Cinnamaldehyde Inhibits Postharvest Gray Mold on Pepper Fruits via Inhibiting Fungal Growth and Triggering Fruit Defense
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain, Media, and Treatment
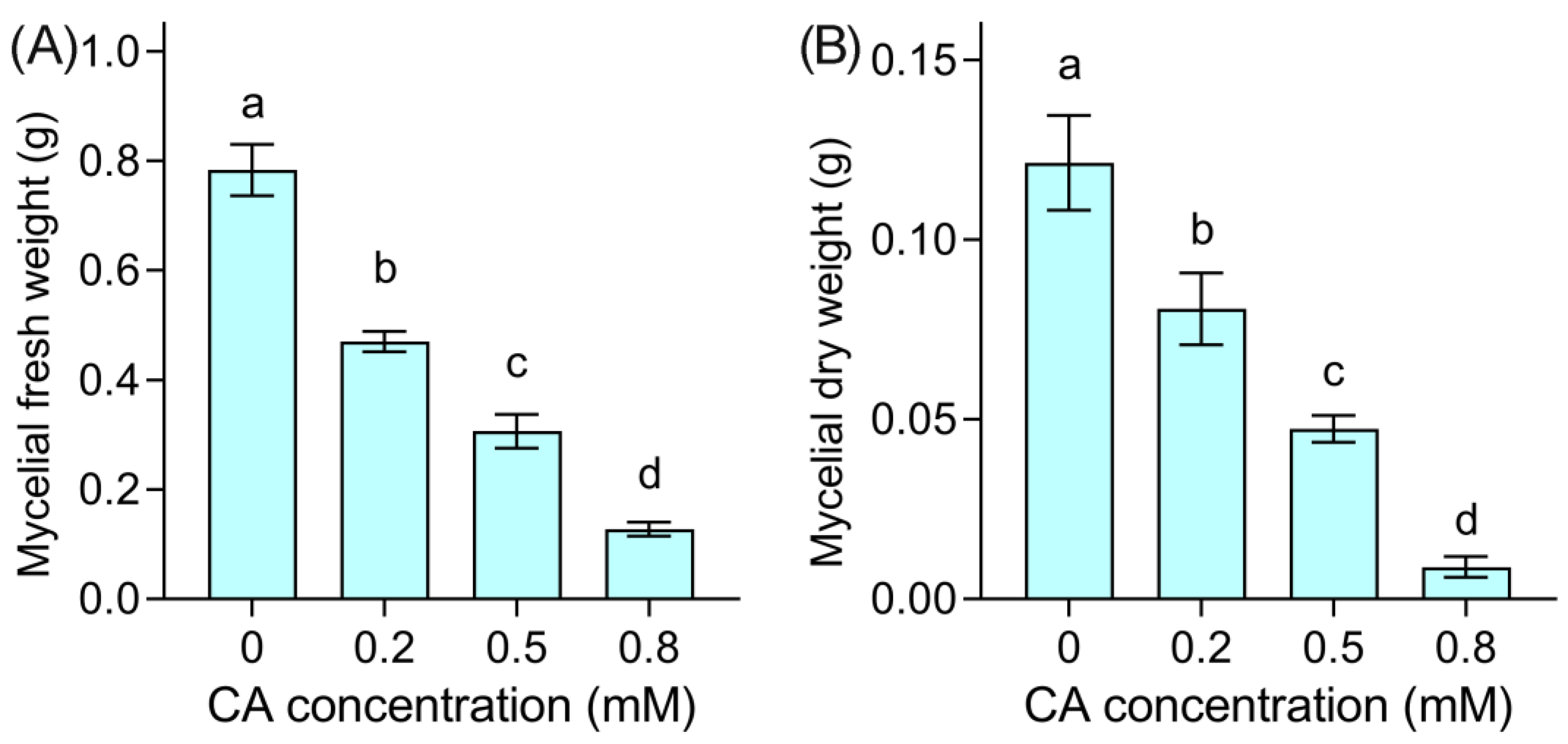
2.2. Determination of Mycelial Biomass
2.3. Spore Production and Germination
2.4. SEM (Scanning Electron Microscopy)
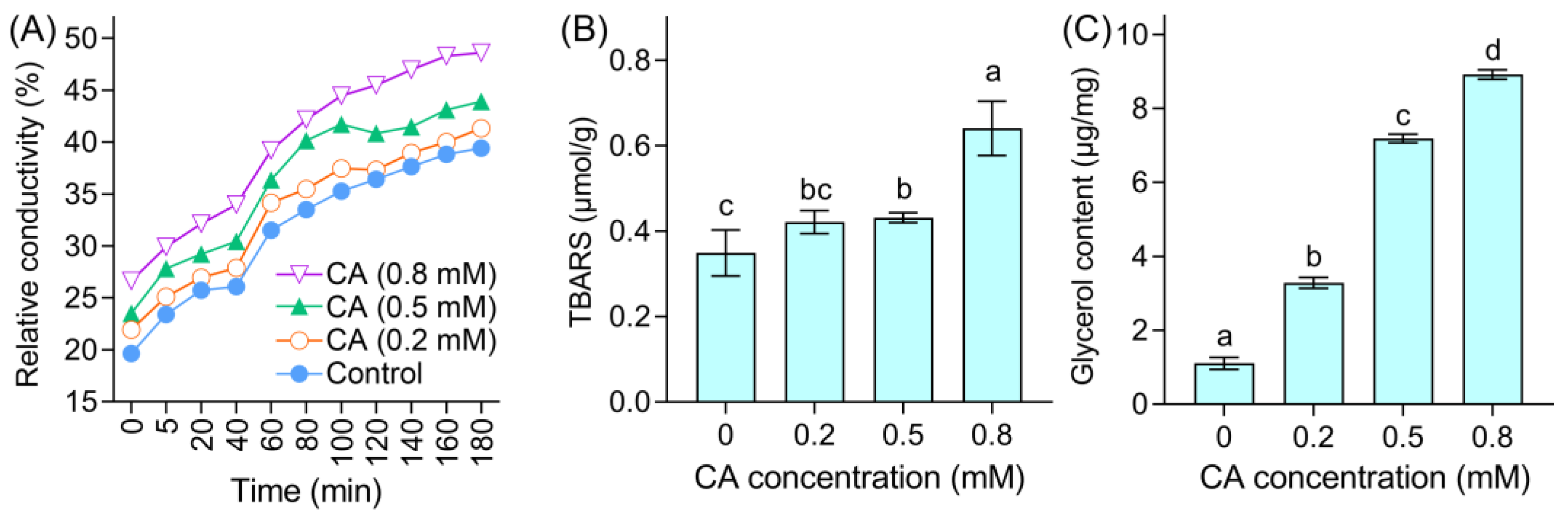
2.5. Meaaurement of Mycelial Conductivity
2.6. Meaaurement of Mycelial TBARS (Thiobarbituric Acid Reactive Substances) Content
2.7. Measurement of Mycelial Glycerol Content
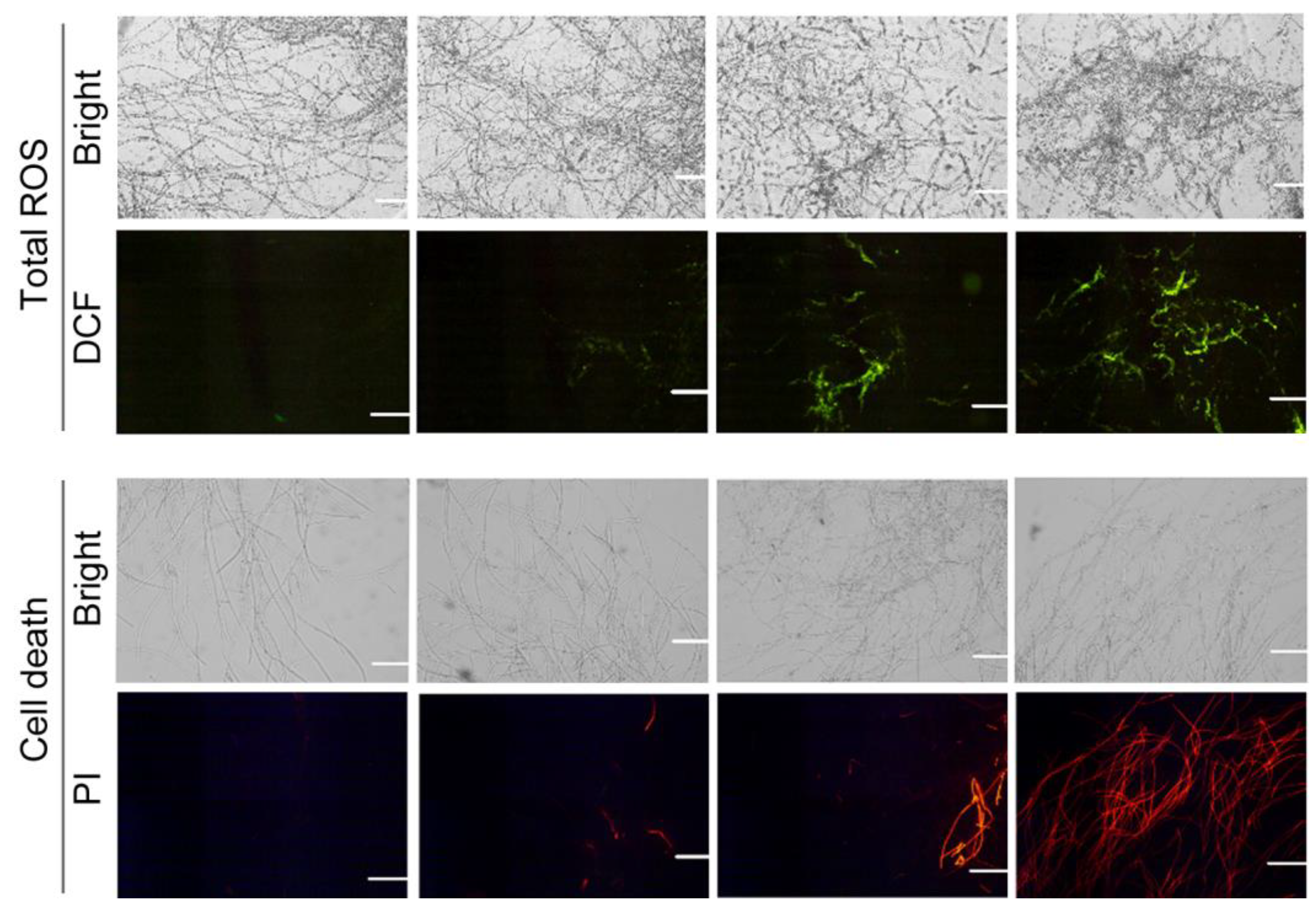
2.8. Histochemical Analysis of ROS (Reactive Oxygen Species) and Cell Death
2.9. CA-Suppressed Development of B. cinerea on Pepper Fruits
2.10. Assay of Defensive Enzyme Activity of Pepper Fruits
2.11. Measurement of Metabolites
2.12. Data Analysis
3. Results
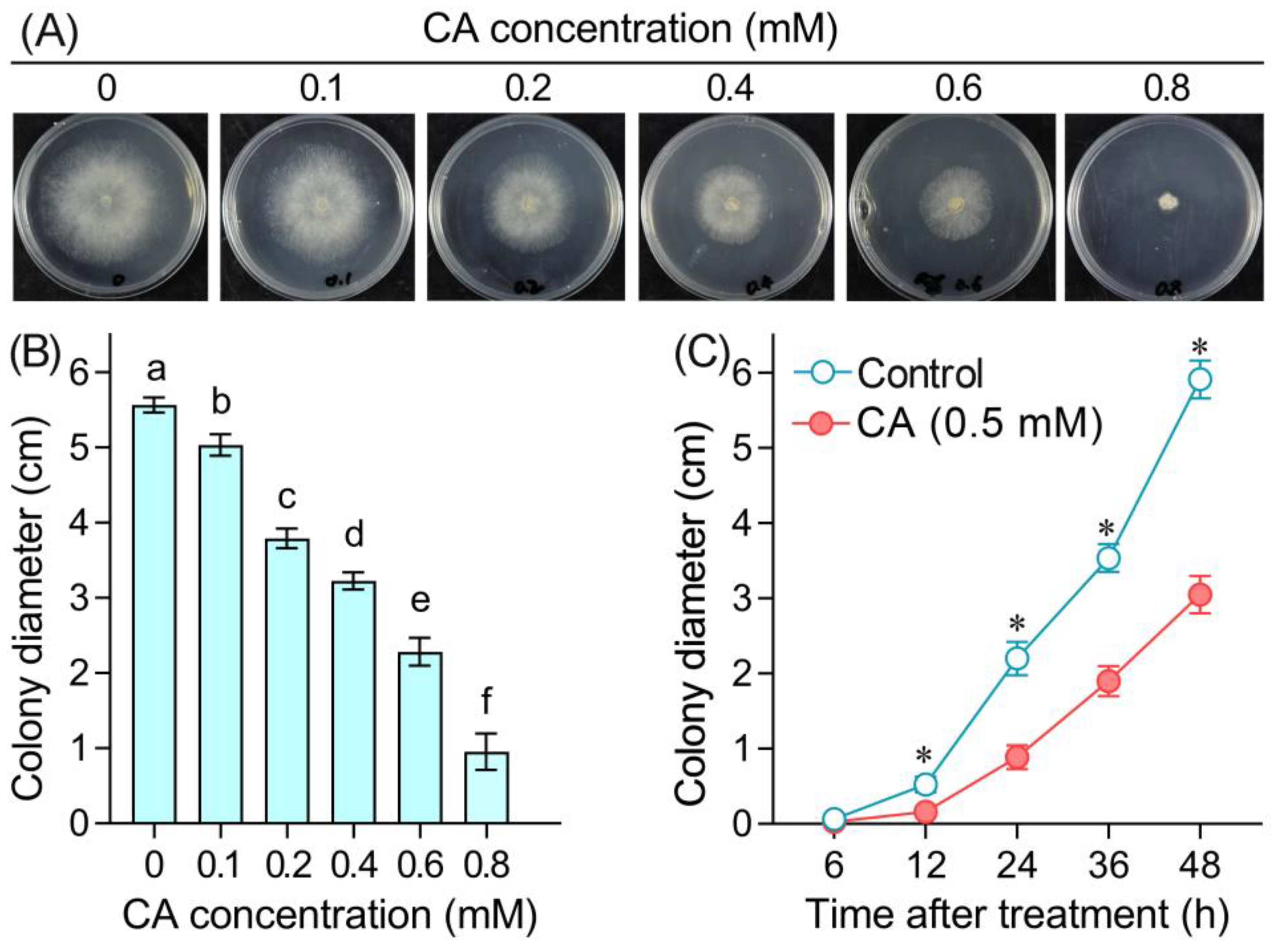
3.1. Mycelial Growth
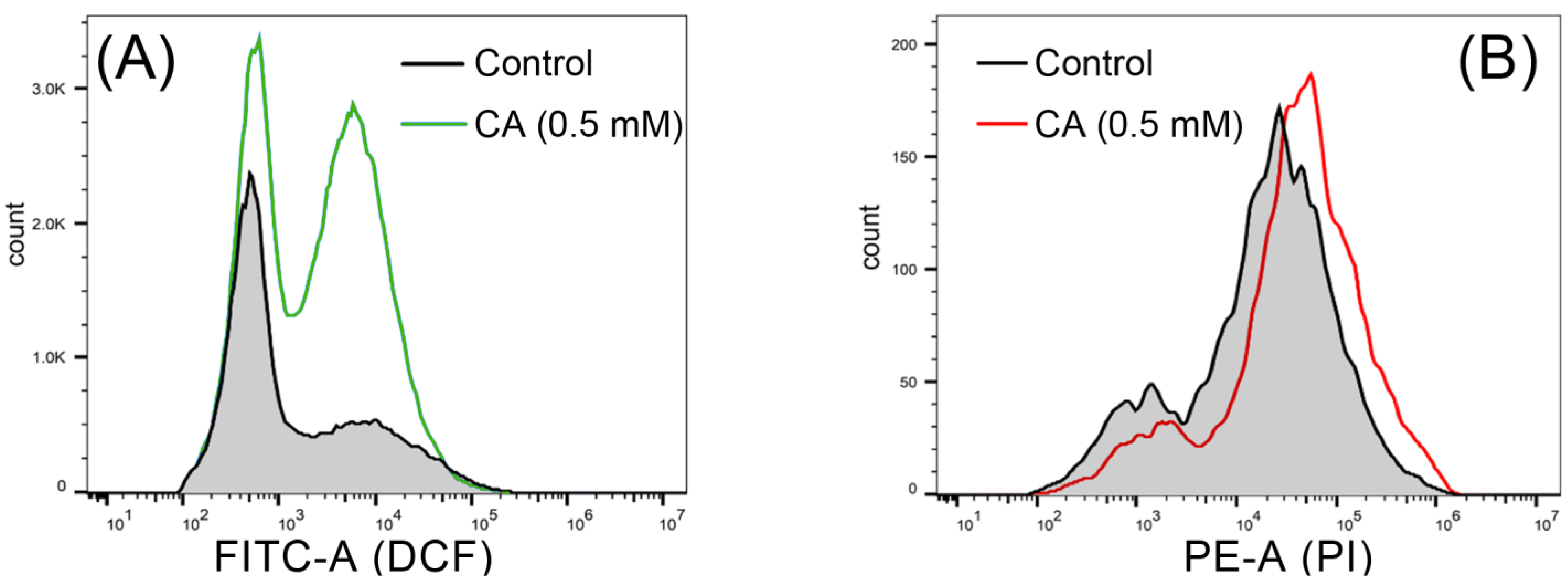
3.2. CA Induced Mycelial Injury of B. cinerea
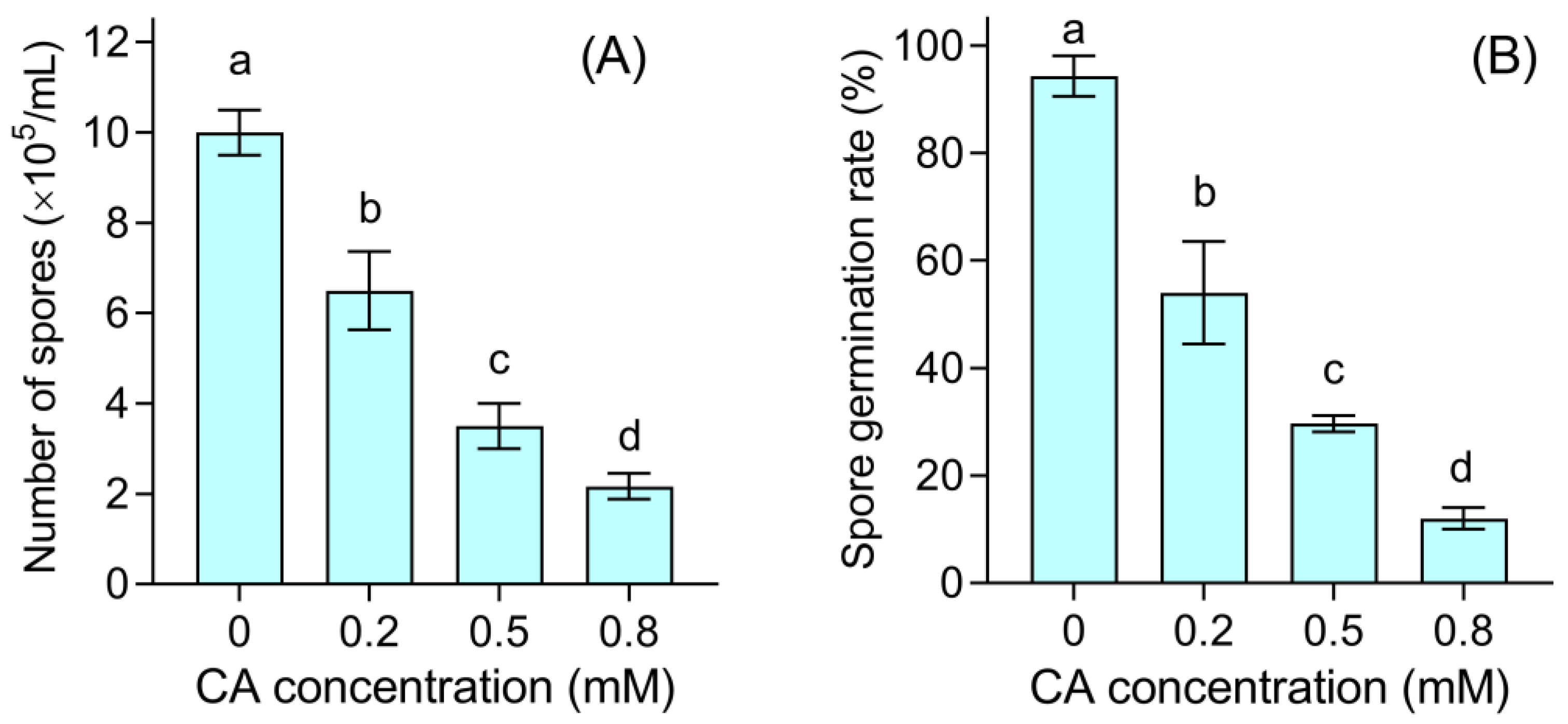
3.3. CA Damaged the Spores of B. cinerea
3.4. Hierarchical Cluster and Correlation Analysis of Physiological Parameters in CA-Treated B. cinerea
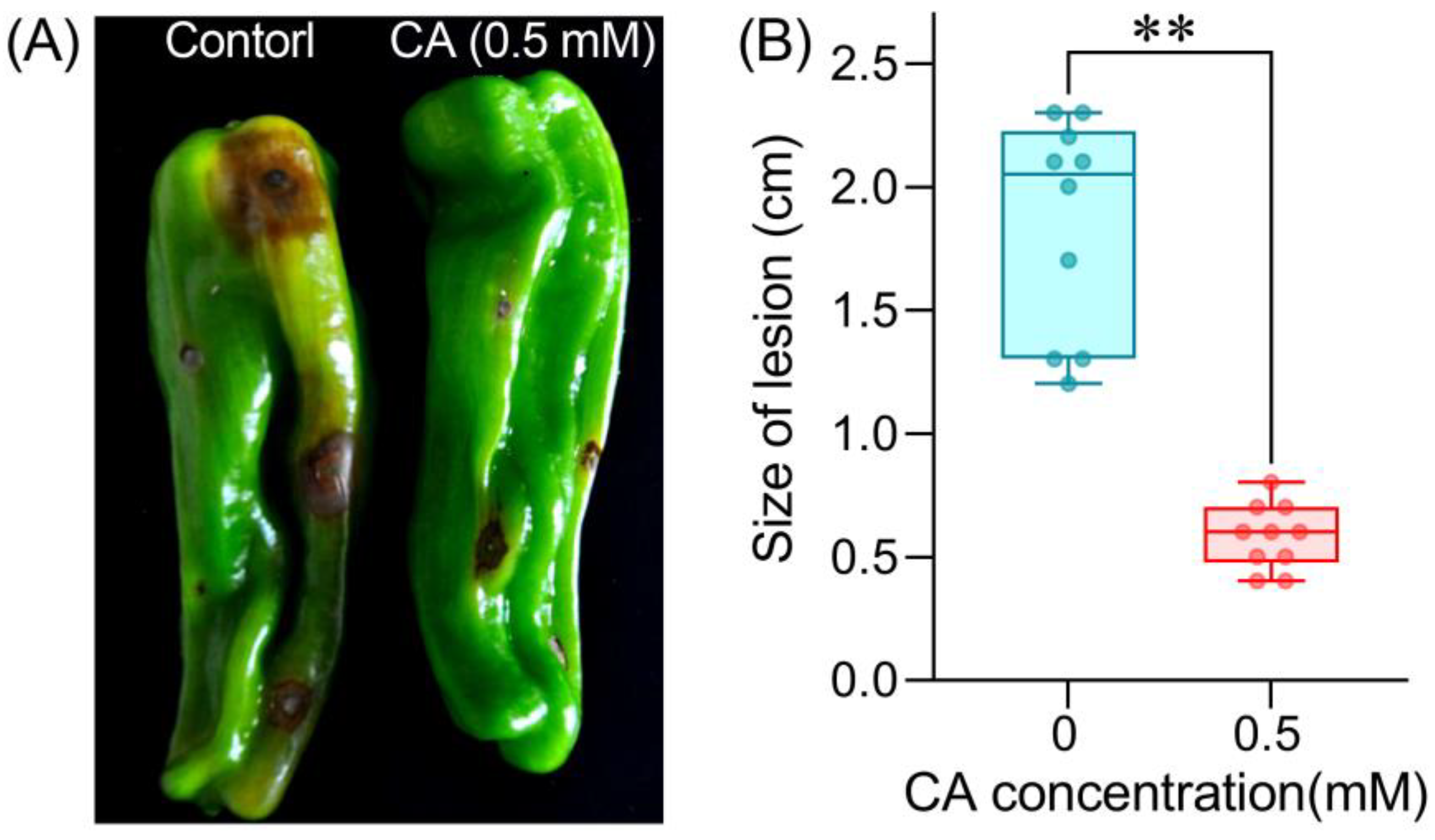
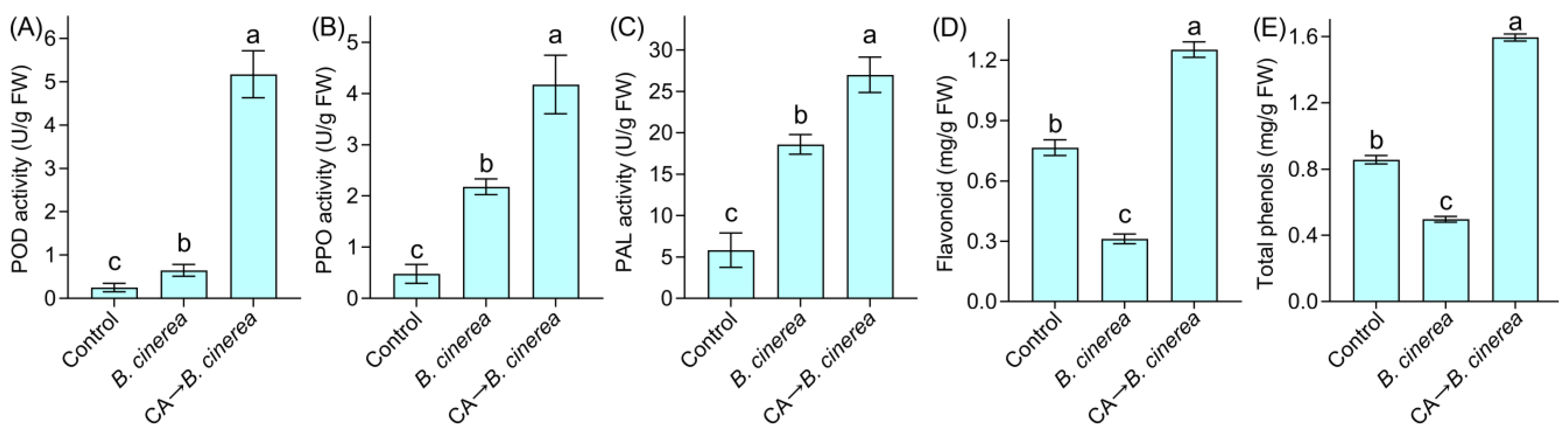
3.5. CA Suppresses the Infection of B. cinerea on Pepper Fruit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, D.; Sharma, R.R. Postharvest Diseases of Fruits and Vegetables and Their Management. In Postharvest Disinfection of Fruits and Vegetables; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–52. [Google Scholar]
- Dean, R.; van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- Krasnow, C.; Ziv, C. Non-chemical approaches to control postharvest gray mold disease in bell peppers. Agronomy 2022, 12, 216. [Google Scholar] [CrossRef]
- O’Neill, T.M.; Shtienberg, D.; Elad, Y. Effect of some host and microclimate factors on infection of tomato stems by Botrytis cinerea. Plant Dis. 1997, 81, 36–40. [Google Scholar] [CrossRef]
- Tsitsigiannis, D.; Antoniou, P.; Tjamos, S.; Paplomatas, E. Major diseases of tomato, pepper and egg plant in green houses. Eur. J. Plant Sci. Biotechnol. 2008, 2, 106–124. [Google Scholar]
- Hausbeck, M.; Moorman, G. Managing Botrytis in greenhouse-grown flower crops. Plant Dis. 1996, 80, 1212–1219. [Google Scholar] [CrossRef]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Postharvest Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Fallik, E.; Grinberg, S.; Alkalai, S.; Yekutieli, O.; Wiseblum, A.; Regev, R.; Beres, H.; Bar-Lev, E. A unique rapid hot water treatment to improve storage quality of sweet pepper. Postharvest Biol. Technol. 1999, 15, 25–32. [Google Scholar] [CrossRef]
- Schirra, M.; D’Aquino, S.; Cabras, P.; Angioni, A. Control of Postharvest Diseases of Fruit by Heat and Fungicides: Efficacy, Residue Levels, and Residue Persistence. A Review. J. Agric. Food Chem. 2011, 59, 8531–8542. [Google Scholar] [CrossRef]
- Li, X.; Zeng, S.; Wisniewski, M.; Droby, S.; Yu, L.; An, F.; Leng, Y.; Wang, C.; Li, X.; He, M.; et al. Current and future trends in the biocontrol of postharvest diseases. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Brito, C.; Hansen, H.; Espinoza, L.; Faúndez, M.; Olea, A.F.; Pino, S.; Díaz, K. Assessing the control of postharvest gray mold disease on tomato fruit using mixtures of essential oils and their respective hydrolates. Plants 2021, 10, 1719. [Google Scholar] [CrossRef]
- Prusky, D.; Romanazzi, G. Induced resistance in fruit and vegetables: A host physiological response limiting postharvest disease development. Annu. Rev. Phytopathol. 2023, 61, 279–300. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement. Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Jayawardana, R.; Galappaththy, P.; Constantine, G.R.; de Vas Gunawardana, N.; Katulanda, P. Efficacy and safety of ‘true’ cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: A systematic review and meta-analysis. Diab. Med. 2012, 29, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Meena, V.; Sree, S.N.; Surya, P.D.V.; Sumanjali, A. A review on pharmacological activities and clinical effects of Cinnamon species. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 653–663. [Google Scholar]
- Sun, Q.; Li, J.; Sun, Y.; Chen, Q.; Zhang, L.; Le, T. The antifungal effects of cinnamaldehyde against Aspergillus niger and its application in bread preservation. Food Chem. 2020, 317, 126405. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.; Huang, Z.; Li, Q.; Wang, E.; Liao, S.; Li, E.; Zou, Y.; Wang, W. Antibacterial mechanism of cinnamaldehyde: Modulation of biosynthesis of phosphatidylethanolamine and phosphatidylglycerol in Staphylococcus aureus and Escherichia coli. J. Agric. Food Chem. 2021, 69, 13628–13636. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Wang, Z.; Cao, H.; Zhang, D. Structure-activity relationships of cinnamaldehyde and eugenol derivatives against plant pathogenic fungi. Ind. Crops Prod. 2017, 97, 388–394. [Google Scholar] [CrossRef]
- Wei, J.; Bi, Y.; Xue, H.; Wang, Y.; Zong, Y.; Prusky, D. Antifungal activity of cinnamaldehyde against Fusarium sambucinum involves inhibition of ergosterol biosynthesis. J. Appl. Microbiol. 2020, 129, 256–265. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, X.; Jing, G.; Ou Yang, Q.; Tao, N. Cinnamaldehyde inhibits the mycelial growth of Geotrichum citri-aurantii and induces defense responses against sour rot in citrus fruit. Postharvest Biol. Technol. 2017, 129, 23–28. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. The extracts of cinnamon and clove as potential biofungicides against strawberry grey mould. Plants 2020, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The fungicidal activity of thymol against Fusarium graminearum via inducing lipid peroxidation and disrupting ergosterol biosynthesis. Molecules 2016, 21, 770. [Google Scholar] [CrossRef] [PubMed]
- Thomulka, K.W.; Abbas, C.G.; Young, D.A.; Lange, J.H. Evaluating median effective concentrations of chemicals with bioluminescent bacteria. Bull. Environ. Contam. Toxicol. 1996, 56, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Sutton, L.M.; Starzyk, M.J. Procedure and analysis of a useful method in determining mycelial dry weights from agar plates. Appl. Microbiol. 1972, 24, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-San Millan, A.; Larraya, L.; Farran, I.; Ancin, M.; Veramendi, J. Successful biocontrol of major postharvest and soil-borne plant pathogenic fungi by antagonistic yeasts. Biol. Control 2021, 160, 104683. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Y.; Lu, H.; Li, P.; Chen, J.; Shi, Z.; Xie, Y.; Mo, H.; Hu, L. Nano-thymol emulsion inhibits Botrytis cinerea to control postharvest gray mold on tomato fruit. Agronomy 2022, 12, 2973. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, F.; Zhuo, R.; Ma, F.; Gong, Y.; Wan, X.; Jiang, M.; Zhang, X. Expression of the laccase gene from a white rot fungus in Pichia pastoris can enhance the resistance of this yeast to H2O2-mediated oxidative stress by stimulating the glutathione-based antioxidative system. Appl. Environ. Microbiol. 2012, 78, 5845–5854. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Ge, C.; Liu, S.; Chen, C.; Zhou, M. Effect of phenylpyrrole fungicide fludioxonil on morphological and physiological characteristics of Sclerotinia sclerotiorum. Pestic. Biochem. Physiol. 2013, 106, 61–67. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, W.; Yang, J.; Chen, J.; Yin, Y.; Shi, Z. Cinnamaldehyde Induces PCD-Like death of Microcystis aeruginosa via reactive oxygen species. Water Air Soil Pollut. 2011, 217, 105–113. [Google Scholar] [CrossRef]
- Firstencel, H.; Butt, T.M.; Carruthers, R.I. A fluorescence microscopy method for determining the viability of entomophthoralean fungal spores. J. Invertebr. Pathol. 1990, 55, 258–264. [Google Scholar] [CrossRef]
- Yao, L.; Ban, F.; Peng, S.; Xu, D.; Li, H.; Mo, H.; Hu, L.; Zhou, X. Exogenous iron induces NADPH oxidases-dependent ferroptosis in the conidia of Aspergillus flavus. J. Agric. Food Chem. 2021, 69, 13608–13617. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.S.; Huang, S.Q.; Guo, K.; Mehta, S.K.; Zhang, P.C.; Yang, Z.M. Metabolic adaptations to mercury-induced oxidative stress in roots of Medicago sativa L. J. Inorg. Biochem. 2007, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, F.; Cui, F.; Sun, W.; Zhang, J.; Qian, L.; Yang, Y.; Wu, D.; Dong, Y.; Jiang, J.; et al. Improved postharvest quality and respiratory activity of straw mushroom (Volvariella volvacea) with ultrasound treatment and controlled relative humidity. Sci. Hortic. 2017, 225, 56–64. [Google Scholar] [CrossRef]
- Ballester, A.R.; Lafuente, M.T.; González-Candelas, L. Spatial study of antioxidant enzymes, peroxidase and phenylalanine ammonia-lyase in the citrus fruit–Penicillium digitatum interaction. Postharvest Biol. Technol. 2006, 39, 115–124. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, Y.; Dong, X.; Zhou, G.; Liu, X.; Gu, Q.; Wei, Q. Metabolic profiling and gene expression analysis unveil differences in flavonoid and lipid metabolisms between ‘Huapi’ kumquat (Fortunella crassifolia Swingle) and its wild type. Front. Plant Sci. 2021, 12, 759968. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84); R Foundation: Vienna, Austria, 2017. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. Application of plant extracts to control postharvest gray mold and susceptibility of apple fruits to B. cinerea from different plant hosts. Foods 2020, 9, 1430. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Yang, K.; Chen, L.; Liu, M.; Geng, Q.; He, X.; Li, Y.; Liu, Y.; Tian, J. Cinnamaldehyde, a promising natural preservative against Aspergillus flavus. Front Microbiol. 2019, 10, 2895. [Google Scholar] [CrossRef]
- Khan, M.S.; Ahmad, I. In vitro antifungal, anti-elastase and anti-keratinase activity of essential oils of Cinnamomum-, Syzygium- and Cymbopogon-species against Aspergillus fumigatus and Trichophyton rubrum. Phytomedicine 2011, 19, 48–55. [Google Scholar] [CrossRef]
- El-Baky, N.A.; Amara, A. Recent approaches towards control of fungal diseases in plants: An updated review. J. Fungi. 2021, 7, 900. [Google Scholar] [CrossRef]
- OuYang, Q.; Duan, X.; Li, L.; Tao, N. Cinnamaldehyde exerts its antifungal activity by disrupting the cell wall integrity of Geotrichum citri-aurantii. Front Microbiol. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Tao, N.; Yang, W.; Jing, G. Cinnamaldehyde damaged the cell membrane of Alternaria alternata and induced the degradation of mycotoxins in vivo. Ind. Crops Prod. 2018, 112, 427–433. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Ma, J.; Liu, L.; Li, J.; Shen, T.; Tian, Y. The major component of cinnamon oil as a natural substitute against Fusarium solani on Astragalus membranaceus. J. Appl. Microbiol. 2022, 132, 3125–3141. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Liu, J.; Cao, B.; Li, B.; Tian, S. Hydrogen Peroxide Acts on Sensitive Mitochondrial Proteins to Induce Death of a Fungal Pathogen Revealed by Proteomic Analysis. PLoS ONE 2011, 6, e21945. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-C.; Fu, H.-Y.; Ho, C.-T.; Tan, D.; Huang, Y.-T.; Pan, M.-H. Induction of apoptosis by cinnamaldehyde from indigenous cinnamon Cinnamomum osmophloeum Kaneh through reactive oxygen species production, glutathione depletion, and caspase activation in human leukemia K562 cells. Food Chem. 2007, 103, 434–443. [Google Scholar] [CrossRef]
- Brown, A.J.; Budge, S.; Kaloriti, D.; Tillmann, A.; Jacobsen, M.D.; Yin, Z.; Ene, I.V.; Bohovych, I.; Sandai, D.; Kastora, S.; et al. Stress adaptation in a pathogenic fungus. J. Exp. Biol. 2014, 217, 144–155. [Google Scholar] [CrossRef]
- Duran, R.; Cary, J.W.; Calvo, A.M. Role of the osmotic stress regulatory pathway in morphogenesis and secondary metabolism in filamentous fungi. Toxins 2010, 2, 367–381. [Google Scholar] [CrossRef]
- Kojima, K.; Takano, Y.; Yoshimi, A.; Tanaka, C.; Kikuchi, T.; Okuno, T. Fungicide activity through activation of a fungal signalling pathway. Mol. Microbiol. 2004, 53, 1785–1796. [Google Scholar] [CrossRef]
- Ren, W.; Liu, N.; Yang, Y.; Yang, Q.; Chen, C.; Gao, Q. The sensor proteins Bcsho1 and Bcsln1 are involved in, though not essential to, vegetative differentiation, pathogenicity and osmotic stress tolerance in Botrytis cinerea. Front Microbiol. 2019, 10, 328. [Google Scholar] [CrossRef]
- Cheung, N.; Tian, L.; Liu, X.; Li, X. The destructive fungal pathogen botrytis cinerea-insights from genes studied with mutant analysis. Pathogens 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wisniewski, M.; Wang, W.; Liu, J.; Liu, Y. Heat-induced oxidative injury contributes to inhibition of Botrytis cinerea spore germination and growth. World J. Microbiol. Biotechnol. 2014, 30, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, X. Recent advances in polyphenol oxidase-mediated plant stress responses. Phytochemistry 2021, 181, 112588. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and Capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, N.; Liu, R.; Bai, H.; Tao, W.; Chen, J.; Shi, Z. Cinnamaldehyde facilitates cadmium tolerance by modulating Ca2+ in Brassica rapa. Water Air Soil Pollut. 2021, 232, 19. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Peng, Y.; Zhou, X.-Q. Cinnamaldehyde improved intestine immune function and alleviated inflammation associated with NF-κB pathways in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Aquac. Rep. 2021, 21, 100837. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Devi, G.; Balasundaram, C.; Van Doan, H.; Jaturasitha, S.; Saravanan, K.; Ringø, E. Impact of cinnamaldehyde on innate immunity and immune gene expression in Channa striatus against Aphanomyces invadans. Fish Shellfish Immunol. 2021, 117, 1–16. [Google Scholar] [CrossRef]
- Gan, Z.; Huang, J.; Chen, J.; Nisar, M.F.; Qi, W. Synthesis and antifungal activities of cinnamaldehyde derivatives against Penicillium digitatum causing Citrus green mold. J. Food Qual. 2020, 2020, 8898692. [Google Scholar] [CrossRef]
- Wang, H.; Peng, Z.; Sun, H. Antifungal activities and mechanisms of trans-cinnamaldehyde and thymol against food-spoilage yeast Zygosaccharomyces rouxii. J. Food Sci. 2022, 87, 1197–1210. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jung, M.; Lee, S.-C.; Huh, M.-J.; Seo, S.-M.; Park, I.-K. Antibacterial mode of action of trans-cinnamaldehyde derived from cinnamon bark (Cinnamomum verum) essential oil against Agrobacterium tumefaciens. Pestic. Biochem. Physiol. 2020, 165, 104546. [Google Scholar] [CrossRef]
- De Angelis, G.; Simonetti, G.; Chronopoulou, L.; Orekhova, A.; Badiali, C.; Petruccelli, V.; Portoghesi, F.; D’Angeli, S.; Brasili, E.; Pasqua, G.; et al. A novel approach to control Botrytis cinerea fungal infections: Uptake and biological activity of antifungals encapsulated in nanoparticle based vectors. Sci. Rep. 2022, 12, 7989. [Google Scholar] [CrossRef]
- Zhang, R.; Cui, Y.; Cheng, M.; Guo, Y.; Wang, X.; Wang, J. Antifungal activity and mechanism of cinnamon essential oil loaded into mesoporous silica nanoparticles. Ind. Crops Prod. 2021, 171, 113846. [Google Scholar] [CrossRef]
- Cionti, C.; Taroni, T.; Sabatini, V.; Meroni, D. Nanostructured oxide-based systems for the pH-triggered release of cinnamaldehyde. Materials 2021, 14, 1536. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Liu, X.; Lu, H.; Zhang, C.; Chen, J.; Shi, Z. Cinnamaldehyde Inhibits Postharvest Gray Mold on Pepper Fruits via Inhibiting Fungal Growth and Triggering Fruit Defense. Foods 2023, 12, 3458. https://doi.org/10.3390/foods12183458
Yang L, Liu X, Lu H, Zhang C, Chen J, Shi Z. Cinnamaldehyde Inhibits Postharvest Gray Mold on Pepper Fruits via Inhibiting Fungal Growth and Triggering Fruit Defense. Foods. 2023; 12(18):3458. https://doi.org/10.3390/foods12183458
Chicago/Turabian StyleYang, Lifei, Xiaoli Liu, Haiyan Lu, Cunzheng Zhang, Jian Chen, and Zhiqi Shi. 2023. "Cinnamaldehyde Inhibits Postharvest Gray Mold on Pepper Fruits via Inhibiting Fungal Growth and Triggering Fruit Defense" Foods 12, no. 18: 3458. https://doi.org/10.3390/foods12183458
APA StyleYang, L., Liu, X., Lu, H., Zhang, C., Chen, J., & Shi, Z. (2023). Cinnamaldehyde Inhibits Postharvest Gray Mold on Pepper Fruits via Inhibiting Fungal Growth and Triggering Fruit Defense. Foods, 12(18), 3458. https://doi.org/10.3390/foods12183458






