Assessment of the Quality Attributes of Oat β-glucan Fortified Reduced-Fat Goat Milk Yogurt Supported by Microfluidization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Milk Blends
2.3. Microfluidization Process and Yogurt Making
2.4. Proximate Composition of Raw Goat Milk
2.5. Proximate Composition and Physico-Chemical Properties of Yogurt Samples
2.6. Oat-β-Glucan Content
2.7. Color Analysis
2.8. Evaluation of Yogurt Gel Properties
2.8.1. Syneresis
2.8.2. Textural Analysis
2.9. Culture Viability of Yogurt Bacteria
2.10. Sensory Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Oat β-glucan Content and Effect of Oat-β Glucan Concentration on Some Characteristics of Yogurt Samples
3.2. Impact of Microfluidization and Oat-β Glucan Concentration on Color Attributes, Syneresis, and General Acceptability of Yogurt Samples
3.3. Derived Color Indices of non-Microfluidized and Microfluidized Oat β-Glucan Fortified Goat Milk Yogurt Samples during Storage Period
3.4. Effect of Microfluidization and Storage Time on Some Physico-Chemical, Proximate Composition, and Textural Characteristics of Yogurt Samples
3.5. Effect of Storage Time on Color, Microbiology, and Texture Attributes of Yogurt Samples
3.6. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haenlein, G.F.W. Goat milk in human nutrition. Small Rumin. Res. 2004, 51, 155–163. [Google Scholar] [CrossRef]
- Raynal-Ljutovac, K.; Lagriffoul, G.; Paccard, P.; Guillet, I.; Chilliard, Y. Composition of goat and sheep milk products: An update. Small Rumin. Res. 2008, 79, 57–72. [Google Scholar] [CrossRef]
- Attaie, R.; Richter, R.L. Size distribution of fat globules in goat milk. J. Dairy. Sci. 2000, 83, 940–944. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Afsar, S.; Day, L. Differences in the microstructure and rheological properties of low-fat yoghurts from goat, sheep and cow milk. Food Res. Int. 2018, 108, 423–429. [Google Scholar] [CrossRef]
- Zee, J.A.; Tirand-Collet, P.; Lavigne, C. Amelioration de la qualite du yogourt fabriqué à partir de lait de chèvre. In Proceedings of the 23rd International Dairy Congress: Brief Communications and Poster Abstracts, Montreal, QC, Canada, 6–12 October 1990; Volume 1, p. 23. [Google Scholar]
- Agnihotri, M.K.; Prasad, V.S.S. Biochemistry and processing of goat milk and milk products. Small Rumin. Res. 1993, 12, 151–170. [Google Scholar] [CrossRef]
- Kalantzopoulos, G. Etat de la recherche sur le lait de chevre en Grece. Lait 1993, 73, 431–441. [Google Scholar] [CrossRef]
- Ambrosoli, R.; Di Stasio, L.; Mazzoco, P. Content of αs1-casein and coagulation properties in goat milk. J. Dairy. Sci. 1988, 71, 24–28. [Google Scholar] [CrossRef]
- Addeo, F.; Masi, P.; Rubino, R. The aptitude of caprine milk to cheese making. Relationship between αs1-casein content and rheological properties of curd and cheese. Dairy. Sci. Abstr. 1992, 54, 472. [Google Scholar]
- Guo, M. Goat’s milk. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Trugo, L., Finglas, P., Eds.; Academic Press: London, UK, 2003; pp. 2944–2949. [Google Scholar]
- Park, Y.W.; Juarez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Pal, M.; Dudhrejiya, T.P.; Pinto, S.; Brahamani, D.; Vijayageetha, V.; Reddy, Y.K.; Kate, P. Goat milk products and their significance. Beverage Food World 2017, 44, 21–25. [Google Scholar]
- Seçkin, A.K.; Baladura, E. Effect of using some dietary fibers on color, texture and sensory properties of strained yogurt. J. Food 2012, 37, 63–69. [Google Scholar]
- Butt, S.M.; Tahir-Nadeem, M.; Iqbal Khan, M.K.; Shabir, R. Oat: Unique among the cereals. Eur. J. Nutr. 2008, 47, 68–79. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Ma, S.; Wang, X.X.; Zheng, X.L. Modification and application of dietary fiber in foods. J. Chem. 2017, 2017, 9340427. [Google Scholar] [CrossRef]
- Dello-Staffolo, M.; Bertola, N.; Martino, M.; Bevilacqua, A. Influence of dietary fiber addition on sensory and rheological properties of yogurt. Int. Dairy. J. 2004, 14, 263–268. [Google Scholar] [CrossRef]
- Mousavi, M.; Heshmati, A.; Garmakhany, A.D.; Vahidinia, A.; Taheri, M. Optimization of the viability of Lactobacillus acidophilus and physico-chemical, textural and sensorial characteristics of flaxseed-enriched stirred probiotic yogurt by using response surface methodology. LWT-Food Sci. Technol. 2019, 102, 80–88. [Google Scholar] [CrossRef]
- Ahmad, A.; Munir, B.; Abrar, M.; Bashir, S.; Adnan, M.; Tabassum, T. Respective of β-glucan as functional ingredient for food industry. J. Nutr. Food Sci. 2012, 2, 133–139. [Google Scholar]
- Sahan, N.; Yasar, K.; Hayaloglu, A.A. Physical, Chemical and Flavour Quality of Non-fat Yogurt as Affected by a β-glukan Hydrocolloidal Composite during Storage. Food Hydrocoll. 2008, 22, 1291–1297. [Google Scholar] [CrossRef]
- Qu, X.; Nazarenko, Y.; Yang, W.; Nie, Y.; Zhang, Y.; Li, B. Effect of oat β-glucan on the rheological characteristics and microstructure of set-type yogurt. Molecules 2021, 26, 4752. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Food labeling: Health claims; oats and coronary heart disease. Interim Final rule. Fed. Regist. 1997, 62, 3583–3601. [Google Scholar]
- Singh, M.; Sanghoon, K.; Sean, X.L. Effect of Prufied Oat β-Glukan on Fermentation of Set-Style Yogurt Mix. J. Food Sci. 2012, 77, 195–201. [Google Scholar] [CrossRef]
- Raikos, V.; Grant, S.B.; Hayes, H.; Ranawana, V. Use of β-glucan from spent brewer’s yeast as a thickener in skimmed yogurt: Physicochemical, textural, and structural properties related to sensory perception. J. Dairy. Sci. 2018, 101, 5821–5831. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Sibakov, N.; de Oliveira Carvalho, M.J.; Lille, M.; Nordlund, E. Impact of enzymatic hydrolysis and microfluidization on the techno-functionality of oat bran in suspension and acid milk gel models. Foods 2022, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Charalampopoulos, D.; Wang, R.; Pandilla, S.S.; Webb, C. Application of cereals and cereal components in functional foods: A review. Int. J. Food Microbiol. 2002, 79, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Inglett, G.E.; Palmquist, D.; Warner, K. Flavor and texture attributes of foods containing β-glucan-rich. LWT-Food Sci. Technol. 2009, 42, 350–357. [Google Scholar] [CrossRef]
- Daou, C.; Zhang, H. Oat beta-glucan: Its role in health promotion and prevention of diseases. Compr. Rev. Food Sci. Food Saf. 2012, 11, 355–365. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Kealy, T.; Mishra, V.K. Effects of β-Glucan Addition to a Probiotic Containing Yogurt. Food Chem. Toxicol. 2007, 72, 405–411. [Google Scholar] [CrossRef]
- Sarantis, S.D.; Eren, N.M.; Kowalcyk, B.; Jimenez–Flores, R.; Alvarez, V.B. Thermodynamic interactions of micellar casein and oat β-glucan in a model food system. Food Hydrocoll. 2021, 115, 106559. [Google Scholar] [CrossRef]
- Sharafbafi, N.; Tosh, S.M.; Alexander, M.; Corredig, M. Phase behaviour, rheological properties, and microstructure of oat β-glucan-milk mixtures. Food Hydrocoll. 2014, 41, 274–280. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, R.; Li, J. Effects of the β-glucan, curdlan, on the fermentation performance, microstructure, rheological and textural properties of set yogurt. LWT-Food Sci. Technol. 2020, 128, 109449. [Google Scholar] [CrossRef]
- Ye, R.; Harte, F. High pressure homogenization to improve the stability of casein–hydroxypropyl cellulose aqueous systems. Food Hydrocoll. 2014, 35, 670–677. [Google Scholar] [CrossRef]
- Ciron, C.I.E.; Gee, V.L.; Kelly, A.L.; Auty, M.A.E. Comparison of the effects of high-pressure microfluidization and conventional homogenization of milk on particle size, water retention and texture of non-fat and low-fat yoghurts. Int. Dairy. J. 2010, 20, 314–320. [Google Scholar] [CrossRef]
- Lanciotti, R.; Vannini, L.; Pittia, P.; Elisabetta, M.; Guerzoni, M.E. Suitability of high-dynamic-pressure- treated milk for the production of yoghurt. Food Microbiol. 2004, 21, 753–760. [Google Scholar] [CrossRef]
- Ciron, C.I.E.; Gee, V.L.; Kelly, A.L.; Auty, M.A.E. Effect of microfluidization of heat-treated milk on rheology and sensory properties of reduced fat yoghurt. Food Hydrocoll. 2011, 25, 170–1476. [Google Scholar] [CrossRef]
- Vargas, M.; Cháfer, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Physicochemical and sensory characteristics of yoghurt produced from mixtures of cows’ and goats’ milk. Int. Dairy J. 2008, 18, 1146–1152. [Google Scholar] [CrossRef]
- Alqahtani, N.K.; Darwish, A.A.; El-Menawy, R.K.; Alnemr, T.M.; Aly, E. Textural and organoleptic attributes and antioxidant activity of goat milk yoghurt with added oat flour. Int. J. Food Prop. 2021, 24, 433–445. [Google Scholar] [CrossRef]
- Kaur, R.; Riar, C.S. Sensory, rheological and chemical characteristics during storage of set type full fat yoghurt fortified with barley β-glucan. J. Food Sci. Technol. 2020, 57, 41–51. [Google Scholar] [CrossRef]
- Brennan, C.S.; Tudorica, C.M. Carbohydrate-based fat replacers in the modification of the rheological, textural and sensory quality of yoghurt: Comparative study of the utilisation of barley beta-glukan, guar gum and inulin. Int. J. Food Sci. Technol. 2008, 43, 824–833. [Google Scholar] [CrossRef]
- Lazaridou, A.; Serafeimidou, A.; Biliaderis, C.G.; Moschakis, T.; Tzanetakis, N. Structure development and acidification kinetics in fermented milk containing oat β-glucan, a yogurt culture and a probiotic strain. Food Hydrocoll. 2014, 39, 204–214. [Google Scholar] [CrossRef]
- TS 1018; İnek Sütü-Çiğ. (Raw Cow’s Milk). Turkish Standards Institute: Ankara, Turkey, 2002.
- No. 149A; Dairy Starter Cultures of Lactic Acid Bacteria (LAB). International Dairy Federation (IDF): Brussels, Belgium, 1997.
- No. 20B; International Dairy Federation (IDF). Determination of Nitrogen Content. International Dairy Federation: Brussels, Belgium, 1993.
- Renner, E. Milchprakticum; Justus-Liebig-Universität: Giessen, Germany, 1993. [Google Scholar]
- Bradley, R.L., Jr.; Arnold, E., Jr.; Barbano, D.M.; Semerad, R.G.; Smith, D.E.; Vines, B.K. Vines. Chemical and physical methods. In Standard Methods for the Examination of Dairy Products; Marshall, R.T., Ed.; American Public Health Association: Washington, DC, USA, 1993; Volume 70, pp. 433–531. [Google Scholar]
- Anonymous. Mixed-Linkage Beta-Glucan Assay Procedure (McLeary Method). 2015. Available online: https://www.megazyme.com/beta-glucan-assay-kit (accessed on 21 November 2015).
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Color measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Robitaille, G.; Tremblay, A.; Moineau, S.; St-Gelais, D.; Vadeboncoeur, C.; Britten, M. Fat-free yogurt made using a galactose-positive exopolysaccharide-producing recombinant strain of Streptococcus thermophilus. J. Dairy. Sci. 2009, 92, 477–482. [Google Scholar] [CrossRef]
- Bodyfelt, F.W.; Tobias, J.; Trout, G.M. The Sensory Evaluation of Dairy Products; Van Nostrand, R., Ed.; Springer Science & Business Media: New York, NY, USA, 1988. [Google Scholar]
- Tribby, D. Yogurt. In The Sensory Evaluation of Dairy Products; Clark, S., Costello, M., Drake, M.A., Bodyfelt, F., Eds.; Springer Science Business Media LLC: New York, NY, USA, 2009; pp. 191–224. [Google Scholar]
- Gee, V.L.; Vasanthan, T.; Temelli, F. Viscosity of model systems enriched with β-glucan as influenced by starter cultures. Int. Dairy. J. 2007, 17, 1083–1088. [Google Scholar] [CrossRef]
- Snart, J.; Bibilonil, R.; Grayson, T.; Lay, C.; Zhang, H.; Allison, G.E.; Laverdiere, J.K.; Temelli, F.; Vasanthan, T.; Bell, R.; et al. Supplementation of the Diet with High-Viscosity Beta-Glucan Results in Enrichment for Lactobacilli in the Rat Cecum. Appl. and Environ. Microbiol. 2005, 72, 1925–1931. [Google Scholar] [CrossRef]
- Donkor, O.N.; Tsangalis, D.; Shah, N.P. Viability of probiotic bacteria and concentrations of organic acids in commercial yoghurts during refrigerated storage. Food Aust. 2007, 59, 121–126. [Google Scholar]
- Valerie, A.R. Viability of Bifidobacteria in Yogurts Containing Oat Beta-Glucan and/or Corn Starch during Cold Storage. Master’s Thesis, Iowa State University, Ames, IA, USA, 2009, unpublished. [Google Scholar]
- Angelov, A.; Gotcheva, V.; Kuncheva, R.; Hristozova, T. Development of a new oat-based probiotic drink. Int. J. Food Microbiol. 2006, 112, 75–80. [Google Scholar] [CrossRef]
- Rosburg, V.; Boylston, T.; White, P. Viability of Bifidobacteria Strains in Yoghurt with Added Oat Beta-Glucan and Corn Starch during Cold Storage. J. Food Sci. 2010, 75, 439–444. [Google Scholar]
- Kearney, N.; Stack, H.M.; Tobin, J.T.; Chaurin, V.; Fenelon, M.A.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Lactobacillus. paracasei NFBC 338 producing recombinant beta-glucan positively influences the functional properties of yoghurt. Int. Dairy. J. 2011, 21, 561–567. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J. Cereal Sci. 2007, 46, 101–118. [Google Scholar] [CrossRef]
- Ketenoglu, O.; Mert, B.; Tekin, A. Effects of microfluidized dietary fibers on stability properties of emulsions. J. Texture Stud. 2014, 45, 295–306. [Google Scholar] [CrossRef]
- Martensson, O.; Andersson, C.; Andersson, K.; Öste, R.; Holst, O. Formulation of an oat-based fermented product and its comparison with yoghurt. J. Sci. Food Agric. 2001, 81, 1314–1321. [Google Scholar] [CrossRef]
- Kurtuldu, O.; Ozcan, T. Effect of β-glucan on the properties of probiotic set yoghurt with Bifidobacterium animalis subsp. lactis strain Bb-12. Int. J. Dairy Technol. 2018, 71, 157–166. [Google Scholar] [CrossRef]
- Pedras, M.M.; Tribst, A.A.; Cristianini, M. Effects of high-pressure homogenisation on physicochemical characteristics of partially skimmed milk. Int. J. Food Sci. Technol. 2014, 49, 861–866. [Google Scholar] [CrossRef]
- Lemay, A.; Paquin, P.; Lacroix, C. Influence of microfluidization of milk on cheddar cheese composition, color, texture, and yield. J. Dairy Sci. 1994, 77, 2870–2879. [Google Scholar] [CrossRef]
- Gervilla, R.; Ferragut, V.; Guamis, B. High Hydrostatic Pressure Effects on Color and Milk- Fat Globule of Ewew’s Milk. J. Food Sci. 2001, 66, 880–885. [Google Scholar] [CrossRef]
- Trujillo, A.J.; Capellas, M.; Saldo, J.; Gervilla, R.; Guamis, B. Applications of high-hydrostatic pressure on milk and dairy products: A Review. Innov. Food Sci. Emerg. Technol. 2002, 3, 295–307. [Google Scholar] [CrossRef]
- Martin-Diana, A.B.; Janer, C.; Pelaez, C.; Requena, T. Development of a fermented goat’s milk containing probiotic bacteria. Int. Dairy. J. 2003, 13, 827–833. [Google Scholar] [CrossRef]
- Iordache, M.; Jelen, P. High pressure microfluidization treatment of heat denatured whey proteins for improved functionality. Innov. Food Sci. Emerg. Technol. 2003, 4, 367–376. [Google Scholar] [CrossRef]
- Penna, A.L.B.; Gurram, S.; Barbosa-Ca’novas, G.V. High hydrostatic pressure processing on microstructure of probiotic low-fat yogurt. Food Res. Int. 2007, 40, 510–519. [Google Scholar] [CrossRef]
- Chen, J.; Gao, D.; Yang, L.; Gao, Y. Effect of microfluidization process on the functional properties of insoluble dietary fiber. Food Res. Int. 2013, 54, 1821–1827. [Google Scholar] [CrossRef]
- Ronkart, S.N.; Paquot, M.; Deroanne, C.; Fougnies, C.; Besbes, S.; Blecker, C.S. Development of gelling properties of inulin by microfluidization. Food Hydrocoll. 2010, 24, 318–324. [Google Scholar] [CrossRef]
- Anli, E.A.; Kıral, A.G.; Sert, D. Physicochemical and Textural Properties of Ready-to-eat yogurt fortified with dried Purslane (Portulaca oleracea L.). J. Food 2021, 46, 229–242. [Google Scholar] [CrossRef]
- Popescu, L.; Ceșco, T.; Gurev, A.; Ghendov-Mosanu, A.; Sturza, R.; Tarna, R. Impact of apple pomace powder on the bioactivity, and the sensory and textural characteristics of yogurt. Foods 2022, 11, 3565. [Google Scholar] [CrossRef]
- Béal, C.; Skokanova, J.; Latrille, E.; Martin, N.; Corrieu, G. Combined effects of culture conditions and storage time on acidification and viscosity of stirred yogurt. J. Dairy. Sci. 1999, 82, 673–681. [Google Scholar] [CrossRef]
- Herrero, A.M.; Requena, T. The effect of supplementing goat’s milk with whey protein concentrate on textural properties of set-type yoghurt. Int. J. Food Sci. Technol. 2006, 41, 87–92. [Google Scholar] [CrossRef]
- Kailasapathy, K. Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT-Food Sci. Technol. 2006, 39, 1221–1227. [Google Scholar] [CrossRef]
- Espírito-Santo, A.P.; Lagazzo, A.; Sousa, A.L.O.P.; Perego, P.; Converti, A.; Oliveira, M.N. Rheology, spontaneous whey separation, microstructure and sensorial characteristics of probiotic yoghurts enriched with passion fruit fiber. Food Res. Int. 2013, 50, 224–231. [Google Scholar] [CrossRef]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy. Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef]
- Rudra, S.G.; Nath, P.; Kaur, C.; Basu, S. Rheological, storage stability and sensory profiling of low-fat yoghurt fortified with red capsicum carotenoids and inulin. J. Food Process. Preserv. 2017, 41, e13067. [Google Scholar] [CrossRef]
- Bruzantin, F.P.; Daniel, J.L.P.; Da Silva, P.P.M.; Spoto, M.H.F. Physicochemical and sensory characteristics of fat-free goat milk yogurt with added stabilizers and skim milk powder fortification. J. Dairy. Sci. 2016, 99, 3316–3324. [Google Scholar] [CrossRef]
- Barrantes, E.; Tamime, A.Y.; Muir, D.D.; Sword, A.M. The effect of substitution of fat by microparticulate whey protein on the quality of set-type yoghurt. J. Soc. Dairy. Technol. 1994, 47, 61–68. [Google Scholar] [CrossRef]
- Domagala, J. Instrumental texture, syneresis and microstructure of yoghurts prepared from goat, cow and sheep milk. Int. J. Food Prop. 2009, 12, 605–615. [Google Scholar] [CrossRef]
- Özcan, T.; Yıldız, E. Sebze püresi ile üretilen yoğurtların tekstürel ve duyusal özelliklerinin belirlenmesi. Turk. J. Agric.-Food Sci. Technol. 2016, 4, 579–587. [Google Scholar] [CrossRef]
- Cayot, P.; Schenker, F.; House, G.; Sulmont-Rosse, C.; Colas, B. Creaminess in relation to consistency and particle size in stirred fat-free yogurt. Int. Dairy. J. 2008, 18, 303–311. [Google Scholar] [CrossRef]
- Lazaridou, A.; Vaikousi, H.; Biliaderis, C.G. Impact of mixed-linkage (1→3, 1→4) β-glucans on physical properties of acid-set skim milk gels. Int. Dairy. J. 2008, 18, 312–322. [Google Scholar] [CrossRef]
- Levy, R.; Okun, Z.; Shpigelman, A. High-pressure homogenization: Principles and applications beyond microbial inactivation. Food Eng. Rev. 2021, 13, 490–508. [Google Scholar] [CrossRef]
- Serra, M.; Trujillo, J.; Guamis, B.; Feragut, V. Flavour profiles and survival of starter cultures of yoghurt produced from high-pressure homogenized milk. Int. Dairy. J. 2009, 19, 100–106. [Google Scholar] [CrossRef]
- Lee, W.J.; Lucey, J.A. Formation and Physical Properties of Yogurt. Asian-Australas. J. Anim. Sci. 2010, 23, 1127–1136. [Google Scholar] [CrossRef]
- Joon, R.; Mishra, S.K.; Brar, G.S.; Singh, P.K.; Panwar, H. Instrumental texture and syneresis analysis of yoghurt prepared from goat and cow milk. Pharma Innov. 2017, 6, 971. [Google Scholar]
- Needs, E.C.; Capellas, M.; Bland, P.; Manoj, P.; MacDougal, D.B.; Gopal, P. Comparison of heat and pressure treatments of skimmed milk, fortified with whey protein concentrate, for set yoghurt preparation: Effects on milk proteins and gel structure. J. Dairy. Res. 2000, 67, 329–348. [Google Scholar] [CrossRef]
- Fernández-Garía, E.; McGregor, J.U.; Traylor, S. The addition of oat fiber and natural alternative sweeteners in the manufacture of plain yogurt. J. Dairy Sci. 1998, 81, 655–663. [Google Scholar] [CrossRef]

| Component (%) | Raw Goat Milk a | Skim Goat Milk Powder b | Oat β-glucan c |
|---|---|---|---|
| Total solid | 14.26 ± 0.83 | 96.61 | 96.97 |
| Fat | 5.13 ± 0.56 | 0.50 | 0.75 |
| Total protein | 4.51 ± 0.30 | - d | 4.25 |
| Ash | 0.78 ± 0.06 | 8.79 | 2.09 |
| pH | 6.67 ± 0.03 | 6.66 | - d |
| Titratable acidity, % LA | 8.37 ± 0.62 | 0.11 | - d |
| β–glucan | - e | - e | 35.00 |
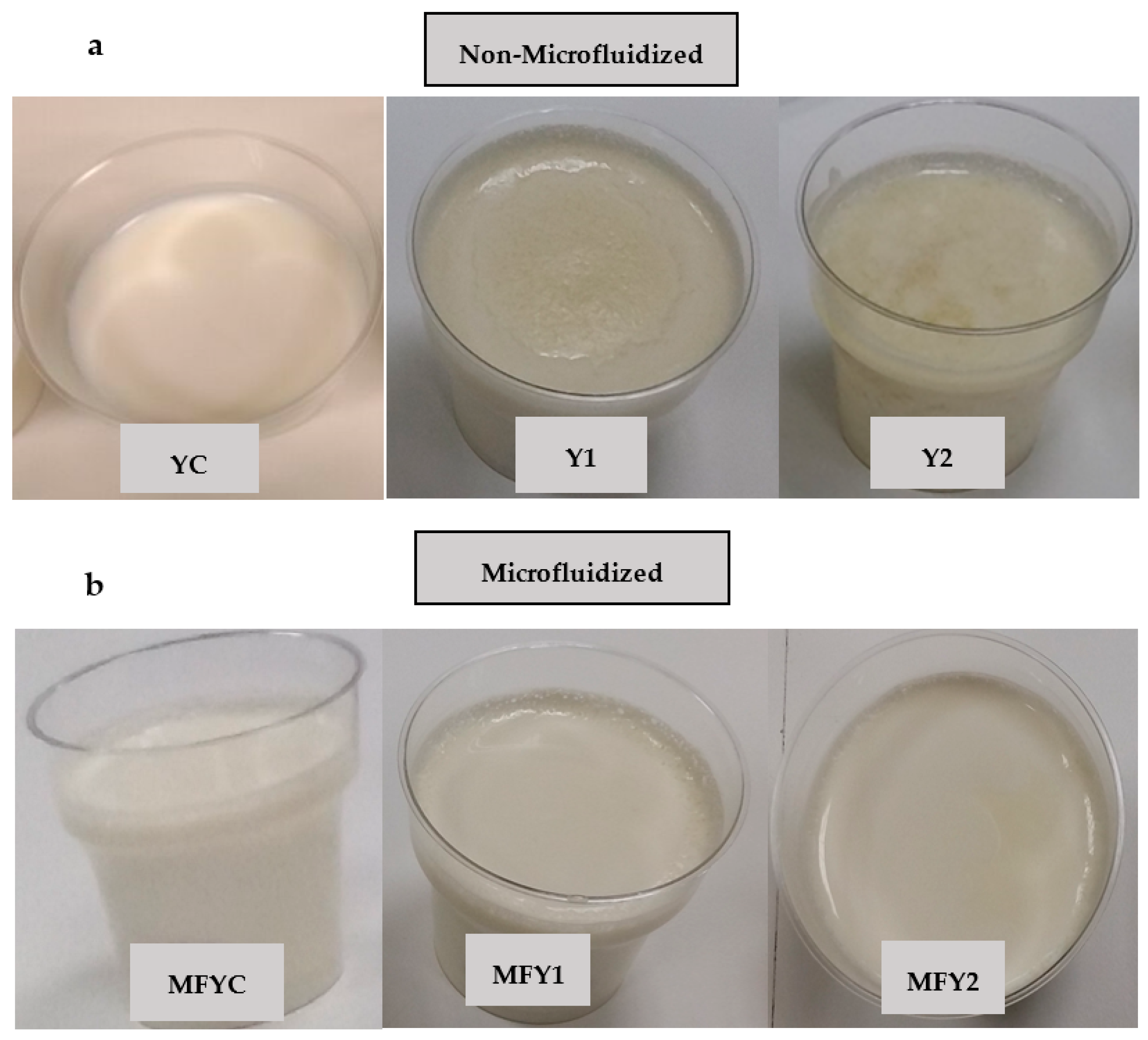
| Details for Milk Blends | ||||
|---|---|---|---|---|
| Sample Code 1 | Oat β Glucan Ratio (%, w/v, on Milk Base) | Microfluidization (MF) | ||
| 0 | 0.25 | 0.50 | ||
| YC | ✓ | - | - | - |
| Y1 | - | ✓ | - | - |
| Y2 | - | - | ✓ | - |
| MFYC | ✓ | - | - | ✓ |
| MFY1 | - | ✓ | - | ✓ |
| MFY2 | - | - | ✓ | ✓ |
| Quality Criteria | Possible Defects in Goat Milk Yogurt |
|---|---|
| Color and appearance The score ranges from 1–5 points 5: defines no defect | Non-uniform color |
| Free whey | |
| Unnatural color | |
| Shrinkage | |
| Surface growth of bacteria | |
| Structure and texture attributes The score ranges from 1–5 points 5: defines no defect | Too thin or too firm |
| Creamy Drinkable Syneresis | |
| Lumpy | |
| Granular | |
| Ropy | |
| Flavor and Odor The score ranges from 1–10 points 10: defines no defect | Lack of yogurt taste and aroma |
| Cooked flavor | |
| Creamy | |
| Cereal flavor | |
| Goaty flavor and odor | |
| Fermented | |
| Sour | |
| Salty | |
| Sweet | |
| Yeasty/Fruity | |
| Foreign |
| Parameter | ANOVA | ||||||
|---|---|---|---|---|---|---|---|
| P-Oat β-Glucan | P-Storage Time | P-MF | P-MF × P-β-Glucan | P-MF × P-Storage Time | P-Oat β-Glucan × P-Storage Time | P-Oat β-Glucan × P-Storage Time × P-MF | |
| Dry matter (%) | ** | NS | NS | NS | ** | NS | NS |
| Lactic Acid (%) | * | NS | NS | NS | ** | NS | NS |
| pH | * | NS | NS | NS | * | NS | NS |
| Ash (%) | NS | NS | NS | NS | ** | NS | NS |
| Oat β-glucan | ** | NS | NS | NS | NS | NS | NS |
| Syneresis (%) | NS | NS | NS | ** | * | NS | NS |
| Firmness (N) | NS | ** | NS | NS | NS | NS | NS |
| Consistency (N×s) | NS | * | NS | NS | NS | NS | NS |
| Cohesiveness (N) | NS | NS | NS | NS | * | NS | NS |
| Index of viscosity (N×s) | * | NS | NS | NS | NS | NS | NS |
| L* | NS | NS | NS | ** | * | NS | NS |
| a* | NS | NS | NS | * | ** | NS | NS |
| b* | NS | * | NS | ** | NS | NS | NS |
| S. thermophilus (log CFU/g) | NS | ** | NS | NS | NS | NS | NS |
| Lb. delbrueckii subsp. bulgaricus (log CFU/g) | NS | ** | NS | NS | NS | NS | NS |
| General acceptability | NS | NS | NS | ** | NS | NS | NS |
| Parameter | ± Sx 1 | ||
|---|---|---|---|
| Control | Oat β-Glucan 0.25% | Oat β-Glucan 0.50% | |
| Dry matter (%) | 15.91 ± 0.1100 B** | 16.21 ± 0.1290 A** | 16.44 ± 0.1300 A** |
| LA (%) | 1.51 ± 0.0155 B* | 1.56 ± 0.0127 A* | 1.49 ± 0.0170 B* |
| pH | 4.29 ± 0.0196 AB* | 4.25 ± 0.0181 B* | 4.33 ± 0.0180 A* |
| Oat β-glucan content (g/100 mL) 2 | - | 0.05 ± 0.0190 B** | 0.11 ± 0.0030 A** |
| Index of viscosity (N × s) | 1.13 ± 0.0668 A* | 1.17 ± 0.0460 A* | 0.70 ± 0.0442 B* |
| Parameter | Yogurt Sample | ± Sx 1 | |
|---|---|---|---|
| Non-Microfluidized | Microfluidized | ||
| L* | Control | 69.63 ± 0.3980 Ab** | 70.30 ± 0.0832 Aa** |
| Oat β-glucan 0.25% | 68.76 ± 0.3830 Bb** | 70.08 ± 0.0989 Aa** | |
| Oat β-glucan 0.50% | 67.26 ± 0.3590 Ca** | 69.46 ± 0.1540 Ba** | |
| a* | Control | −2.12 ± 0.1050 Aa* | −2.08 ± 0.0814 Aa* |
| Oat β-glucan 0.25% | −2.23 ± 0.1720 Aa* | −2.00 ± 0.0551 Aa* | |
| Oat β-glucan 0.50% | −2.70 ± 0.1160 Bb* | −2.15 ± 0.0786 Aa | |
| b* | Control | 7.90 ± 0.3750 Ca** | 8.31 ± 0.0889 Ba** |
| Oat β-glucan 0.25% | 8.52 ± 0.4060 Ba** | 8.53 ± 0.0535 ABa** | |
| Oat β-glucan 0.50% | 9.26 ± 0.3300 Aa** | 8.80 ± 0.0484 Ab** | |
| Syneresis (%) | Control | 12.53 ± 0.434 Ba** | 12.81 ± 0.969 Aa** |
| Oat β-glucan 0.25% | 21.88 ± 0.853 Aa** | 10.34 ± 0.785 Ab** | |
| Oat β-glucan 0.50% | 24.59 ± 2.280 Aa** | 15.08 ± 1.400 Ab** | |
| General acceptability | Control | 3.62 ± 0.125 Aa** | 3.27 ± 0.163 Aa** |
| Oat β-glucan 0.25% | 3.71 ± 0.132 Aa** | 3.83 ± 0.122 Aa** | |
| Oat β-glucan 0.50% | 1.80 ± 0.210 Bb** | 3.49 ± 0.168 Aa** | |
| Color Indices | Sample | Non-Microfluidized | Sample | Microfluidized | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Storage Time (Day) | Storage Time (Day) | |||||||||
| 1 | 7 | 14 | 21 | 1 | 7 | 14 | 21 | |||
| ΔE | YC | - | - | - | - | MFYC | - | - | - | - |
| Y1 | 1.13 ± 0.17 | 1.02 ± 0.13 | 1.13 ± 0.08 | 1.34 ± 0.09 | MFY1 | 0.38 ± 0.06 | 0.33 ± 0.06 | 0.42 ± 0.08 | 0.46 ± 0.07 | |
| Y2 | 2.12 ± 0.20 | 2.15 ± 0.04 | 1.75 ± 0.05 | 1.21 ± 0.26 | MFY2 | 0.89 ± 0.24 | 0.94 ± 0.17 | 0.63 ± 0.04 | 0.44 ± 0.13 | |
| Chroma | YC | 7.06 ± 1.42 | 8.45 ± 0.08 | 8.55 ± 0.16 | 8.69 ± 0.21 | MFYC | 8.44 ± 0.08 | 8.63 ± 0.11 | 8.67 ± 0.19 | 8.54 ± 0.17 |
| Y1 | 7.66 ± 1.51 | 9.19 ± 0.02 | 9.09 ± 0.12 | 9.39 ± 0.13 | MFY1 | 8.66 ± 0.03 | 8.76 ± 0.08 | 8.84 ± 0.11 | 8.84 ± 0.10 | |
| Y2 | 8.73 ± 1.25 | 9.99 ± 0.09 | 10.07 ± 0.16 | 9.85 ± 0.11 | MFY2 | 8.97 ± 0.13 | 9.04 ± 0.05 | 9.17 ± 0.11 | 9.05 ± 0.03 | |
| Hue Angle | YC | 107.55 ± 4.40 | 103.79 ± 0.65 | 103.46 ± 0.20 | 106.98 ± 1.72 | MFYC | 104.24 ± 1.52 | 104.00 ± 1.29 | 103.95 ± 1.67 | 104.20 ± 1.78 |
| Y1 | 107.52 ± 5.70 | 102.55 ± 1.45 | 103.33 ± 1.79 | 107.42 ± 2.94 | MFY1 | 103.32 ± 0.96 | 103.16 ± 0.94 | 103.09 ± 0.91 | 103.02 ± 1.07 | |
| Y2 | 107.43 ± 3.16 | 105.03 ± 0.92 | 104.65 ± 0.55 | 108.64 ± 2.09 | MFY2 | 103.94 ± 0.96 | 103.63 ± 0.91 | 103.55 ± 1.28 | 103.69 ± 1.56 | |
| Whiteness Index | YC | 67.64 ± 1.18 | 68.83 ± 0.24 | 68.46 ± 0.45 | 69.12 ± 0.09 | MFYC | 69.16 ± 0.06 | 69.16 ± 0.28 | 69.09 ± 0.27 | 68.96 ± 0.16 |
| Y1 | 66.59 ± 1.10 | 68.02 ± 0.16 | 67.53 ± 0.28 | 67.88 ± 0.11 | MFY1 | 68.97 ± 0.15 | 68.87 ± 0.25 | 68.74 ± 0.26 | 68.70 ± 0.26 | |
| Y2 | 64.77 ± 0.79 | 65.92 ± 0.19 | 65.92 ± 0.24 | 66.74 ± 0.34 | MFY2 | 68.15 ± 0.39 | 67.99 ± 0.45 | 68.15 ± 0.22 | 68.29 ± 0.16 | |
| Yellowness Index | YC | 13.99 ± 2.85 | 16.74 ± 0.10 | 17.05 ± 0.24 | 16.84 ± 0.29 | MFYC | 16.61 ± 0.27 | 16.98 ± 0.37 | 17.09 ± 0.55 | 16.84 ± 0.50 |
| Y1 | 15.37 ± 3.19 | 18.45 ± 0.16 | 18.34 ± 0.40 | 18.42 ± 0.05 | MFY1 | 17.14 ± 0.09 | 17.37 ± 0.26 | 17.56 ± 0.35 | 17.57 ± 0.34 | |
| Y2 | 18.01 ± 2.59 | 20.43 ± 0.17 | 20.64 ± 0.35 | 19.51 ± 0.43 | MFY2 | 17.90 ± 0.08 | 18.11 ± 0.12 | 18.33 ± 0.37 | 18.04 ± 0.21 | |
| Parameter | Storage Period (day) | Sample | |
|---|---|---|---|
| ± Sx 1 | |||
| Non-Microfluidized | Microfluidized | ||
| Dry matter (%) | 1 | 16.73 ± 0.0923 Aa** | 15.58 ± 0.1620 Bb** |
| 7 | 16.62 ± 0.1160 Aa** | 15.92 ± 0.2050 Ab** | |
| 14 | 16.63 ± 0.1010 Aa** | 15.60 ± 0.1300 Bb** | |
| 21 | 16.66 ± 0.1300 Aa** | 15.75 ± 0.1630 ABb** | |
| Lactic Acid (%) | 1 | 1.41 ± 0.0266 Ca** | 1.46 ± 0.0125 Ba** |
| 7 | 1.53 ± 0.0250 Ba** | 1.53 ± 0.0090 Aa** | |
| 14 | 1.56 ± 0.0195 ABa** | 1.54 ± 0.0096 Aa** | |
| 21 | 1.59 ± 0.0175 Aa** | 1.52 ± 0.0277 Ab** | |
| pH | 1 | 4.41 ± 0.0186 Aa* | 4.37 ± 0.0247 Aa* |
| 7 | 4.33 ± 0.0287 Ba* | 4.21 ± 0.0147 BCb* | |
| 14 | 4.29 ± 0.0166 BCa* | 4.24 ± 0.0250 Bb* | |
| 21 | 4.26 ± 0.0173 Ca* | 4.19 ± 0.0187 Cb* | |
| Ash (%) | 1 | 1.40 ± 0.0104 Aa** | 1.09 ± 0.0482 Bb** |
| 7 | 1.39 ± 0.0105 Aa** | 1.25 ± 0.0080 Ab** | |
| 14 | 1.40 ± 0.0047 Aa** | 1.28 ± 0.0044 Ab** | |
| 21 | 1.40 ± 0.0074 Aa** | 1.28 ± 0.0062 Ab** | |
| L | 1 | 67.34 ± 0.7740 Bb* | 69.99 ± 0.1940 Aa* |
| 7 | 68.93 ± 0.4020 Ab* | 69.93 ± 0.2400 Aa* | |
| 14 | 68.64 ± 0.3660 Ab* | 69.95 ± 0.1650 Aa* | |
| 21 | 69.29 ± 0.3320 Aa* | 69.91 ± 0.1210 Aa* | |
| a | 1 | −2.14 ± 0.1170 Aa** | −2.08 ± 0.0901 Aa** |
| 7 | −2.21 ± 0.1310 Aa** | −2.07 ± 0.0751 Aa** | |
| 14 | −2.22 ± 0.1190 Aa** | −2.08 ± 0.0836 Aa** | |
| 21 | −2.84 ± 0.2100 Bb** | −2.08 ± 0.1010 Aa** | |
| Syneresis (%) | 1 | 18.30 ± 2.390 Ba* | 12.50 ± 1.460 ABb* |
| 7 | 18.43 ± 2.050 Ba* | 11.58 ± 1.320 Bb* | |
| 14 | 22.77 ± 2.970 Aa* | 12.23 ± 1.090 ABb* | |
| 21 | 19.16 ± 2.040 Ba* | 14.66 ± 1.600 Ab* | |
| Cohesiveness (N) | 1 | 0.68 ± 0.0456 Ba* | 0.68 ± 0.0727 Aa* |
| 7 | 0.72 ± 0.039 Aa* | 0.68 ± 0.0746 Ab* | |
| 14 | 0.72 ± 0.0452 Aa* | 0.70 ± 0.0739 Aa* | |
| 21 | 0.73 ± 0.0347 Aa* | 0.67 ± 0.0721 Ab* | |
| Parameter | Storage Time (Day) | ± Sx 1 |
|---|---|---|
| b* | 1 | 7.96 ± 0.3960 B* |
| 7 | 8.75 ± 0.1180 A* | |
| 14 | 8.80 ± 0.1280 A* | |
| 21 | 8.70 ± 0.1030 A* | |
| Firmness (N) | 1 | 1.43 ± 0.147 B** |
| 7 | 1.50 ± 0.151 AB** | |
| 14 | 1.51 ± 0.161 A** | |
| 21 | 1.53 ± 0.159 A** | |
| Consistency (N×s) | 1 | 14.45 ± 1.68 B* |
| 7 | 14.96 ± 1.72 A* | |
| 14 | 15.12 ± 1.73 A* | |
| 21 | 15.15 ± 1.76 A* | |
| S. thermophilus (log CFU/g) | 1 | 9.93 ± 0.209 B** |
| 7 | 10.98 ± 0.369 A** | |
| 14 | 10.23 ± 0.276 B** | |
| 21 | 10.13 ± 0.293 B** | |
| Lb. delbrueckii subsp. bulgaricus (log CFU/g) | 1 | 8.62 ± 0.183 A** |
| 7 | 8.08 ± 0.127 B** | |
| 14 | 7.36 ± 0.142 C** | |
| 21 | 6.85 ± 0.217 D** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anli, E.A.; Gursel, A.; Gursoy, A.; Mert, B. Assessment of the Quality Attributes of Oat β-glucan Fortified Reduced-Fat Goat Milk Yogurt Supported by Microfluidization. Foods 2023, 12, 3457. https://doi.org/10.3390/foods12183457
Anli EA, Gursel A, Gursoy A, Mert B. Assessment of the Quality Attributes of Oat β-glucan Fortified Reduced-Fat Goat Milk Yogurt Supported by Microfluidization. Foods. 2023; 12(18):3457. https://doi.org/10.3390/foods12183457
Chicago/Turabian StyleAnli, Elif Ayse, Asuman Gursel, Ayse Gursoy, and Behic Mert. 2023. "Assessment of the Quality Attributes of Oat β-glucan Fortified Reduced-Fat Goat Milk Yogurt Supported by Microfluidization" Foods 12, no. 18: 3457. https://doi.org/10.3390/foods12183457





