula, B.; Bae, J.-Y.; Majrashi, T.; Wu, T.-Y.; Wang, Y.-H.; Wang, M.; Ali, Z.; Wu, Y.-C.; Khan, I.A. Targeted and non-targeted analysis of annonaceous alkaloids and acetogenins from Asimina and Annona species using UHPLC-QToF-MS. J. Pharm. Biomed. Anal. 2018, 159, 548–566. [Google Scholar] [CrossRef]
- Allegrand, J.; Touboul, D.; Schmitz-Afonso, I.; Guérineau, V.; Giuliani, A.; Le Ven, J.; Champy, P.; Laprévote, O. Structural study of acetogenins by tandem mass spectrometry under high and low collision energy. Rapid Commun. Mass Spectrom. 2010, 24, 3602–3608. [Google Scholar] [CrossRef]
- METLIN. METLIN Simple Search. Available online: https://metlin.scripps.edu/landing_page.php?pgcontent=simple_search (accessed on 7 June 2023).
- Tsugawa, H.; Nakabayashi, R.; Mori, T.; Yamada, Y.; Takahashi, M.; Rai, A.; Sugiyama, R.; Yamamoto, H.; Nakaya, T.; Yamazaki, M.; et al. A cheminformatics approach to characterize metabolomes in stable-isotope-labeled organisms. Nat. Methods 2019, 16, 295–298. [Google Scholar] [CrossRef]
- Cunha, A.G.; Brito, E.S.; Moura, C.F.H.; Ribeiro, P.R.V.; Miranda, M.R.A. UPLC–qTOF-MS/MS-based phenolic profile and their biosynthetic enzyme activity used to discriminate between cashew apple (Anacardium occidentale L.) maturation stages. J. Chromatogr. B 2017, 1051, 24–32. [Google Scholar] [CrossRef]
- Roesler, R.; Catharino, R.R.; Malta, L.G.; Eberlin, M.N.; Pastore, G. Antioxidant activity of Annona crassiflora: Characterization of major components by electrospray ionization mass spectrometry. Food Chem. 2007, 104, 1048–1054. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Baldwin, E.A. Flavor and aroma of fresh-cut fruits and vegetables. In Fresh-Cut Fruits and Vegetables Science, Technology, and Market; Lamikanra, O., Ed.; CRC Press: Boca Raton, USA, 2002; pp. 391–425. ISBN 9781420031874. [Google Scholar]
- Cui, B.; Liu, S.-M.; Zheng, T. Chemotaxonomic Identification of Key Taste and Nutritional Components in ‘Shushanggan Apricot’ Fruits by Widely Targeted Metabolomics. Molecules 2022, 27, 3870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Y.; Yang, Z.; Song, X.; Ma, Y.; Zhao, J.; Wang, X.; Liu, H.; Fan, L. Widely target metabolomics analysis of the differences in metabolites of licorice under drought stress. Ind. Crops Prod. 2023, 202, 117071. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Silva, L.M.A.; de Brito, E.S.; Wurlitzer, N.J.; Fernandes, F.A.N.; Rabelo, M.C.; Fonteles, T.V.; Rodrigues, S. Evaluation of thermal and non-thermal processing effect on non-prebiotic and prebiotic acerola juices using 1H qNMR and GC–MS coupled to chemometrics. Food Chem. 2018, 265, 23–31. [Google Scholar] [CrossRef]
- Cardoso, L.d.M.; Oliveira, D.d.S.; Bedetti, S.d.F.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Araticum (Annona crassiflora Mart.) from the Brazilian Cerrado: Chemical composition and bioactive compounds. Fruits 2013, 68, 121–134. [Google Scholar] [CrossRef]
- Wailzer, B.; Klocker, J.; Wolschann, P.; Buchbauer, G. Structural Features for Furan-Derived Fruity and Meaty Aroma Impressions. Nat. Prod. Commun. 2016, 11, 1475–1479. [Google Scholar] [CrossRef]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transciption. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research Advances in Their Phytochemistry, Biological Activities, and Pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef]
- Kim, C.-W.; Choi, K.-C. Potential Roles of Iridoid Glycosides and Their Underlying Mechanisms against Diverse Cancer Growth and Metastasis: Do They Have an Inhibitory Effect on Cancer Progression? Nutrients 2021, 13, 2974. [Google Scholar] [CrossRef]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation Is a Major Regulator of Phenylpropanoid Availability and Biological Activity in Plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef]
- Mascellani, A.; Leiss, K.; Bac-Molenaar, J.; Malanik, M.; Marsik, P.; Hernandez Olesinski, E.; Tauchen, J.; Kloucek, P.; Smejkal, K.; Havlik, J. Polyketide Derivatives in the Resistance of Gerbera hybrida to Powdery Mildew. Front. Plant Sci. 2022, 12, 790907. [Google Scholar] [CrossRef]
- Ramos, A.L.C.C.; Silva, M.R.; Mendonça, H.d.O.P.; Mazzinghy, A.C.d.C.; Silva, V.D.M.; Botelho, B.G.; Augusti, R.; de Seixas Boavida Ferreira, R.M.; de Sousa, I.M.N.; Batista-Santos, P.; et al. Use of pulp, peel, and seed of Annona crassiflora Mart. in elaborating extracts for fingerprint analysis using paper spray mass spectrometry. Food Res. Int. 2022, 160, 111687. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Ueberbacher, B.T.; Oberdorfer, G.; Gruber, K.; Faber, K. Epoxide-Hydrolase-Initiated Hydrolysis/Rearrangement Cascade of a Methylene-Interrupted Bis-Epoxide Yields Chiral THF Moieties without Involvement of a “Cyclase. ” ChemBioChem 2009, 10, 1697–1704. [Google Scholar] [CrossRef]
- Alali, F.Q.; Liu, X.-X.; McLaughlin, J.L. Annonaceous Acetogenins: Recent Progress. J. Nat. Prod. 1999, 62, 504–540. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.-C.; Liou, J.-R.; Wu, T.-Y.; Chang, F.-R.; Wu, Y.-C. Acetogenins from Annonaceae. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Switzerland, 2016; Volume 101, pp. 113–230. ISBN 978-3-319-22692-7. [Google Scholar]
- Lim, G.-H.; Singhal, R.; Kachroo, A.; Kachroo, P. Fatty Acid– and Lipid-Mediated Signaling in Plant Defense. Annu. Rev. Phytopathol. 2017, 55, 505–536. [Google Scholar] [CrossRef]
- Tartaglio, V.; Rennie, E.A.; Cahoon, R.; Wang, G.; Baidoo, E.; Mortimer, J.C.; Cahoon, E.B.; Scheller, H.V. Glycosylation of inositol phosphorylceramide sphingolipids is required for normal growth and reproduction in Arabidopsis. Plant J. 2017, 89, 278–290. [Google Scholar] [CrossRef]
- Göbel, C.; Feussner, I.; Schmidt, A.; Scheel, D.; Sanchez-Serrano, J.; Hamberg, M.; Rosahl, S. Oxylipin Profiling Reveals the Preferential Stimulation of the 9-Lipoxygenase Pathway in Elicitor-treated Potato Cells. J. Biol. Chem. 2001, 276, 6267–6273. [Google Scholar] [CrossRef] [PubMed]
- Egydio, A.P.M.; Catarina, C.S.; Floh, E.I.S.; Santos, D.Y.A.C. dos Free amino acid composition of Annona (Annonaceae) fruit species of economic interest. Ind. Crops Prod. 2013, 45, 373–376. [Google Scholar] [CrossRef]
- Arruda, H.S.; Pereira, G.A.; Pastore, G.M. Oligosaccharide profile in Brazilian Cerrado fruit araticum (Annona crassiflora Mart.). LWT 2017, 76, 278–283. [Google Scholar] [CrossRef]
- Zullo, V.; Iuliano, A.; Guazzelli, L. Sugar-Based Ionic Liquids: Multifaceted Challenges and Intriguing Potential. Molecules 2021, 26, 2052. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Jin, J.-Y.; Zhong, N.; Cai, X.; Zou, S.-P.; Zhou, H.-Y.; Liu, Z.-Q.; Zheng, Y.-G. Strengthening the (R)-pantoate pathway to produce D-pantothenic acid based on systematic metabolic analysis. Food Biosci. 2021, 43, 101283. [Google Scholar] [CrossRef]
- Kawai, F.; Yamada, H.; Ogata, K. Enzymatic Formation of D-Pantothemc Acid-β-Glucoside by Various β-Glucosidases. Agric. Biol. Chem. 1974, 38, 831–836. [Google Scholar] [CrossRef]
- Olaiya, C.O.; Gbadegesin, M.A.; Nwauzoma, A.B. Bioregulators as tools for plant growth, development, defence and improvement. Afr. J. Biotechnol. 2013, 12, 4987–4999. [Google Scholar] [CrossRef]
- Christmann, A.; Moes, D.; Himmelbach, A.; Yang, Y.; Tang, Y.; Grill, E. Integration of Abscisic Acid Signalling into Plant Responses. Plant Biol. 2006, 8, 314–325. [Google Scholar] [CrossRef]
- Moco, S.; Capanoglu, E.; Tikunov, Y.; Bino, R.J.; Boyacioglu, D.; Hall, R.D.; Vervoort, J.; De Vos, R.C.H. Tissue specialization at the metabolite level is perceived during the development of tomato fruit. J. Exp. Bot. 2007, 58, 4131–4146. [Google Scholar] [CrossRef]
- Berger, A.; Schinnerl, J. Taxonomical and phytochemical diversity of Costa Rica Palicoureeae and Psychotrieae (Rubiaceae). Acta ZooBot Austria 2019, 156, 231–248. [Google Scholar]
- Placines, C.; Castañeda-Loaiza, V.; João Rodrigues, M.; Pereira, C.G.; Stefanucci, A.; Mollica, A.; Zengin, G.; Llorent-Martínez, E.J.; Castilho, P.C.; Custódio, L. Phenolic Profile, Toxicity, Enzyme Inhibition, In Silico Studies, and Antioxidant Properties of Cakile maritima Scop. (Brassicaceae) from Southern Portugal. Plants 2020, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Palomo, E.S.; Pérez-Coello, M.S.; Díaz-Maroto, M.C.; González Viñas, M.A.; Cabezudo, M.D. Contribution of free and glycosidically-bound volatile compounds to the aroma of muscat “a petit grains” wines and effect of skin contact. Food Chem. 2006, 95, 279–289. [Google Scholar] [CrossRef]
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically bound aroma precursors in fruits: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef] [PubMed]

| TPC (mg GAE/g) | TEAC (µmol TE/g) | ORAC (µmol TE/g) | Refs. | |||
|---|---|---|---|---|---|---|
| dw | fw | dw | fw | dw | fw | |
| 21.74 ± 0.30 | 6.68 ± 0.09 | 218.11 ± 5.54 | 67.07 ± 1.70 | 172.90 ± 6.17 | 53.17 ± 1.90 | Our study |
| 11.46 | - | 115.30 | - | 140.07 | - | [8] |
| 46.70 | - | 683.65 | - | 546.26 | - | [7] |
| 45.58 | - | 184.81 | - | - | - | [11] |
| 26.20 | - | 231.79 | - | 337.25 | - | [22] |
| 15.89 | - | 94.66 | - | - | - | [14] |
| 12.45 | - | - | - | - | - | [23] |
| 5.80–10.95 | - | - | - | - | - | [24] |
| - | 4.81–10.08 | - | 23.16–93.76 | - | - | [25] |
| - | 7.39 | - | 131.58 | - | - | [26] |
| - | 7.28 | - | 132.16 | - | - | [27] |
| - | 4.34 | - | - | - | - | [28] |
| - | 2.90 | - | - | - | - | [9] |
| - | 2.58 | - | - | - | - | [10] |
| - | 2.22 | - | - | - | - | [29] |
| - | 2.11–2.61 | - | - | - | - | [30] |
| No. | Identified/Tentatively Annotated Compound | r.t. (min) | Experimental Mass (m/z) | Calculated Mass | MS/MS Fragment Ions (m/z) | Molecular Formula | Error (ppm) | Refs. |
|---|---|---|---|---|---|---|---|---|
| Organic acids and derivatives | ||||||||
| 1 | Malic acid | 0.67 | 133.0145 [M − H]− | 134.0217 | 133, 115 | C4H6O5 | 1.67 | [35] |
| 2 | Citric acid | 0.73 | 191.0205 [M − H]− | 192.0278 | 191, 129, 111 | C6H8O7 | 3.54 | [36] |
| 3 | 2-Furoic acid * | 0.75 | 111.0088 [M − H]− | 112.0162 | 111, 109, 106 | C5H4O3 | −1.39 | [37] |
| 4 | n-Propylmalic acid * | 1.49 | 175.0622 [M − H]− | 176.0695 | 131, 115, 113 | C7H12O5 | −5.86 | [15] |
| Jasmonates and derivatives | ||||||||
| 5 | Dihydroxyjasmononic acid hexoside isomer 1 * | 2.74 | 403.1618 [M − H]− | 404.1701 | 223, 161 | C18H28O10 | −4.46 | [38] |
| 6 | Dihydroxyjasmononic acid hexoside isomer 2 * | 2.92 | 403.1612 [M − H]− | 404.1685 | 241, 223, 179, 149 | C18H28O10 | −0.71 | [38] |
| 7 | Tuberonic acid hexoside isomer 1 * | 3.65 | 387.1671 [M − H]− | 388.1745 | 387, 163, 119 | C18H28O9 | −3.11 | [39] |
| 8 | Tuberonic acid hexoside isomer 2 * | 4.09 | 387.1664 [M − H]− | 388.1738 | 387, 163, 119 | C18H28O9 | −1.21 | [39] |
| 9 | Tuberonic acid hexoside isomer 3 * | 4.48 | 387.1654 [M − H]− | 388.1727 | 387, 207, 163, 119 | C18H28O9 | 1.63 | [39] |
| 10 | Tuberonic acid hexoside isomer 4 * | 4.65 | 387.1658 [M − H]− | 388.1731 | 387, 207, 163, 119 | C18H28O9 | 0.54 | [39] |
| 11 | (−)-11-hydroxy-9,10-dihydrojasmonic acid 11-β-D-glucoside * | 5.30 | 389.1823 [M − H]− | 390.1895 | 389, 227, 133, 101 | C18H30O9 | −1.36 | [40] |
| Iridoids | ||||||||
| 12 | Ajugol * | 2.83 | 393.1399 [M + COOH]− | 348.1417 | 161, 141, 135, 119 | C15H24O9 | 0.89 | [41] |
| 13 | Aucubin * | 3.05 | 391.1253 [M + COOH]− | 346.1272 | 183, 168, 151, 123 | C15H22O9 | −2.35 | [41] |
| 14 | Aldosecologanin isomer 1 * | 11.02 | 757.2612 [M − H]− | 758.2633 | 595 | C34H46O19 | −7.53 | [42] |
| 15 | Aldosecologanin isomer 2 * | 11.41 | 757.2616 [M − H]− | 758.2633 | 595 | C34H46O19 | −8.06 | [42] |
| Flavonoids and derivatives | ||||||||
| 16 | Procyanidin A dimer | 2.61 | 575.1195 [M − H]− | 576.1267 | 449, 423, 407, 289, 125 | C30H24O12 | 0.18 | [43] |
| 17 | Catechin | 3.39 | 289.0726 [M − H]− | 290.0799 | 289, 245, 151, 137, 123, 109 | C15H14O6 | −2.97 | [44] |
| 18 | Procyanidin B dimer isomer 1 * | 3.52 | 577.1353 [M − H]− | 578.1426 | 577, 451, 425, 407, 289, 161, 125 | C30H26O12 | −0.25 | [45] |
| 19 | Procyanidin B trimer isomer 1 | 4.24 | 865.1998 [M − H]− | 866.2069 | 865, 713, 695, 577, 543, 451, 425, 287, 125 | C45H38O18 | −0.84 | [45] |
| 20 | Procyanidin B dimer isomer 2 | 4.44 | 577.1354 [M − H]− | 578.1428 | 577, 451, 425, 407, 289, 161, 125 | C30H26O12 | −0.59 | [45] |
| 21 | Dihydrokaempferol hexoside * | 4.59 | 449.1091 [M − H]− | 450.1165 | 449, 287, 269, 259, 179, 151 | C21H22O11 | −0.53 | [46] |
| 22 | Epicatechin | 4.70 | 289.0721 [M − H]− | 290.0794 | 289, 245, 221, 205, 151, 137, 125, 123, 109 | C15H14O6 | −2.30 | [44] |
| 23 | Procyanidin B dimer isomer 3 * | 4.78 | 577.1364 [M − H]− | 578.1438 | 577, 451, 425, 407, 289, 161, 125 | C30H26O12 | −2.29 | [45] |
| 24 | Procyanidin B trimer isomer 2 * | 4.90 | 865.1976 [M − H]− | 866.2050 | 865, 713, 695, 577, 543, 451, 425, 287, 125 | C45H38O18 | 0.93 | [45] |
| 25 | Procyanidin B tetramer isomer 1 * | 5.08 | 1153.2637 [M − H]− | 1154.2709 | 1027, 865, 695, 577, 575, 533, 451, 449, 413, 287, 125 | C60H50O24 | −1.51 | [45] |
| 26 | Procyanidin B trimer isomer 3 * | 5.30 | 865.1993 [M − H]− | 866.2067 | 865, 713, 695, 577, 543, 451, 425, 287, 125 | C45H38O18 | −1.04 | [45] |
| 27 | Procyanidin B trimer isomer 4 * | 5.56 | 865.1998 [M − H]− | 866.2070 | 865, 713, 695, 577, 543, 451, 425, 287, 125 | C45H38O18 | −0.71 | [45] |
| 28 | Dihydroquercetin hexoside * | 5.99 | 465.1045 [M − H]− | 466.1118 | 285, 151 | C21H22O12 | −1.39 | [47] |
| 29 | Procyanidin B tetramer isomer 2 * | 6.04 | 1153.2599 [M − H]− | 1154.2672 | 1027, 1001, 983, 863, 533, 407, 289, 297 | C60H50O24 | 1.77 | [45] |
| 30 | Procyanidin B dimer isomer 4 * | 6.19 | 577.1350 [M − H]− | 578.1423 | 577, 451, 425, 407, 289, 161, 125 | C30H26O12 | 0.15 | [45] |
| 31 | Procyanidin B trimer isomer 5 * | 6.30 | 865.1967 [M − H]− | 866.2040 | 865, 713, 695, 577, 543, 451, 425, 287, 125 | C45H38O18 | 2.14 | [45] |
| 32 | Procyanidin B tetramer isomer 3 * | 6.77 | 1153.2605 [M − H]− | 1154.2675 | 1027, 1001, 983, 865, 863, 739, 695, 577, 575, 451, 449, 423, 413, 407, 289, 287, 125 | C60H50O24 | 1.44 | [45] |
| 33 | Procyanidin B trimer isomer 6 * | 6.92 | 865.1978 [M − H]− | 866.2049 | 865, 713, 695, 577, 543, 451, 425, 287, 125 | C45H38O18 | −1.03 | [45] |
| 34 | Procyanidin B tetramer isomer 4 * | 7.21 | 1153.2611 [M − H]− | 1154.2683 | 1027, 1001, 865, 863, 575, 451, 459, 423, 413, 405, 289, 287 | C60H50O24 | 0.75 | [45] |
| 35 | Procyanidin B trimer isomer 7 * | 7.60 | 865.1987 [M − H]− | 866.2060 | 865, 713, 695, 577, 543, 451, 425, 287, 125 | C45H38O18 | 0.27 | [45] |
| 36 | Procyanidin B tetramer isomer 5 * | 7.64 | 1153.2618 [M − H]− | 1154.2685 | 1027, 983, 865, 863, 739, 695, 577, 575, 451, 449, 407, 289, 287, 125 | C60H50O24 | 0.61 | [45] |
| 37 | Flavanomarein * | 7.74 | 449.1088 [M − H]− | 450.1160 | 449, 269, 179, 151, 135 | C21H22O11 | 0.54 | [48] |
| 38 | Procyanidin B tetramer isomer 6 * | 8.03 | 1153.2621 [M − H]− | 1154.2696 | 1001, 983, 863, 695, 577, 575, 451, 449, 413, 407, 289, 287, 125 | C60H50O24 | −0.36 | [45] |
| 39 | Quercetin-3-O-pentosylhexoside isomer 1 * | 8.20 | 595.1307 [M − H]− | 596.1381 | 595, 301, 300, 271, 179, 151 | C26H28O16 | −0.56 | [49] |
| 40 | Quercetin-3-O-pentosylhexoside isomer 2 * | 8.46 | 595.1317 [M − H]− | 596.1387 | 595, 301, 300, 271, 179, 151 | C26H28O16 | −1.63 | [49] |
| 41 | Procyanidin B dimer isomer 5 * | 8.74 | 577.1362 [M − H]− | 578.1435 | 577, 451, 425, 407, 289, 161, 125 | C30H26O12 | −1.77 | [45] |
| 42 | Procyanidin B trimer isomer 8 * | 8.77 | 865.1990 [M − H]− | 866.2063 | 865, 713, 695, 577, 543, 451, 425, 287, 125 | C45H38O18 | −0.53 | [45] |
| 43 | Rutin | 8.85 | 609.1456 [M − H]− | 610.1528 | 609, 343, 301, 300, 271, 255, 151 | C27H30O16 | 0.96 | [49] |
| 44 | Kaempferol-3-O-hexosylpentoside * | 9.07 | 579.1359 [M − H]− | 580.1432 | 579, 285, 284, 255 | C26H28O15 | −0.72 | [49] |
| 45 | Hesperidin | 9.20 | 609.1474 [M − H]− | 610.1549 | 609, 301, 300, 255 | C28H34O15 | 3.01 | [25] |
| 46 | Quercetin-3-O-β-D-glucoside | 9.24 | 463.0890 [M − H]− | 464.0963 | 463, 301, 300, 271, 255, 243, 211, 163, 151 | C21H20O12 | −1.74 | [50] |
| 47 | Phloretin-C-diglycoside * | 9.33 | 597.1844 [M − H]− | 598.1915 | 417, 387, 357, 345, 315, 239, 209 | C27H34O15 | −2.89 | [51] |
| 48 | Procyanidin B trimer isomer 9 * | 9.37 | 865.1997 [M − H]− | 866.2070 | 865, 713, 695, 577, 543, 451, 425, 287, 125 | C45H38O18 | −1.35 | [45] |
| 49 | Procyanidin B tetramer isomer 7 * | 9.46 | 1153.2618 [M − H]− | 1154.2691 | 1027, 1001, 983, 865, 577, 575, 449, 423, 413, 289, 287, 125 | C60H50O24 | 0.07 | [45] |
| 50 | Kaempferol-3-O-rutinoside | 9.89 | 593.1511 [M − H]− | 594.1584 | 593, 285, 284, 255, 227, 151, 107 | C27H30O15 | 0.08 | [44] |
| 51 | Kaempferol-3-O-β-D-glucoside | 10.08 | 447.0948 [M − H]− | 448.1020 | 447, 285, 284, 255, 227 | C21H20O11 | 3.08 | [44] |
| 52 | Quercetin pentoside * | 10.28 | 433.0766 [M − H]− | 434.0839 | 433, 301, 300, 271, 255, 227, 151 | C20H18O11 | 2.32 | [44] |
| 53 | Kaempferol hexoside isomer 1 * | 10.34 | 447.0941 [M − H]− | 448.1017 | 447, 285, 284, 255, 227, 211, 151 | C21H20O11 | 2.37 | [44] |
| 54 | Kaempferol deoxyhexosylhexoside * | 10.58 | 593.1505 [M − H]− | 594.1580 | 593, 285, 284 | C27H30O15 | 0.74 | [44] |
| 55 | Isorhamnetin-3-O-rutinoside * | 10.72 | 623.1611 [M − H]− | 624.1684 | 315, 300, 151 | C28H32O16 | 1.09 | [52] |
| 56 | Kaempferol hexoside isomer 2 * | 10.83 | 447.0927 [M − H]− | 448.1000 | 447, 285, 284, 255, 227, 151 | C21H20O11 | −1.39 | [44] |
| 57 | Isorhamnetin hexoside * | 11.25 | 477.1052 [M − H]− | 478.1124 | 477, 315, 314, 299, 271, 257, 243, 179, 151, 107 | C22H22O12 | −2.63 | [50] |
| 58 | (epi)Catechin-ethyl trimer * | 11.67 | 893.2313 [M − H]− | 894.2371 | 893, 603, 577, 451, 407, 315, 289, 125 | C47H42O18 | −2.24 | [45] |
| 59 | Luteolin * | 12.12 | 285.0416 [M − H]− | 286.0477 | 285, 199, 175, 151, 133, 121 | C15H10O6 | −5.96 | [50] |
| Non-flavonoid phenolic compounds and derivatives | ||||||||
| 60 | Protocatechuic acid hexoside * | 1.31 | 315.0722 [M − H]− | 316.0794 | 315, 153, 152, 123, 109, 108 | C13H16O9 | −1.90 | [50] |
| 61 | Hydroxytyrosol hexoside | 1.36 | 315.1095 [M − H]− | 316.1169 | 315, 153, 123, 108 | C14H20O8 | −3.31 | [53] |
| 62 | Hydroxybenzoic acid hexoside * | 1.57 | 299.0792 [M − H]− | 300.0865 | 137 | C13H16O8 | −6.75 | [40] |
| 63 | Leonuriside A * | 1.62 | 331.1039 [M − H]− | 332.1114 | 169, 153, 125 | C14H20O9 | −1.95 | [54] |
| 64 | Caffeoylsucrose isomer 1 * | 1.70 | 503.1417 [M − H]− | 504.1492 | 503, 341, 281, 161 | C21H28O14 | 1.70 | [55] |
| 65 | Hydroxytyrosol hexosylpentoside * | 1.75 | 447.1506 [M − H]− | 448.1579 | 153, 123 | C19H28O12 | 0.47 | [56] |
| 66 | Vanillic acid hexoside isomer 1 * | 1.96 | 329.0892 [M − H]− | 330.0966 | 329, 167, 152, 123, 108 | C14H18O9 | −4.71 | [57] |
| 67 | Dihydroxybenzoic acid pentoside isomer 1 * | 2.01 | 285.0629 [M − H]− | 286.0700 | 285, 152, 108 | C12H14O8 | −4.09 | [58] |
| 68 | Caffeoylsucrose isomer 2 * | 2.21 | 503.1409 [M − H]− | 504.1482 | 503, 341, 281, 179, 161, 135 | C21H28O14 | −0.13 | [55] |
| 69 | Caffeic acid hexoside isomer 1 * | 2.34 | 341.0887 [M − H]− | 342.0959 | 341, 179, 161, 135 | C15H18O9 | 1.88 | [43] |
| 70 | Dihydrocoumaroyl hexoside (3-(2-hydroxyphenyl)-propanoic acid hexose or dihydromelilotoside) | 2.40 | 327.1086 [M − H]− | 328.1158 | 165, 147 | C15H20O8 | −0.09 | [59] |
| 71 | Syringic acid hexoside | 2.78 | 359.0988 [M − H]− | 360.1061 | 197, 182, 167, 153, 138, 123 | C15H20O10 | 1.38 | [57] |
| 72 | Caffeic acid hexoside isomer 2 * | 2.95 | 341.0880 [M − H]− | 342.0954 | 341, 179, 161, 135 | C15H18O9 | 0.18 | [43] |
| 73 | Vanillic acid hexoside isomer 2 * | 3.05 | 329.0882 [M − H]− | 330.0955 | 329, 167, 123 | C14H18O9 | −1.18 | [57] |
| 74 | Dihydroxybenzoic acid pentoside isomer 2 * | 3.18 | 285.0621 [M − H]− | 286.0695 | 285, 153, 152, 109, 108 | C12H14O8 | −2.06 | [58] |
| 75 | Coniferin * | 3.35 | 387.1296 [M + COOH]− | 342.1315 | 343, 180, 179, 164 | C16H22O8 | 0.02 | [60] |
| 76 | Caffeic acid hexoside isomer 3 * | 3.39 | 341.0886 [M − H]− | 342.0959 | 341, 179, 161, 135 | C15H18O9 | 1.79 | [43] |
| 77 | p-Coumaric acid hexoside | 3.44 | 325.0931 [M − H]− | 326.1004 | 163, 145, 117 | C15H18O8 | −0.77 | [25] |
| 78 | Caffeic acid | 3.70 | 179.0352 [M − H]− | 180.0425 | 179, 135, 134, 107 | C9H8O4 | −1.63 | [15] |
| 79 | Vanillic acid hexoside isomer 3 * | 3.91 | 329.0886 [M − H]− | 330.0959 | 329, 167, 123 | C14H18O9 | −2.48 | [57] |
| 80 | Caffeoylquinic acid (Chlorogenic acid) | 4.17 | 353.0879 [M − H]− | 354.0953 | 191, 179, 161, 135 | C16H18O9 | −0.65 | [50] |
| 81 | Ferulic acid hexoside | 4.41 | 355.1039 [M − H]− | 356.1112 | 193, 175, 134 | C16H20O9 | −0.30 | [25] |
| 82 | Methylsyringin* | 5.86 | 385.1510 [M − H]− | 386.1581 | 223, 179, 161 | C18H26O9 | −1.20 | [41] |
| 83 | Caffeoylshikimic acid* | 6.47 | 335.0781 [M − H]− | 336.0854 | 179, 161, 135 | C16H16O8 | −2.59 | [61] |
| 84 | Ferulic acid | 6.73 | 193.0508 [M − H]− | 194.0581 | 134 | C10H10O4 | −0.97 | [62] |
| 85 | Phloroacetophenone 6′-[xylosyl-(1→6)-glucoside] * | 7.38 | 489.1617 [M − H]− | 490.1691 | 168 | C21H30O13 | −1.03 | [41] |
| 86 | Caffeoyltyramine isomer 1 * | 8.12 | 298.1094 [M − H]− | 299.1167 | 298, 178, 135 | C17H17NO4 | −0.23 | [15] |
| 87 | Pinoresinol hexoside * | 9.73 | 519.1864 [M − H]− | 520.1937 | 357, 342, 311, 151, 136 | C26H32O11 | 1.40 | [63] |
| 88 | Pinoresinol * | 9.76 | 357.1339 [M − H]− | 358.1411 | 342, 311, 151, 136 | C20H22O6 | 1.57 | [64] |
| 89 | Caffeoyltyramine isomer 2 * | 10.49 | 298.1100 [M − H]− | 299.1172 | 298, 178, 161, 135 | C17H17NO4 | 1.43 | [15] |
| 90 | Syringaresinol-O-β-D-glucopyranoside * | 10.72 | 579.2076 [M − H]− | 580.2148 | 417, 181 | C28H36O13 | 1.37 | [65] |
| 91 | Caffeic acid ethyl ester * | 10.89 | 207.0657 [M − H]− | 208.0729 | 179, 161, 135, 133 | C11H12O4 | 3.11 | [66] |
| 92 | Lavandulifolioside * | 10.98 | 755.2463 [M − H]− | 756.2477 | 593 | C34H44O19 | −8.47 | [67] |
| 93 | N-Feruloyltyramine * | 11.93 | 312.1241 [M − H]− | 313.1314 | 312, 297, 178, 135 | C18H19NO4 | 0.12 | [68] |
| 94 | 4-Hydroxyphenyl-hexanoic acid * | 12.43 | 207.1035 [M − H]− | 208.1109 | 193, 149, 135, 119 | C12H16O3 | −4.70 | [69] |
| 95 | p-Coumaric acid methyl ester | 12.66 | 177.0917 [M − H]− | 178.0991 | 163, 145 | C11H14O2 | 1.72 | [25] |
| 96 | Verimol H * | 12.97 | 327.1612 [M − H]− | 328.1675 | 327, 165, 145 | C20H24O4 | −2.80 | [41] |
| 97 | p-Decycloxybenzoic acid * | 13.42 | 277.1816 [M − H]− | 278.1889 | 233, 205 | C17H26O3 | −2.46 | [70] |
| Alkaloids | ||||||||
| 98 | Bakankoside * | 2.18 | 356.1363 [M − H]− | 357.1435 | 195, 194, 123 | C16H23NO8 | −3.06 | [41] |
| 99 | Isoboldine | 5.99 | 326.1407 [M − H]− | 327.1481 | 326, 312, 311, 297, 296, 281, 268, 267, 253, 252, 29, 225, 197 | C19H21NO4 | −3.23 | [71] |
| 100 | Damascenine * | 13.10 | 194.0831 [M − H]− | 195.0904 | 179, 149 | C10H13NO3 | −4.58 | [72] |
| Annonaceous acetogenins | ||||||||
| 101 | Unknown acetogenin 1 * | 13.31 | 627.4472 [M − H]− | 628.4545 | 565, 501, 197, 127 | C35H64O9 | 0.88 | [73] |
| 102 | Unknown acetogenin 2 * | 13.40 | 671.4750 [M − H]− | 672.4823 | 609, 545, 127 | C37H68O10 | −1.52 | [73] |
| 103 | Unknown acetogenin 3 * | 13.49 | 655.4792 [M − H]− | 656.4864 | 637, 611, 127 | C37H68O9 | −0.16 | [73] |
| 104 | Unknown acetogenin 4 * | 13.53 | 627.4488 [M − H]− | 628.4564 | 609, 583, 565, 501, 127 | C35H64O9 | −2.23 | [73] |
| 105 | Unknown acetogenin 5 * | 13.66 | 627.4481 [M − H]− | 628.4554 | 609, 583, 565, 501, 127 | C35H64O9 | −0.58 | [73] |
| 106 | Unknown acetogenin 6 * | 13.83 | 653.4630 [M − H]− | 654.4707 | 635, 609, 599, 591, 581, 527, 491, 127 | C37H66O9 | −0.15 | [73] |
| 107 | Unknown acetogenin 7 * | 13.88 | 609.4377 [M − H]− | 610.4463 | 547, 483, 197, 127 | C35H62O8 | −3.01 | [73] |
| 108 | Unknown acetogenin 8 * | 13.89 | 655.4789 [M − H]− | 656.4863 | 637, 619, 611, 593, 583, 565, 529, 197, 127 | C37H68O9 | −0.61 | [73] |
| 109 | Unknown acetogenin 9 * | 13.98 | 611.4526 [M − H]− | 612.4604 | 593, 575, 567, 549, 197, 127 | C35H64O8 | −0.48 | [73] |
| 110 | Unknown acetogenin 10 * | 14.09 | 637.4694 [M − H]− | 638.4766 | 619, 593, 575, 525, 511, 493, 127 | C37H66O8 | −1.33 | [73] |
| 111 | Unknown acetogenin 11 * | 14.14 | 609.4370 [M − H]− | 610.4489 | 591, 573, 547, 483, 465, 127 | C35H62O8 | −7.26 | [73] |
| 112 | Unknown acetogenin 12 * | 14.22 | 639.4852 [M − H]− | 640.4923 | 621, 603, 595, 577, 527, 513, 127 | C37H68O8 | −1.38 | [73] |
| 113 | Unknown acetogenin 13 * | 14.35 | 637.4694 [M − H]− | 638.4766 | 619, 593, 575, 511, 197, 127 | C37H66O8 | −1.34 | [73] |
| 114 | Unknown acetogenin 14 * | 14.53 | 621.4720 [M − H]− | 622.4821 | 577, 509, 197, 127 | C37H66O7 | −2.01 | [73] |
| 115 | Unknown acetogenin 15 * | 14.57 | 595.4589 [M − H]− | 596.4661 | 577, 533, 197, 127 | C35H64O7 | −1.57 | [73] |
| 116 | Unknown acetogenin 16 * | 14.57 | 623.4892 [M − H]− | 624.4965 | 579, 561, 127 | C37H68O7 | 0.05 | [73] |
| 117 | Unknown acetogenin 17 * | 14.66 | 619.4587 [M − H]− | 620.4675 | 601, 575, 507, 127 | C37H64O7 | −3.75 | [73] |
| 118 | Unknown acetogenin 18 * | 14.79 | 623.4899 [M − H]− | 624.4972 | 561, 497, 127 | C37H68O7 | −1.12 | [73] |
| Fatty acids and derivatives | ||||||||
| 119 | n-Hydroxyhexanoic acid hexoside * | 1.79 | 293.1244 [M − H]− | 294.1316 | 173, 131 | C12H22O8 | −0.34 | [51] |
| 120 | 6E-Octene-2,4-diynoic acid * | 2.35 | 133.0298 [M − H]− | 134.0371 | 133, 115 | C8H6O2 | −2.03 | [74] |
| 121 | Prenyl arabinosyl-(1→6)-glucoside * | 2.40 | 379.1615 [M − H]− | 380.1688 | 191, 149, 131 | C16H28O10 | −1.56 | [41] |
| 122 | Pentyl-pentosylhexoside * | 3.18 | 381.1771 [M − H]− | 382.1844 | 249, 161, 113, 101 | C16H30O10 | −1.26 | [51] |
| 123 | 1-(3-Methylbutanoyl)-6-apiosylglucose * | 3.70 | 395.1555 [M − H]− | 396.1629 | 395, 249, 163, 161, 113, 101 | C16H28O11 | 0.78 | [41] |
| 124 | 8:1 + 2O fatty acyl hexoside isomer 1 * | 4.22 | 321.1559 [M − H]− | 322.1628 | 321, 159 | C14H26O8 | −1.18 | [75] |
| 125 | 8:1 + 2O fatty acyl hexoside isomer 2 * | 4.56 | 321.1555 [M − H]− | 322.1628 | 159 | C14H26O8 | 0.29 | [75] |
| 126 | 8:1 + 2O fatty acyl hexoside isomer 3 * | 6.51 | 321.1556 [M − H]− | 322.1629 | 159 | C14H26O8 | −0.35 | [75] |
| 127 | 9,12,13-Trihydroxy-octadecadienoic acid * | 12.32 | 327.2191 [M − H]− | 328.2265 | 229, 211, 197, 183, 171 | C18H32O5 | −4.61 | [51] |
| 128 | 11-Hydroperoxy-octadecatrienoic acid * | 13.05 | 309.2080 [M − H]− | 310.2156 | 291, 209, 185, 121 | C18H30O4 | −3.77 | [65] |
| Other compounds | ||||||||
| 129 | L-Arginine * | 0.58 | 173.1053 [M − H]− | 174.1126 | 131, 127 | C6H14N4O2 | −5.57 | [71] |
| 130 | Glucuronic or galacturonic acid * | 0.62 | 193.0365 [M − H]− | 194.0437 | 177, 130, 113, 103 | C6H10O7 | −5.52 | [71] |
| 131 | Gluconic acid | 0.62 | 195.0513 [M − H]− | 196.0586 | 195, 177, 129 | C6H12O7 | −1.74 | [35] |
| 132 | Pantothenic acid hexoside * | 1.27 | 380.1572 [M − H]− | 381.1644 | 380, 218, 146 | C15H27NO10 | −2.31 | [46] |
| 133 | Dehydrophaseic acid hexoside * | 2.92 | 443.1919 [M − H]− | 444.1992 | 281, 237, 219, 189, 161, 153, 143, 119 | C21H32O10 | 0.88 | [76] |
| 134 | Benzyl-pentosylhexoside * | 2.92 | 401.1454 [M − H]− | 402.1528 | 269, 161, 113, 101 | C18H26O10 | −0.51 | [51] |
| 135 | Methylbenzoic acid * | 3.74 | 135.0454 [M − H]− | 136.0527 | 135, 134, 119, 117, 107 | C8H8O2 | −1.91 | [71] |
| 136 | 1-Hexanol arabinosylglucoside * | 5.95 | 441.1976 [M + COOH]− | 396.1992 | 395, 263 | C17H32O10 | 0.81 | [41] |
| 137 | Roseoside * | 5.00 | 431.1921 [M + COOH]− | 386.1948 | 385, 153 | C19H30O8 | −1.80 | [35] |
| 138 | (1S,2S,4R,8S)-p-Menthane-1,2,8,9-tetrol-2-glucoside * | 6.11 | 359.0988 [M − H]− | 366.1888 | 203, 157 | C16H30O9 | 0.42 | [41] |
| 139 | Abscisic acid * | 11.41 | 263.1286 [M − H]− | 264.1359 | 219, 204, 203, 199, 173, 171, 163, 153, 149, 139, 137 | C15H20O4 | 1.00 | [71] |
| Tentatively Annotated Annonaceous Acetogenins | Precursor Molecular Ion ([M − H]−) (m/z) | MS/MS Fragment Ions (m/z) | Specific Fragmentation Pathway |
|---|---|---|---|
| Unknown acetogenin 1 | 627.4472 | 565.4436 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule |
| 501.4063 [M-H-7H2O]− | Fragment ion resulting from the successive loss of seven water molecules | ||
| 197.1874 [C13H25O]− | Fragment ion produced by cleavage in the alpha of the first hydroxyl group starting from the methyl extremity | ||
| 127.0396 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 2 | 671.4750 | 609.4686 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule |
| 545.4426 [M-H-7H2O]− | Fragment ion resulting from the successive loss of seven water molecules | ||
| 127.0416 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 3 | 655.4792 | 637.4572 [M-H-H2O]− | Fragment ion resulting from the loss of one water molecule |
| 611.4484 [M-H-CO2]− | Fragment ion resulting from the loss of one CO2 molecule | ||
| 127.0395 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 4 | 627.4488 | 609.4336 [M-H-H2O]− | Fragment ion resulting from the loss of one water molecule |
| 583.3684 [M-H-CO2]− | Fragment ion resulting from the loss of one CO2 molecule | ||
| 565.4461 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule | ||
| 501.4140 [M-H-7H2O]− | Fragment ion resulting from the successive loss of seven water molecules | ||
| 127.0395 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 5 | 627.4481 | 609.4395 [M-H-H2O]− | Fragment ion resulting from the loss of one water molecule |
| 583.3489 [M-H-CO2]− | Fragment ion resulting from the loss of one CO2 molecule | ||
| 565.4556 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule | ||
| 501.4124 [M-H-7H2O]− | Fragment ion resulting from the successive loss of seven water molecules | ||
| 127.0398 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 6 | 653.4630 | 635.4531 [M-H-H2O]− | Fragment ion resulting from the loss of one water molecule |
| 609.4309 [M-H-CO2]− | Fragment ion resulting from the loss of one CO2 molecule | ||
| 599.4428 [M-H-3H2O]− | Fragment ion resulting from the successive loss of three water molecules | ||
| 591.4620 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule | ||
| 581.4394 [M-H-4H2O]− | Fragment ion resulting from the successive loss of four water molecules | ||
| 527.4343 [M-H-7H2O]− | Fragment ion resulting from the successive loss of seven water molecules | ||
| 491.4131 [M-H-9H2O]− | Fragment ion resulting from the successive loss of nine water molecules | ||
| 127.0403 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 7 | 609.4377 | 547.4355 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule |
| 483.4054 [M-H-7H2O]− | Fragment ion resulting from the successive loss of seven water molecules | ||
| 197.1920 [C13H25O]− | Fragment ion produced by cleavage in the alpha of the first hydroxyl group starting from the methyl extremity | ||
| 127.0402 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 8 | 655.4789 | 637.4616 [M-H-H2O]− | Fragment ion resulting from the loss of one water molecule |
| 619.4591 [M-H-2H2O]− | Fragment ion resulting from the successive loss of two water molecules | ||
| 611.4513 [M-H-CO2]− | Fragment ion resulting from the loss of one CO2 molecule | ||
| 593.4770 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule | ||
| 583.4575 [M-H-4H2O]− | Fragment ion resulting from the successive loss of four water molecules | ||
| 565.4437 [M-H-5H2O]− | Fragment ion resulting from the successive loss of five water molecules | ||
| 529.4359 [M-H-7H2O]− | Fragment ion resulting from the successive loss of seven water molecules | ||
| 197.1921 [C13H25O]− | Fragment ion produced by cleavage in the alpha of the first hydroxyl group starting from the methyl extremity | ||
| 127.0405 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 9 | 611.4526 | 593.4368 [M-H-H2O]− | Fragment ion resulting from the loss of one water molecule |
| 575.4350 [M-H-2H2O]− | Fragment ion resulting from the successive loss of two water molecules | ||
| 567.4716 [M-H-CO2]− | Fragment ion resulting from the loss of one CO2 molecule | ||
| 549.4527 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule | ||
| 197.1918 [C13H25O]− | Fragment ion produced by cleavage in the alpha of the first hydroxyl group starting from the methyl extremity | ||
| 127.0400 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 10 | 637.4694 | 619.4571 [M-H-H2O]− | Fragment ion resulting from the loss of one water molecule |
| 593.4720 [M-H-CO2]− | Fragment ion resulting from the loss of one CO2 molecule | ||
| 575.4471 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule | ||
| 525.4318 [M-C6H8O2]− | Fragment ion resulting from the loss of the terminal γ-lactone ring | ||
| 511.4320 [M-H-7H2O]− | Fragment ion resulting from the successive loss of seven water molecules | ||
| 493.4218 [M-H-8H2O]− | Fragment ion resulting from the successive loss of eight water molecules | ||
| 127.0401 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 11 | 609.4370 | 591.4309 [M-H-H2O]− | Fragment ion resulting from the loss of one water molecule |
| 573.4091 [M-H-2H2O]− | Fragment ion resulting from the successive loss of two water molecules | ||
| 547.4392 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule | ||
| 483.4055 [M-H-7H2O]− | Fragment ion resulting from the successive loss of seven water molecules | ||
| 465.3989 [M-H-8H2O]− | Fragment ion resulting from the successive loss of eight water molecules | ||
| 127.0401 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 12 | 639.4852 | 621.4720 [M-H-H2O]− | Fragment ion resulting from the loss of one water molecule |
| 603.4564 [M-H-2H2O]− | Fragment ion resulting from the successive loss of two water molecules | ||
| 595.4555 [M-H-CO2]− | Fragment ion resulting from the loss of one CO2 molecule | ||
| 577.4825 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule | ||
| 527.4503 [M-C6H8O2]− | Fragment ion resulting from the loss of the terminal γ-lactone ring | ||
| 513.4373 [M-H-7H2O]− | Fragment ion resulting from the successive loss of seven water molecules | ||
| 127.0406 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 13 | 637.4694 | 619.4532 [M-H-H2O]− | Fragment ion resulting from the loss of one water molecule |
| 593.4496 [M-H-CO2]− | Fragment ion resulting from the loss of one CO2 molecule | ||
| 575.4707 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule | ||
| 511.4383 [M-H-7H2O]− | Fragment ion resulting from the successive loss of seven water molecules | ||
| 197.1955 [C13H25O]− | Fragment ion produced by cleavage in the alpha of the first hydroxyl group starting from the methyl extremity | ||
| 127.0402 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 14 | 621.4720 | 577.4801 [M-H-CO2]− | Fragment ion resulting from the loss of one CO2 molecule |
| 509.4241 [M-C6H8O2]− | Fragment ion resulting from the loss of the terminal γ-lactone ring | ||
| 197.1937 [C13H25O]− | Fragment ion produced by cleavage in the alpha of the first hydroxyl group starting from the methyl extremity | ||
| 127.0390 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 15 | 595.4589 | 577.4471 [M-H-H2O]− | Fragment ion resulting from the loss of one water molecule |
| 533.4516 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule | ||
| 197.1914 [C13H25O]− | Fragment ion produced by cleavage in the alpha of the first hydroxyl group starting from the methyl extremity | ||
| 127.0397 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 16 | 623.4892 | 579.4922 [M-H-CO2]− | Fragment ion resulting from the loss of one CO2 molecule |
| 561.9243 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule | ||
| 127.0406 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 17 | 619.4587 | 601.4414 [M-H-H2O]− | Fragment ion resulting from the loss of one water molecule |
| 575.2319 [M-H-CO2]− | Fragment ion resulting from the loss of one CO2 molecule | ||
| 507.2350 [M-C6H8O2]− | Fragment ion resulting from the loss of the terminal γ-lactone ring | ||
| 127.0399 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring | ||
| Unknown acetogenin 18 | 623.4899 | 561.4874 [M-H-CO2-H2O]− | Fragment ion resulting from the successive loss of one water molecule and one CO2 molecule |
| 497.4376 [M-H-7H2O]− | Fragment ion resulting from the successive loss of seven water molecules | ||
| 127.0404 [C6H7O3]− | Fragment ion referring to the tetrahydrofuran ring |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arruda, H.S.; Angolini, C.F.F.; Eberlin, M.N.; Pastore, G.M.; Marostica Junior, M.R. UHPLC-ESI-QTOF-MS/MS Profiling of Phytochemicals from Araticum Fruit (Annona crassiflora Mart.) and Its Antioxidant Activity. Foods 2023, 12, 3456. https://doi.org/10.3390/foods12183456
Arruda HS, Angolini CFF, Eberlin MN, Pastore GM, Marostica Junior MR. UHPLC-ESI-QTOF-MS/MS Profiling of Phytochemicals from Araticum Fruit (Annona crassiflora Mart.) and Its Antioxidant Activity. Foods. 2023; 12(18):3456. https://doi.org/10.3390/foods12183456
Chicago/Turabian StyleArruda, Henrique Silvano, Célio Fernando Figueiredo Angolini, Marcos Nogueira Eberlin, Glaucia Maria Pastore, and Mario Roberto Marostica Junior. 2023. "UHPLC-ESI-QTOF-MS/MS Profiling of Phytochemicals from Araticum Fruit (Annona crassiflora Mart.) and Its Antioxidant Activity" Foods 12, no. 18: 3456. https://doi.org/10.3390/foods12183456
APA StyleArruda, H. S., Angolini, C. F. F., Eberlin, M. N., Pastore, G. M., & Marostica Junior, M. R. (2023). UHPLC-ESI-QTOF-MS/MS Profiling of Phytochemicals from Araticum Fruit (Annona crassiflora Mart.) and Its Antioxidant Activity. Foods, 12(18), 3456. https://doi.org/10.3390/foods12183456