Neuroimaging Approach: Effects of Hot and Cold Germinated Wheat Beverages on Electroencephalographic (EEG) Activity of the Human Brain
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Participants
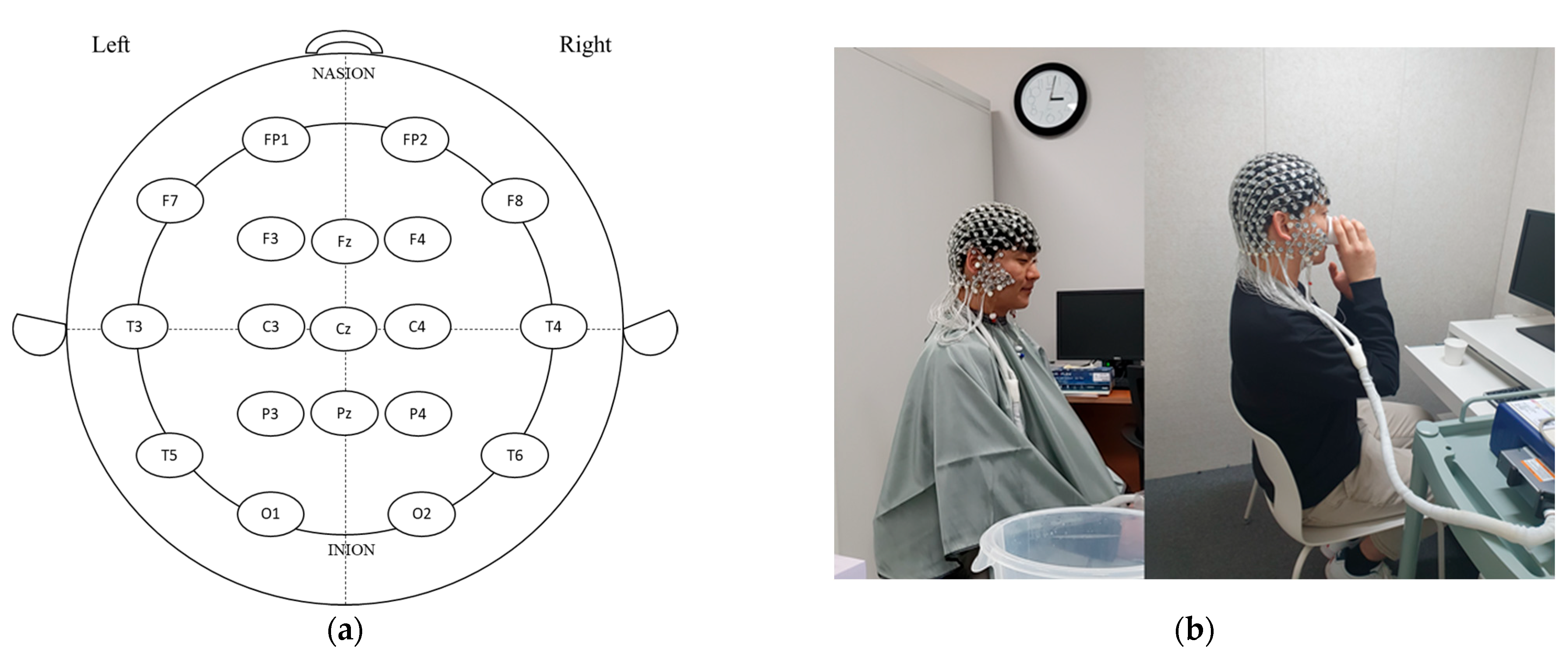
2.3. Electroencephalography (EEG) Data Acquisition
2.4. Data Processing and Statistical Analysis
3. Results
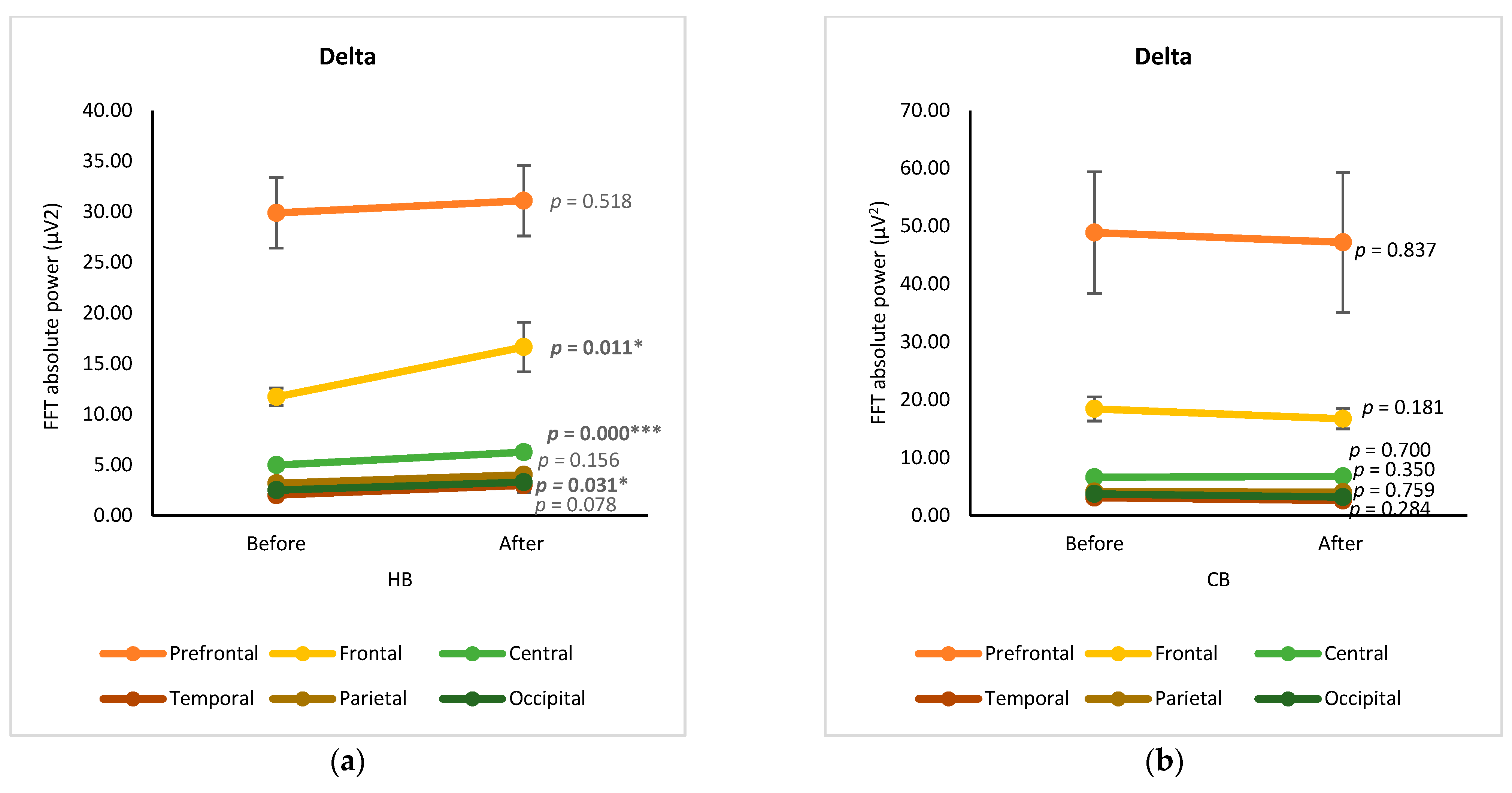
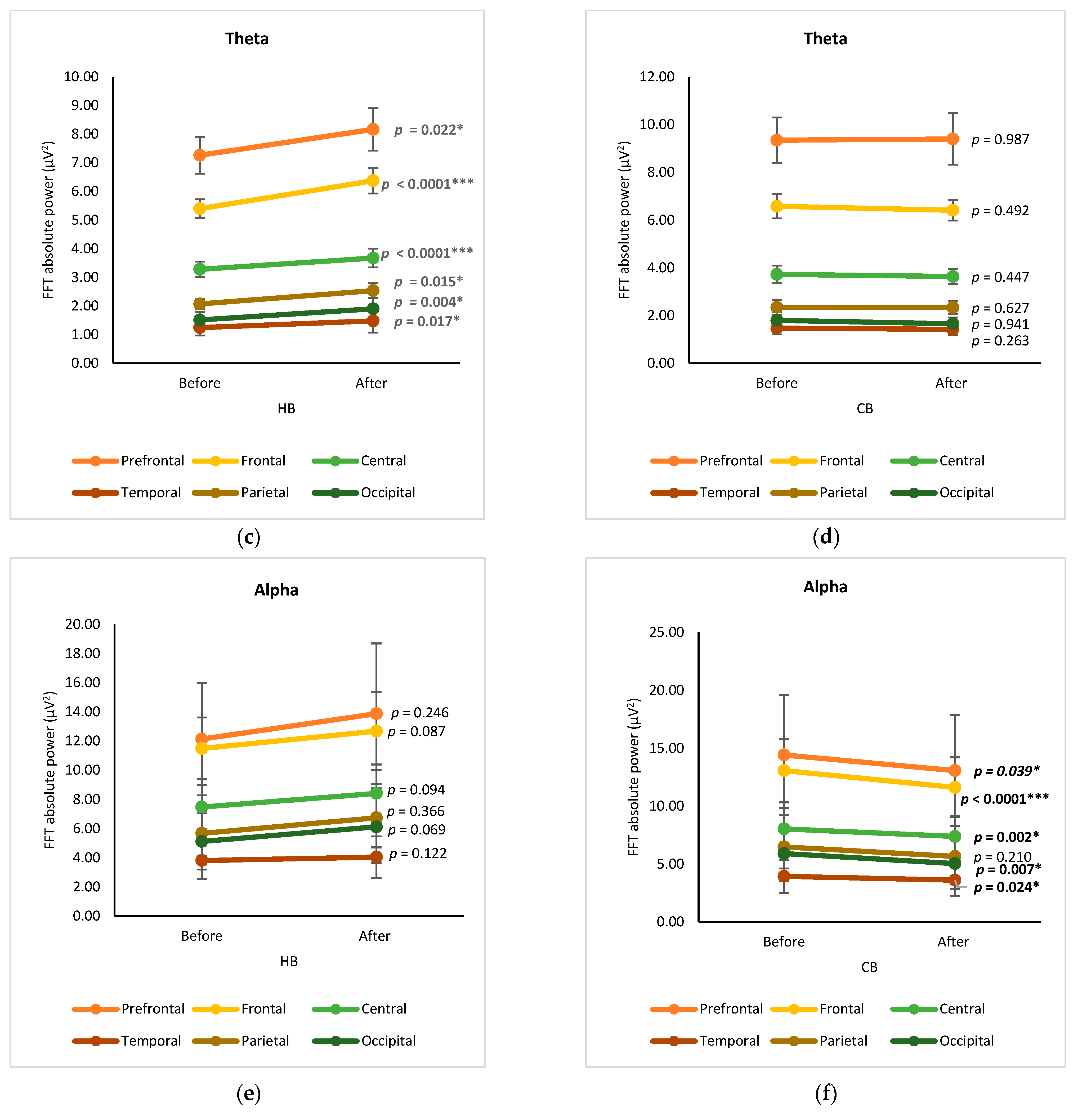
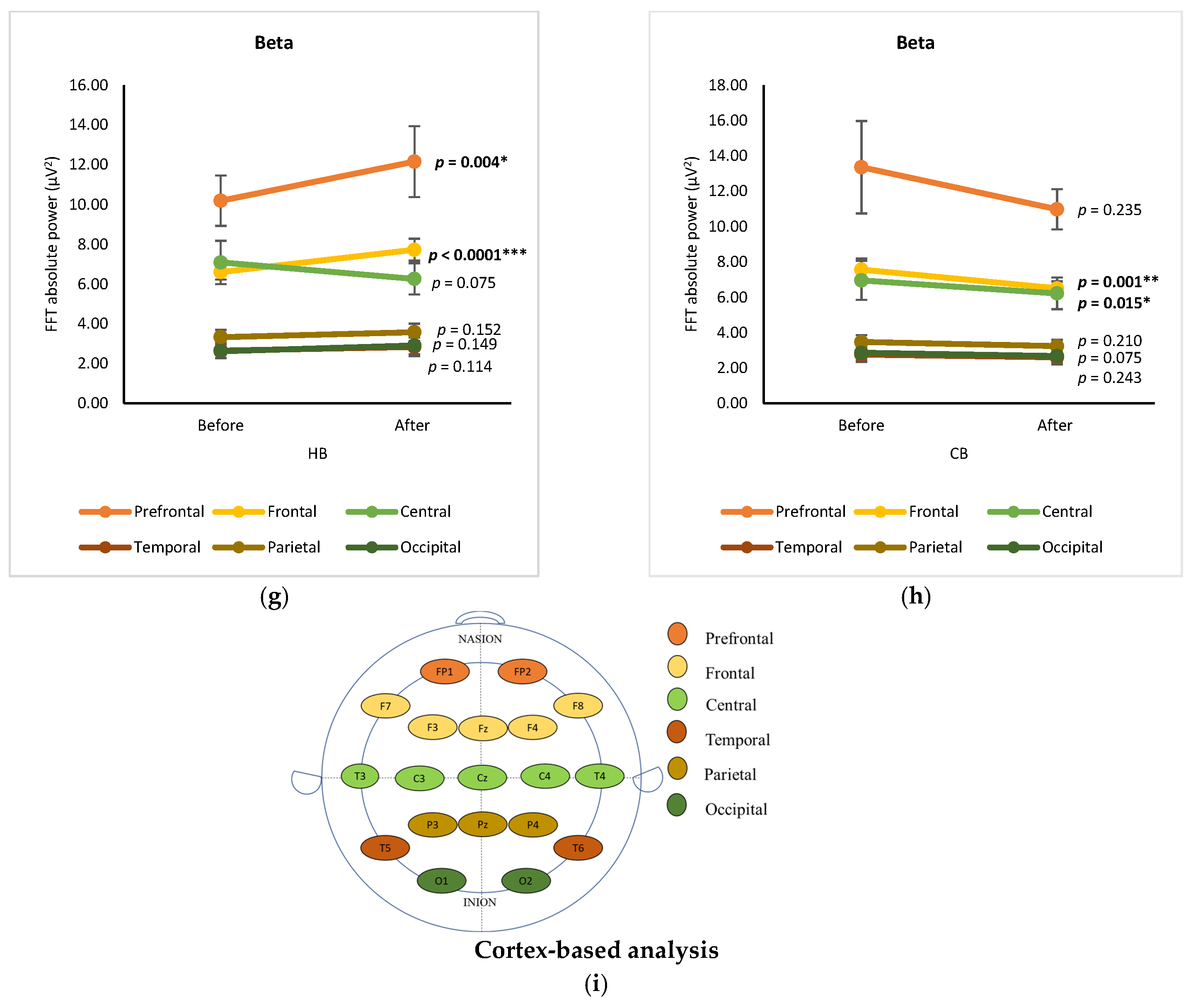
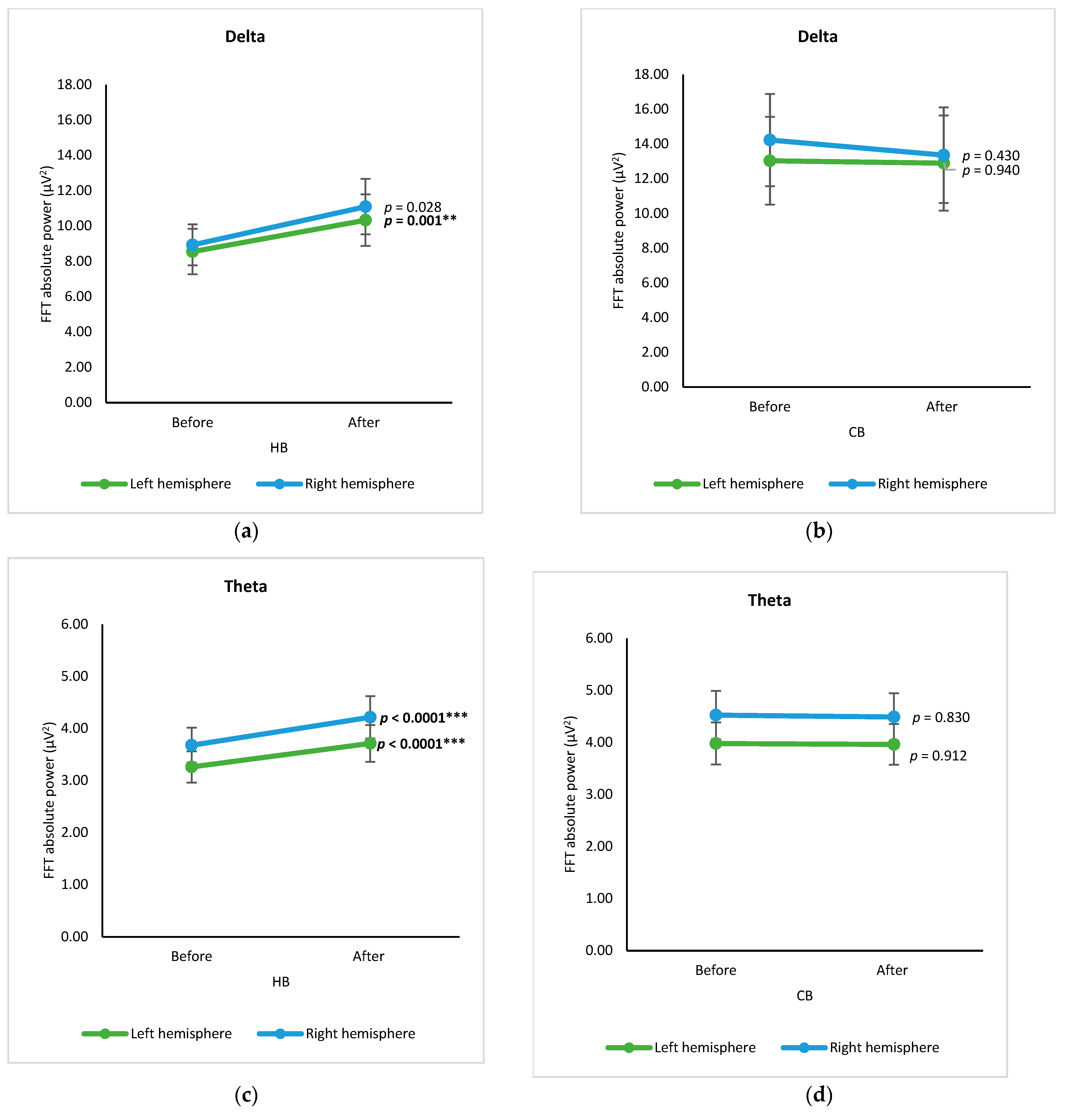
3.1. Cortex-Based Analysis
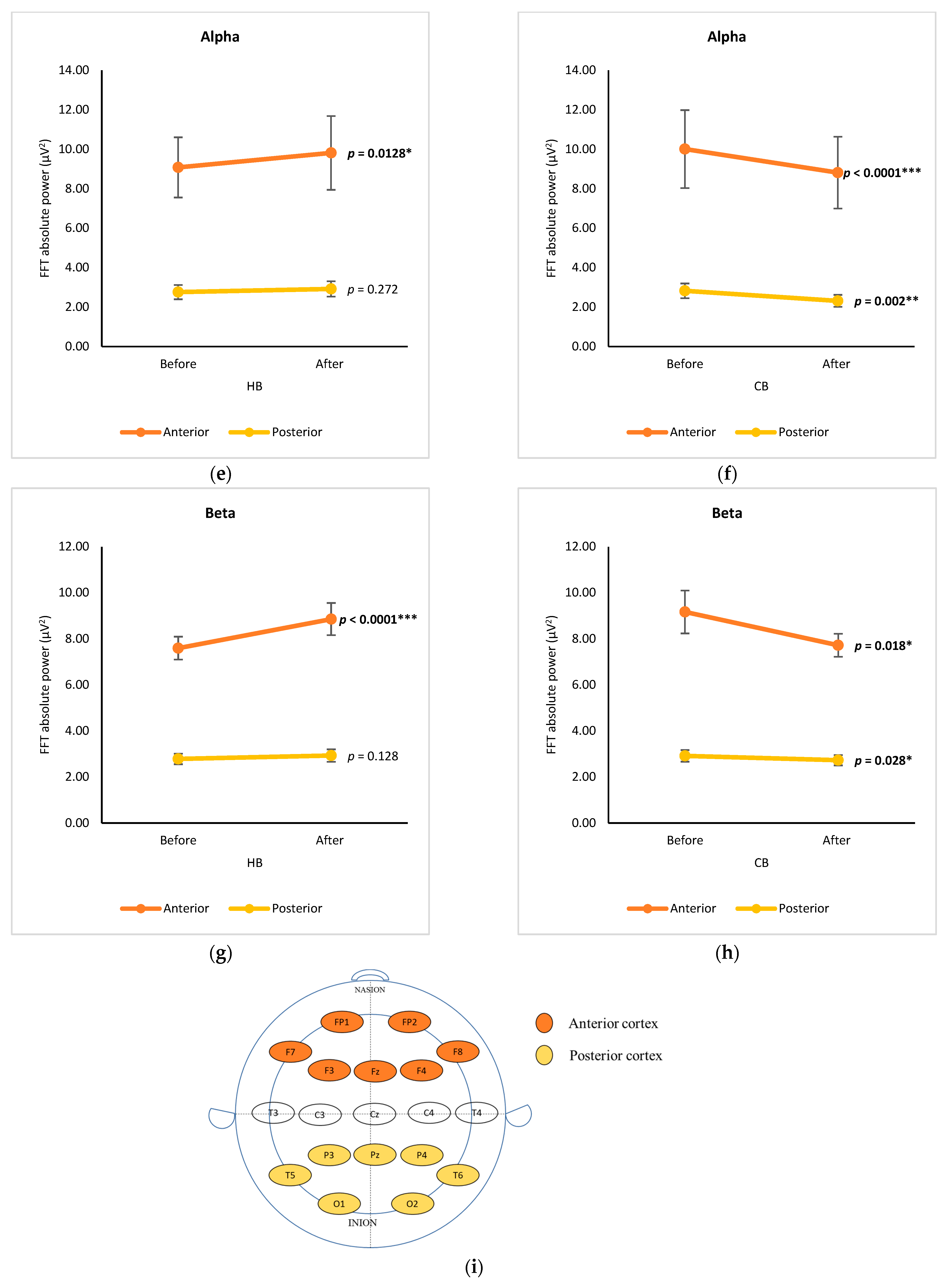
3.2. Anterior-Posterior Analysis
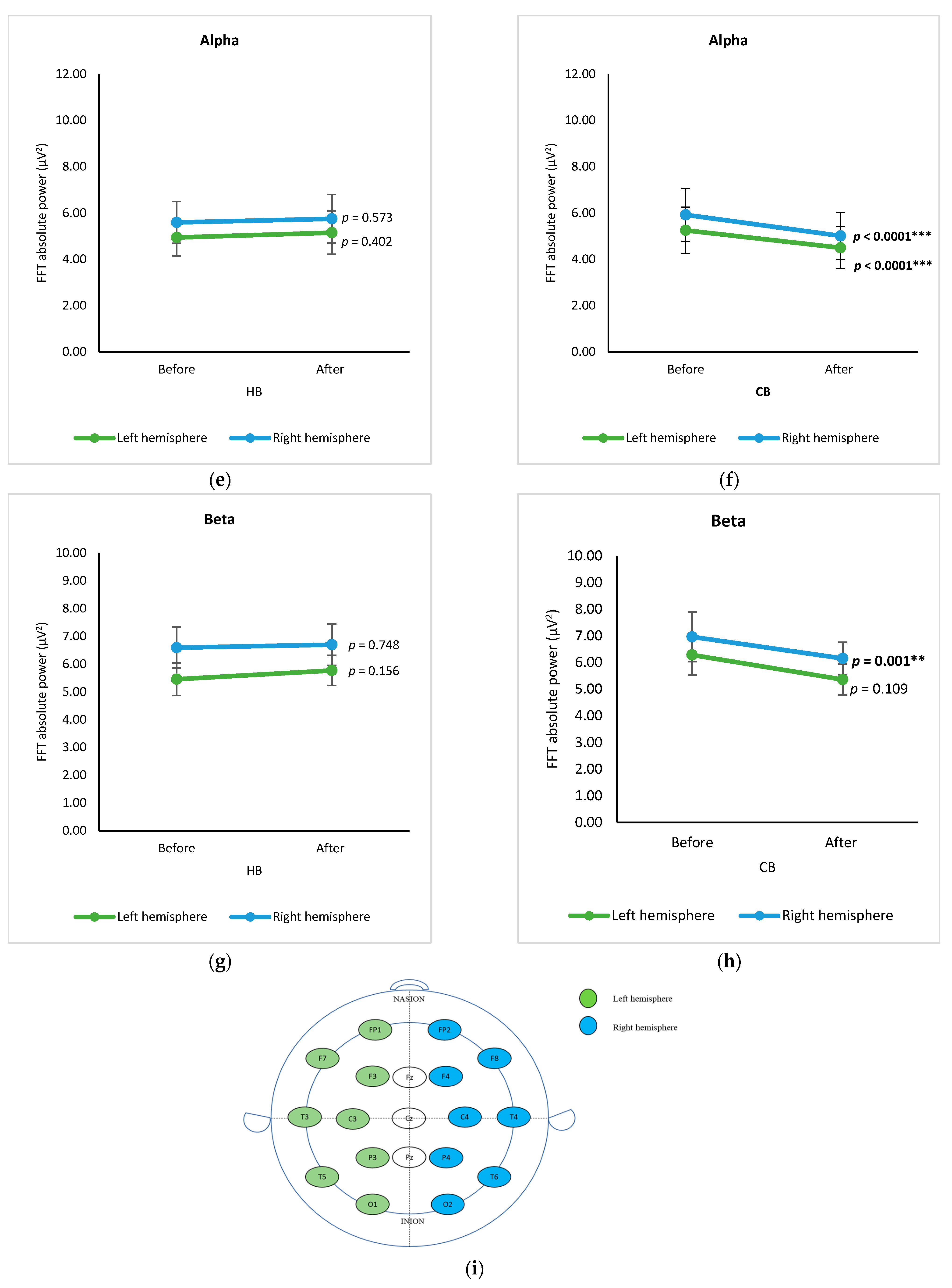
3.3. Hemisphere-Based Analysis
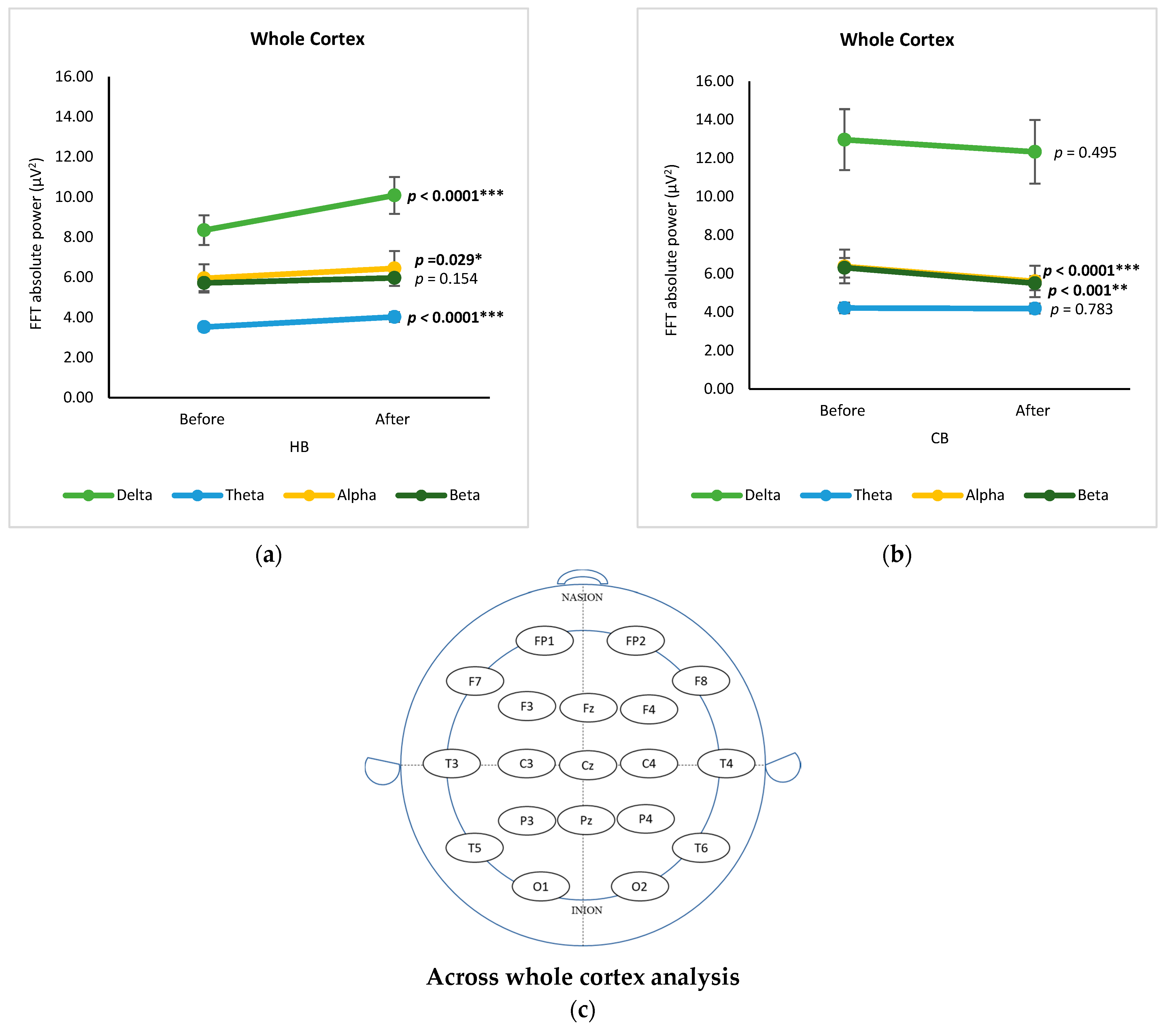
3.4. Across Whole Cortex Analysis
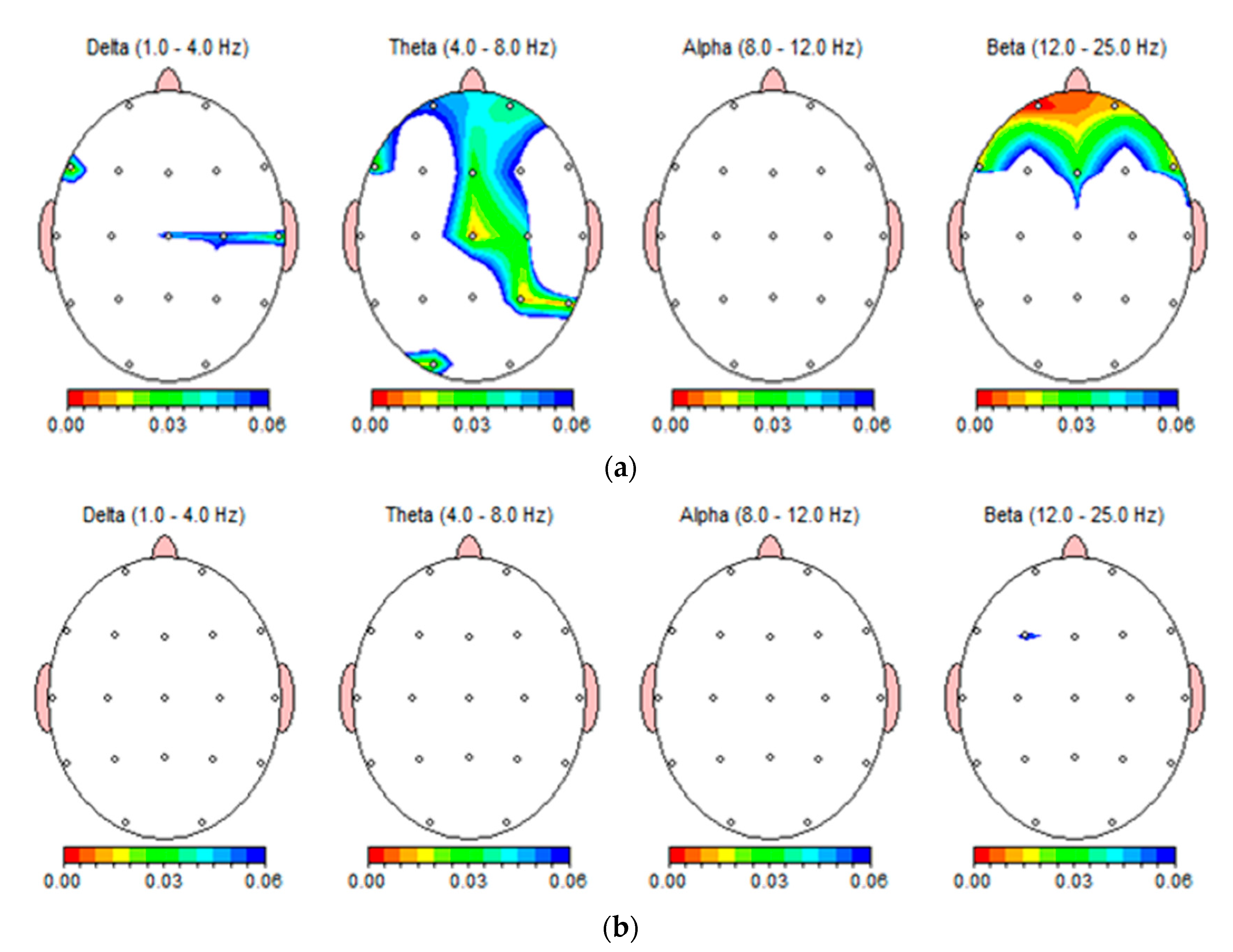
3.5. Topographic Analysis of the Whole Cortex
4. Discussion
5. Limitations and Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aung, T.; Kim, B.R.; Kim, M.J. Optimized Roasting Conditions of Germinated Wheat for a Novel Cereal Beverage and Its Sensory Properties. Foods 2022, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Kim, B.R.; Kim, M.J. Comparative Flavor Profile of Roasted Germinated Wheat (Triticum aestivum L.) Beverages Served Hot and Cold Using Electronic Sensors Combined with Chemometric Statistical Analysis. Foods 2022, 11, 3099. [Google Scholar] [CrossRef]
- Aung, T.; Kim, B.R.; Kim, S.; Shin, E.C.; Kim, M.J. Comparative volatiles, amino acids, and phenolic compounds and characteristics of roasted germinated wheat (Triticum aestivum L.) during beverage preparation. LWT 2023, 173, 114412. [Google Scholar] [CrossRef]
- Cho, S.; Moazzem, M.S. Recent applications of potentiometric electronic tongue and electronic nose in sensory evaluation. Prev. Nutr. Food Sci. 2022, 27, 354. [Google Scholar] [CrossRef] [PubMed]
- Songsamoe, S.; Saengwong-ngam, R.; Koomhin, P.; Matan, N. Understanding consumer physiological and emotional responses to food products using electroencephalography (EEG). Trends Food Sci. Technol. 2019, 93, 167–173. [Google Scholar] [CrossRef]
- Ahmad, R.F.; Malik, A.S.; Kamel, N.; Reza, F. EEG-fMRI combination for better understanding of brain functions: Pros and cons. In Proceedings of the 2015 IEEE International Conference on Signal and Image Processing Applications (ICSIPA), Kuala Lumpur, Malaysia, 19–21 October 2015; pp. 278–281. [Google Scholar]
- Sowndhararajan, K.; Kim, S. Influence of fragrances on human psychophysiological activity: With special reference to human electroencephalographic response. Sci. Pharm. 2016, 84, 724–752. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, M. A review of electroencephalogram signal processing methods for brain-controlled robots. Cogn. Robot. 2021, 1, 111–124. [Google Scholar] [CrossRef]
- Malik, A.S.; Khairuddin, R.N.H.R.; Amin, H.U.; Smith, M.L.; Kamel, N.; Abdullah, J.M.; Fawzy, S.M.; Shim, S. EEG based evaluation of stereoscopic 3D displays for viewer discomfort. Biomed. Eng. Online 2015, 14, 1–21. [Google Scholar] [CrossRef]
- Minnerly, C.; Shokry, I.M.; To, W.; Callanan, J.J.; Tao, R. Characteristic changes in EEG spectral powers of patients with opioid-use disorder as compared with those with methamphetamine-and alcohol-use disorders. PLoS ONE 2021, 16, e0248794. [Google Scholar] [CrossRef]
- Walsh, A.M.; Duncan, S.E.; Bell, M.A.; O’Keefe, S.F.; Gallagher, D.L. Breakfast meals and emotions: Implicit and explicit assessment of the visual experience. J. Sens. Stud. 2017, 32, e12265. [Google Scholar] [CrossRef]
- Pérez-Elvira, R.; Oltra-Cucarella, J.; Carrobles, J.A.; Teodoru, M.; Bacila, C.; Neamtu, B. Individual alpha peak frequency, an important biomarker for live z-score training neurofeedback in adolescents with learning disabilities. Brain Sci. 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Lagopoulos, J.; Xu, J.; Rasmussen, I.; Vik, A.; Malhi, G.S.; Eliassen, C.F.; Arntsen, I.E.; Sæther, J.G.; Hollup, S.; Holen, A. Increased theta and alpha EEG activity during nondirective meditation. J. Altern. Complement. Med. 2009, 15, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Dimpfel, W.; Kler, A.; Kriesl, E.; Lehnfeld, R.; Keplinger-Dimpfel, I. Neurophysiological characterization of a functionally active drink containing extracts of ginkgo and ginseng by source density analysis of the human EEG. Nutr. Neurosci. 2006, 9, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Ajjimaporn, A.; Noppongsakit, P.; Ramyarangsi, P.; Siripornpanich, V.; Chaunchaiyakul, R. A low-dose of caffeine suppresses EEG alpha power and improves working memory in healthy University males. Physiol. Behav. 2022, 256, 113955. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Staudigl, T.; Fellner, M.-C. Oscillatory power decreases and long-term memory: The information via desynchronization hypothesis. Front. Hum. Neurosci. 2012, 6, 74. [Google Scholar] [CrossRef]
- Scholey, A.; Downey, L.A.; Ciorciari, J.; Pipingas, A.; Nolidin, K.; Finn, M.; Wines, M.; Catchlove, S.; Terrens, A.; Barlow, E. Acute neurocognitive effects of epigallocatechin gallate (EGCG). Appetite 2012, 58, 767–770. [Google Scholar] [CrossRef]
- Takahashi, T.; Murata, T.; Hamada, T.; Omori, M.; Kosaka, H.; Kikuchi, M.; Yoshida, H.; Wada, Y. Changes in EEG and autonomic nervous activity during meditation and their association with personality traits. Int. J. Psychophysiol. 2005, 55, 199–207. [Google Scholar] [CrossRef]
- Meng, J.; Mundahl, J.H.; Streitz, T.D.; Maile, K.; Gulachek, N.S.; He, J.; He, B. Effects of soft drinks on resting state EEG and brain–computer interface performance. IEEE Access 2017, 5, 18756–18764. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, X.; Blank, I.; Liu, Y. Investigating the influence of monosodium L-glutamate on brain responses via scalp-electroencephalogram (scalp-EEG). Food Sci. Hum. Wellness 2022, 11, 1233–1239. [Google Scholar] [CrossRef]
- Klemm, W.; Lutes, S.; Hendrix, D.; Warrenburg, S. Topographical EEG maps of human responses to odors. Chem. Senses 1992, 17, 347–361. [Google Scholar] [CrossRef]
- Martin, G.N. Human electroencephalographic (EEG) response to olfactory stimulation: Two experiments using the aroma of food. Int. J. Psychophysiol. 1998, 30, 287–302. [Google Scholar] [CrossRef] [PubMed]
- TS, L.I. Alteration of human EEG by odor administration. Psychobiology 1988, 16, 281–284. [Google Scholar]
- Cho, H.; Sowndhararajan, K.; Jung, J.-W.; Jhoo, J.-W.; Kim, S. Fragrant chemicals in the supercritical carbon dioxide extract of Magnolia kobus DC. Flower buds increase the concentration state of brain function. J. Essent. Oil Bear. Plants 2015, 18, 1059–1069. [Google Scholar] [CrossRef]
- Watson, A.; Okello, E.; Brooker, H.; Lester, S.; McDougall, G.; Wesnes, K. The impact of blackcurrant juice on attention, mood and brain wave spectral activity in young healthy volunteers. Nutr. Neurosci. 2019, 22, 596–606. [Google Scholar] [CrossRef]
- Hoffmann, E.; Keppel Hesselink, J.M.; da Silveira Barbosa, Y.-W. Effects of a psychedelic, tropical tea, ayahuasca, on the electroencephalographic (EEG) activity of the human brain during a shamanistic ritual. MAPS Bull. 2001, 11, 25–30. [Google Scholar]
- Boyle, N.B.; Billington, J.; Lawton, C.; Quadt, F.; Dye, L. A combination of green tea, rhodiola, magnesium and B vitamins modulates brain activity and protects against the effects of induced social stress in healthy volunteers. Nutr. Neurosci. 2022, 25, 1845–1859. [Google Scholar] [CrossRef]
- Okello, E.J.; Abadi, A.M.; Abadi, S.A. Effects of green and black tea consumption on brain wave activities in healthy volunteers as measured by a simplified electroencephalogram (EEG): A feasibility study. Nutr. Neurosci. 2016, 19, 196–205. [Google Scholar] [CrossRef]
- Labbe, D.; Martin, N.; Le Coutre, J.; Hudry, J. Impact of refreshing perception on mood, cognitive performance and brain oscillations: An exploratory study. Food Qual. Prefer. 2011, 22, 92–100. [Google Scholar] [CrossRef]
- Yoto, A.; Murao, S.; Motoki, M.; Yokoyama, Y.; Horie, N.; Takeshima, K.; Masuda, K.; Kim, M.; Yokogoshi, H. Oral intake of γ-aminobutyric acid affects mood and activities of central nervous system during stressed condition induced by mental tasks. Amino Acids 2012, 43, 1331–1337. [Google Scholar] [CrossRef]
- Murthy, S.V.; Fathima, S.N.; Mote, R. Hydroalcoholic extract of ashwagandha improves sleep by modulating GABA/histamine receptors and EEG slow-wave pattern in in vitro-in vivo experimental models. Prev. Nutr. Food Sci. 2022, 27, 108. [Google Scholar] [CrossRef]
- Mirshekari Jahangiri, H.; Sarkaki, A.; Farbood, Y.; Dianat, M.; Goudarzi, G. Gallic acid affects blood-brain barrier permeability, behaviors, hippocampus local EEG, and brain oxidative stress in ischemic rats exposed to dusty particulate matter. Environ. Sci. Pollut. Res. 2020, 27, 5281–5292. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, M.; Kirch, W. Effects of caffeine on topographic quantitative EEG. Neuropsychobiology 2002, 45, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhao, M.; Liu, J.; Zheng, C. Electroencephalogram and electrocardiograph assessment of mental fatigue in a driving simulator. Accid. Anal. Prev. 2012, 45, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Doppelmayr, M.; Schwaiger, J.; Winkler, T.; Gruber, W. Theta oscillations and the ERP old/new effect: Independent phenomena? Clin. Neurophysiol. 2000, 111, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Scarpelli, S.; Marzano, C.; D’Atri, A.; Gorgoni, M.; Ferrara, M.; De Gennaro, L. State-or trait-like individual differences in dream recall: Preliminary findings from a within-subjects study of multiple nap REM sleep awakenings. Front. Psychol. 2015, 6, 928. [Google Scholar] [CrossRef][Green Version]
- Ueno, K.; Ishii, R.; Ueda, M.; Yuri, T.; Hata, M.; Naito, Y. Frontal midline theta rhythm and gamma activity measured by sheet-type wearable EEG device. Front. Hum. Neurosci. 2023, 17, 1145282. [Google Scholar] [CrossRef]
- Wischnewski, M.; Joergensen, M.L.; Compen, B.; Schutter, D.J. Frontal beta transcranial alternating current stimulation improves reversal learning. Cereb. Cortex 2020, 30, 3286–3295. [Google Scholar] [CrossRef]
- Marrone, G.; Serra, A.; Miele, L.; Biolato, M.; Liguori, A.; Grieco, A.; Gasbarrini, A. Branched chain amino acids in hepatic encephalopathy and sarcopenia in liver cirrhosis: Evidence and uncertainties. World J. Gastroenterol. 2023, 29, 2905. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Branched-chain amino acids and brain function. J. Nutr. 2005, 135, 1539S–1546S. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aung, T.; Kim, B.R.; Kwak, H.S.; Kim, M.J. Neuroimaging Approach: Effects of Hot and Cold Germinated Wheat Beverages on Electroencephalographic (EEG) Activity of the Human Brain. Foods 2023, 12, 3493. https://doi.org/10.3390/foods12183493
Aung T, Kim BR, Kwak HS, Kim MJ. Neuroimaging Approach: Effects of Hot and Cold Germinated Wheat Beverages on Electroencephalographic (EEG) Activity of the Human Brain. Foods. 2023; 12(18):3493. https://doi.org/10.3390/foods12183493
Chicago/Turabian StyleAung, Thinzar, Bo Ram Kim, Han Sub Kwak, and Mi Jeong Kim. 2023. "Neuroimaging Approach: Effects of Hot and Cold Germinated Wheat Beverages on Electroencephalographic (EEG) Activity of the Human Brain" Foods 12, no. 18: 3493. https://doi.org/10.3390/foods12183493
APA StyleAung, T., Kim, B. R., Kwak, H. S., & Kim, M. J. (2023). Neuroimaging Approach: Effects of Hot and Cold Germinated Wheat Beverages on Electroencephalographic (EEG) Activity of the Human Brain. Foods, 12(18), 3493. https://doi.org/10.3390/foods12183493





