Assessment of the Fruit Chemical Characteristics and Antioxidant Activity of Different Mulberry Cultivars (Morus spp.) in Semi-Arid, Sandy Regions of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Mulberry Sample Preparation
2.3. Chemicals and Reagents
2.4. Mulberry Conventional Quality Assay
2.5. Phytochemical Extraction of Mulberry
2.6. Total Anthocyanins Contents Assay (TAC)
2.7. Total Phenolics Contents Assay (TPC)
2.8. Total Flavonoid Contents Assay (TFC)
2.9. HPLC-DAD
2.9.1. Analysis of Flavonoid and Anthocyanin
2.9.2. Analysis of Phenolics
2.10. Antioxidant Activity Assay
2.11. HPLC Condition Used for Sugar Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Indicators of Four Mulberry Genotypes
3.1.1. Mulberry Conventional Quality
3.1.2. Phenolics and Their Metabolites Mulberries
3.2. Antioxidant Activity of Four Mulberry Genetypes
3.3. TAC, TPC, and TFC of Four Mulberry Genetypes
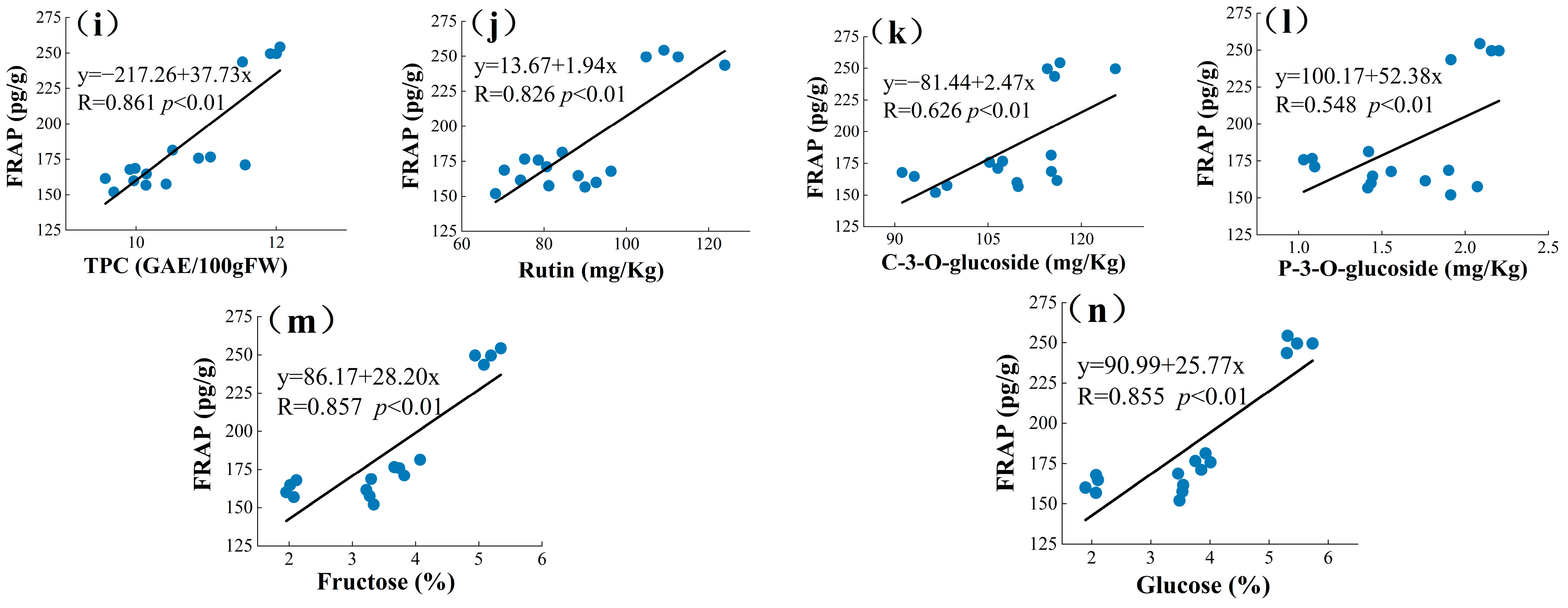
3.4. Principal Component Analysis (PCA) and Pearson’s Correlation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rohela, G.K.; Muttanna, P.S.; Kumar, R.; Chowdhury, S.R. Mulberry (Morus spp.): An ideal plant for sustainable development. Trees For. People 2020, 2, 100011. [Google Scholar] [CrossRef]
- Sarkar, T.; Mogili, T.; Sivaprasad, V. Improvement of abiotic stress adaptive traits in Mulberry (Morus spp.): An update on biotechnological interventions. 3 Biotech 2017, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Pel, P.; Chae, H.S.; Nhoek, P.; Kim, Y.M.; Chin, Y.W. Chemical constituents with proprotein convertase subtilisin/kexin type 9 mRNA expression inhibitory activity from dried immature Morus alba fruits. J. Agric. Food Chem. 2017, 65, 5316–5321. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, H.-J.; Bucheli, P.; Zhang, P.F.; Wei, D.Z.; Lu, Y.H. Phytochemical Profiles of Different Mulberry (Morus spp.) Species from China. J. Agric. Food Chem. 2009, 57, 9133–9140. [Google Scholar] [CrossRef]
- Sun, Z.; Yin, Y.; Zhu, W.; Zhou, Y. Morphological, Physiological, and Biochemical Composition of Mulberry (Morus spp.) under Drought Stress. Forests 2023, 14, 949. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.G.; Linhardt, R.J.; Liao, S.T.; Wu, H.; Zou, Y.X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Dhiman, S.; Kumar, V.; Mehta, C.M.; Gat, Y.; Kaur, S. Bioactive compounds, health benefits and utilization of Morus spp.–A comprehensive review. J. Hortic. Sci. Biotechnol. 2020, 95, 8–18. [Google Scholar] [CrossRef]
- Yang, X.; Yang, L.; Zheng, H. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem. Toxicol. 2010, 48, 2374–2379. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.F.; Lou, X.; Li, X. Effects of mulberry fruit (Morus alba L.) consumption on health outcomes: A mini-review. Antioxidant 2018, 7, 69. [Google Scholar] [CrossRef]
- Mnaa, S.; Aniess, W.; Olwy, Y.; Shaker, E. Antioxidant activity of white (Morus alba L.) and black (Morus nigra L.) berries against CC14 hepatotoxic agent. Adv. Tech. Biol Med. 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.Y.; Ganie, N.A.; Wani, D.M.; Wani, A.W.; Dar, S.Q.; Khan, A.H.; A Khan, N.; Manzar, M.S.; Dehghani, M.H. The phenolic components extracted from mulberry fruits as bioactive compounds against cancer: A review. Phytother. Res. 2023, 37, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lay, H.L. Characteristics of fruit growth, component analysis and antioxidant activity of mulberry (Morus spp.). Sci. Hortic. 2013, 162, 285–292. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, W.; Zhang, A.; Mu, H.; Bai, H.; Duan, J.; Wu, C. Variation in antioxidant activities of polysaccharides from Fructus Jujubae in South Xinjiang area. Int. J. Biol. Macromol. 2013, 57, 278–284. [Google Scholar] [CrossRef]
- Khan, M.S.; Chen, C.; Fu, X. The effect of geographic variation on chemical composition, antioxidant and hypoglycemic activities of Morus alba L. polysaccharides. J. Food Process. Preserv. 2019, 43, e14206. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, S.; Zhou, M.; Shang, X.; Yang, W.; Fu, X. Geographic variation in water-soluble polysaccharide content and antioxidant activities of Cyclocarya paliurus leaves. Ind. Crops Prod. 2018, 121, 180–186. [Google Scholar] [CrossRef]
- Gundogdu, M.; Muradoglu, F.; Sensoy, R.I.G.; Yilmaz, H. Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Sci. Hortic. 2011, 132, 37–41. [Google Scholar] [CrossRef]
- Mahmood, T.; Anwar, F.; Abbas, M.; Boyce, M.C.; Saari, N. Compositional variation in sugars and organic acids at different maturity stages in selected small fruits from Pakistan. Int. J. Mol. Sci. 2012, 13, 1380–1392. [Google Scholar] [CrossRef]
- Sun, R.; Sun, L.; Han, C. Partial-least-squares and canonical-correlation analysis of chemical constituents and active ingredients of new types of Chinese mulberries. Food Sci. Nutr. 2018, 6, 1950–1959. [Google Scholar] [CrossRef]
- Okatan, V. Phenolic compounds and phytochemicals in fruits of black mulberry (Morus nigra L.) genetypes from the Aegean region in Turkey. Folia Hortic. 2018, 30, 93–101. [Google Scholar] [CrossRef]
- Paunovi, S.M.; Maskovic, P.; Milinkovi, M. Determination of primary metabolites, vitamins and minerals in black mulberry (Morus nigra) berries depending on altitude. Erwerbs-Obstbau 2020, 62, 355–360. [Google Scholar] [CrossRef]
- Aramwit, P.; Bang, N.; Srichana, T. The properties and stability of anthocyanins in mulberry fruits. Food Res. Int. 2010, 43, 1093–1097. [Google Scholar] [CrossRef]
- Shih, P.H.; Chan, Y.C.; Liao, J.W.; Wang, M.F.; Yen, G.C. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J. Nutr. Biochem. 2010, 21, 598–605. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Ding, S.D.; Ding, X.L. Comparison of antioxidant capacities of extracts from five cultivars of Chinese jujube. Process Biochem. 2005, 40, 3607–3613. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Bao, T.; Gowd, V. Mulberry fruit extract affords protection against ethyl carbamate-induced cytotoxicity and oxidative stress. Oxid. Med. Cell. Longev. 2017, 2017, 1594963. [Google Scholar] [CrossRef]
- Gao, Y.; Qiu, G.Y.; Shimizu, H.; Tobe, K.; Sun, B.; Wang, J. A 10-year study on techniques for vegetation restoration in a desertified Salt Lake area. J. Arid Environ. 2002, 524, 483–497. [Google Scholar] [CrossRef]
- Nunezmir, G.C.; Iannone, B.V.I.; Curtis, K.; Fei, S.L.; Oliet, J.A. Evaluating the evolution of forest restoration research in a changing world: A big literature review. New Forest 2015, 46, 669–682. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, Y.; Yin, Y.; Zhu, X.; Zhu, W.; Zhou, Y. Comparison of soil bacterial community and functional characteristics following afforestation in the semi-arid areas. Peer J. 2019, 7, e7141. [Google Scholar] [CrossRef]
- China Weather Network. Available online: http://www.weather.com.cn/radar/ (accessed on 1 December 2021.).
- Mars, M.; Marrakchi, M. Diversity of pomegranate (Punica granatum L.) germplasm in Tunisia. Genet. Resour. Crop Evol. 1999, 46, 461–467. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990; pp. 220–240. [Google Scholar]
- Lin, Y.; Wang, Y.H.; Li, B.; Tan, H.; Li, D.N.; Li, L.; Liu, X.; Han, J.C.; Meng, X.J. Comparative transcriptome analysis of genes involved in anthocyanin synthesis in blueberry. Plant Physiol. Biochem Hem. 2018, 127, 561–572. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299C, pp. 152–178. [Google Scholar] [CrossRef]
- Xie, Y.H.; Zheng, Y.X.; Dai, X.L.; Wang, Q.X.; Cao, J.G.; Xiao, J.B. Seasonal dynamics of total flavonoid contents and antioxidant activity of Dryopteris erythrosora. Food Chem. 2015, 186, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Nayab, S.; Razzaq, K.; Ullah, S.; Rajwana, I.A.; Naz, A. Genetypes and harvest maturity influence the nutritional fruit quality of mulberry. Sci. Hortic. 2020, 266, 109311. [Google Scholar] [CrossRef]
- Shulman, Y.; Fainberstein, L.; Lavee, S. Pomegranate fruit development and maturation. J. Hortic. Sci. 1984, 59, 265–274. [Google Scholar] [CrossRef]
- Zaouay, F.; Mena, P.; Cristina, G.V.; Mars, M. Antioxidant activity and physico-chemical properties of Tunisian grown pomegranate (Punica granatum L.) cultivars. Ind. Crops Prod. 2012, 40, 81–89. [Google Scholar] [CrossRef]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Ahmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef]
- Rao, A.V.; Snyder, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Gao, X.; Hou, Q.; Ma, Z. Differences of sugar components in different mulberry cultivars during its ripening. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 446, p. 032058. [Google Scholar] [CrossRef]
- Eyduran, S.P.; Ercisli, S.; Akin, M.; Beyhan, O.; Geçer, M.K.; Eyduran, E.; Erturk, Y.E. Organic acids, sugars, vitamin C, antioxidant capacity, and phenolic compounds in fruits of white (Morus alba L.) and black (Morus nigra L.) mulberry genetypes. J. Appl. Bot. Food Qual. 2015, 88, 134–138. [Google Scholar] [CrossRef]
- Gundogdu, M.; Canan, I.; Gecer, M.K.; Kan, T.; Ercisli, S. Phenolic compounds, bioactive content and antioxidant capacity of the fruits of mulberry (Morus spp.) germplasm in Turkey. Folia Hortic. 2017, 29, 251–262. [Google Scholar] [CrossRef]
- Pch, H. Evidence for health benefits of plant phenols: Local or systemic effects? J. Sci. Food Agric. 2001, 81, 842–852. [Google Scholar] [CrossRef]
- You, Q.; Chen, F.; Wang, X.; Jiang, Y.; Lin, S. Anti-diabetic activities of phenolic compounds in muscadine against alpha-glucosidase and pancreatic lipase. LWT–Food Sci. Technol. 2012, 46, 164–168. [Google Scholar] [CrossRef]
- Suh, H.J.; Noh, D.O.; Kang, C.S.; Kim, J.M.; Lee, S.W. Thermal kinetics of color degradation of mulberry fruit extract. Mol. Nutr. Food Res. 2003, 47, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Nie, W.J. Nutritional composition and in vitro antioxidant capacity of black mulberry (Morus nigra L.) fruits from Xinjiang province. Food Sci. 2014, 35, 126–129. [Google Scholar] [CrossRef]
- Duarte, R.; Carvalho, A.; Gadelha, D.; Braga, V. Rutin reduces oxidative stress in animals with renovascular hypertension. BMC Proc. 2014, 8 (Suppl. S4), P65. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Liu, R.; Xue, B.; Luo, J.; Gao, L.; Wang, Y.; Ou, S.; Li, S.; Peng, X. Impact and consequences of polyphenols and fructooligosaccharide interplay on gut microbiota in rats. Food Funct. 2017, 8, 1925–1932. [Google Scholar] [CrossRef]
- Huang, J.; Lin, X.; Xue, B.; Luo, J.; Gao, L.; Wang, Y.; Ou, S.; Peng, X. Impact of polyphenols combined with high-fat diet on rats’ gut microbiota. J. Funct. Foods 2016, 26, 763–771. [Google Scholar] [CrossRef]
- Lin, S.; Hu, J.; Zhou, X.; Cheung, P.C.K. Inhibition of vascular endothelial growth factor-induced angiogenesis by chlorogenic acid via targeting the vascular endothelial growth factor receptor 2-mediated signaling pathway. J. Funct. Foods 2017, 32, 285–295. [Google Scholar] [CrossRef]
- Wang, Z.; Lam, K.-L.; Hu, J.; Ge, S.; Zhou, A.; Zhou, A.; Zheng, B.; Zeng, S.; Lin, S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019, 7, 579–588. [Google Scholar] [CrossRef]
- Polumackanycz, M.; Sledzinski, T.; Goyke, E.; Wesolowski, M.; Viapiana, A. A comparative study on the phenolic composition and biological activities of Morus alba L. commercial samples. Molecules 2019, 24, 3082–3100. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Yi, W.; Hao, J.Y.; Hu, R.Z.; Cong, C.; Yao, X.H.; Zhao, W.G.; Liu, Z.Y.; Long, L. Evaluation of the alkaloid, polyphenols, and antioxidant contents of various mulberry cultivars from different planting areas in eastern China. Ind. Crops Prod. 2018, 122, 298–307. [Google Scholar] [CrossRef]
- Farahani, M.; Salehi-Arjmand, H.; Khadivi, A.; Akramian, M. Chemical characterization and antioxidant activities of Morus alba var. nigra fruits. Sci. Hortic. 2019, 253, 120–127. [Google Scholar] [CrossRef]
- Gecer, M.K.; Akin, M.; Gundogdu, M.; Eyduran, S.P.; Ercisli, S.; Eyduran, E. Organic acids, sugars, phenolic compounds, and some horticultural characteristics of black and white mulberry accessions from Eastern Anatolia. Can. J. Plant Sci. 2016, 96, 27–33. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT-Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Lee, Y.; Hwang, K.T. Changes in physicochemical properties of mulberry fruits (Morus alba L.) during ripening. Sci. Hortic. 2017, 217, 189–196. [Google Scholar] [CrossRef]
- Bao, T.; Xu, Y.; Gowd, V.; Zhao, J.; Xie, J.; Liang, W.; Chen, W. Systematic study on phytochemicals and antioxidant activity of some new and common mulberry cultivars in China. J. Funct. Food. 2016, 25, 537–547. [Google Scholar] [CrossRef]
- Okatan, V. Antioxidant properties and phenolic profile of the most widely appreciated cultivated berry species: A comparative study. Folia Hortic. 2020, 32, 79–85. [Google Scholar] [CrossRef]
- Yao, X.H.; Shen, Y.S.; Hu, R.Z.; Xu, M.; Chen, T. The antioxidant activity and composition of the seed oil of mulberry cultivars. Food Biosci. 2020, 37, 100709. [Google Scholar] [CrossRef]
- Mamipour, S.; Yahoo, M.; Jalalvandi, S. An empirical analysis of the relationship between the environment, economy, and society: Results of a PCA-VAR model for Iran. Ecol. Indic. 2019, 102, 760–769. [Google Scholar] [CrossRef]
- Patra, B.; Schluttenhofer, C.; Wu, Y.; Pattanaik, S.; Yuan, L. Transcriptional Regulation of Secondary Metabolite Biosynthesis in Plants. Biochim. Biophys. Acta-Gene Regul. Mech. 2013, 1829, 1236–1247. [Google Scholar] [CrossRef]
- Yu, Y.F.; Li, H.Y.; Zhang, B.; Wang, Y.W.; Shi, X.P.; Huang, J.Z.; Yang, J.Y.; Zhang, Y.F.; Deng, Z.Y. Nutritional and functional components of mulberry leaves from different varieties: Evaluation of their potential as food materials. Int. J. Food Prop. 2018, 21, 1495–1507. [Google Scholar] [CrossRef]



| Geno Types | Origin | Location | Yield (kg/hm2) | PH (cm) | DBH (cm) | BL (mm) | BWI (mm) | BWE (g) | MC (%) |
|---|---|---|---|---|---|---|---|---|---|
| Shen sang 1 | Huludao, LN | 120°51′ E, 40°45′ N | 71.70 | 235.67 ± 6.28 a | 12.50 ± 0.7 a | 23.81 ± 1.16 bc | 12.25 ± 0.49 a | 3.11 ± 0.01 a | 78.83 ± 0.1 c |
| A’er xiang | Fuxin, LN | 122°25′ E, 42°49′ N | 61.33 | 179.00 ± 7.3 c | 11.12 ± 1.08 ab | 27.71 ± 0.87 a | 12.72 ± 1.04 a | 3.14 ± 0.02 a | 84.59 ± 0.52 b |
| Fujia | Tieling, LN | 123°48′ E, 43°17′ N | 67.98 | 177.44 ± 7.16 c | 8.83 ± 1.06 b | 22.23 ± 1.02 c | 12.93 ± 0.68 a | 2.62 ± 0.04 b | 87.02 ± 0.15 a |
| Ji’an | Tonghua, JL | 125°92′ E, 41°49′ N | 65.29 | 196.50 ± 6.56 b | 13.79 ± 1.32 a | 24.81 ± 0.85 b | 11.73 ± 0.82 ab | 3.06 ± 0.03 a | 83.49 ± 0.08 b |
| Genotypes | CPC (%) | Ash (%) | TSS (%) | Mineral Elements | Free Sugars | ||||
|---|---|---|---|---|---|---|---|---|---|
| K (mg/g) | Ca (mg/g) | Fe (mg/kg) | Zn (mg/kg) | Fru (%) | Glu (%) | ||||
| Shen sang 1 | 1.53 ± 0.07 b | 0.83 ± 0.03 b | 17 ± 0.19 a | 2.05 ± 0.09 b | 1.81 ± 0.07 b | 470.78 ± 13.67 a | 27.22 ± 0.62 a | 5.14 ± 0.09 a | 5.46 ± 0.1 a |
| A’er xiang | 1.7 ± 0.08 a | 0.83 ± 0.06 b | 13.21 ± 0.09 b | 1.97 ± 0.12 b | 1.98 ± 0.14 b | 378.01 ± 12.25 b | 27.61 ± 2.14 a | 3.83 ±0.05 b | 3.89 ± 0.05 b |
| Fujia | 1.77 ± 0 a | 0.77 ± 0.06 b | 8.99 ± 0.16 d | 1.94 ± 0.02 b | 1.39 ± 0.02 c | 347.2 ± 12.4 b | 28.63 ± 1.36 a | 2.04 ± 0.05 d | 2.04 ± 0.05 d |
| Ji’an | 1.24 ± 0.04 c | 1.12 ± 0.02 a | 12.11 ± 0.21 c | 2.35 ± 0.03 a | 2.27 ± 0.04 a | 467.32 ± 13.05 a | 27.57 ± 2.1 a | 3.28 ± 0.02 c | 3.51 ± 0.02 c |
| Genetypes | Rutin (mg/kg) | Quercetin (mg/kg) | Chlorogenic Acid (mg/kg) | C-3-O-Glucoside (mg/kg) | P-3-O-Glucoside (mg/kg) |
|---|---|---|---|---|---|
| Shensang 1 | 112.63 ± 3.09 a | 13.11 ± 1.06 b | 52.55 ± 0.42 b | 118.08 ± 2.5 a | 2.09 ± 0.06 a |
| A’erxiang | 79.75 ± 1.91 c | 15.55 ± 0.63 a | 54.67 ± 2.55 b | 108.58 ± 2.23 ab | 1.16 ± 0.09 a |
| Fujia | 91.82 ± 1.74 b | 9.73 ± 0.71 c | 52.99 ± 0.9 b | 100.96 ± 3.1 b | 1.46 ± 0.03 b |
| Ji’an | 73.51 ± 2.85 c | 4.04 ± 1.03 d | 103.78 ± 2.38 a | 106.56 ± 4.27 ab | 1.91 ± 0.06 a |
| Genetypes | DPPH (pg/g) | FRAP (pg/g) | TAC (mg/100gFW) | TFC (mg/100gFW) | TPC (GAE/100gFW) |
|---|---|---|---|---|---|
| Shensang 1 | 294.86 ± 3.89 a | 249.86 ± 1.88 a | 567.6 ± 3.43 a | 1.10 ± 0.05 a | 11.87 ± 0.12 a |
| A’erxiang | 289.19 ± 2.5 a | 177.02 ± 1.97 b | 441.02 ± 2.99 b | 0.66 ± 0.01 c | 11.01 ± 0.22 b |
| Fujia | 250.22 ± 4.44 b | 159.12 ± 2.14 c | 300.16 ± 2.47 d | 0.82 ± 0.02 b | 10.04 ± 0.06 c |
| Ji’an | 239.69 ± 3.42 b | 159.57 ± 2.07 c | 404.53 ± 3.9 c | 0.74 ± 0.05 b c | 9.92 ± 0.19 c |
| PC1 62.86% | PC2 17.33% | PC3 11.08% | |
|---|---|---|---|
| DPPH 1 | 0.559 | −0.730 | 0.260 |
| FRAP | 0.984 | −0.127 | 0.025 |
| Total soluble solids | 0.931 | 0.328 | −0.128 |
| Total anthocyanins contents | 0.931 | 0.328 | −0.128 |
| Total flavonoid contents | 0.771 | −0.290 | 0.433 |
| Total phenolics contents | 0.875 | −0.090 | −0.406 |
| Rutin | 0.763 | −0.547 | 0.148 |
| C-3-O-glucoside | 0.695 | 0.215 | 0.018 |
| P-3-O-glucoside | 0.559 | 0.200 | 0.735 |
| Chlorogenic acid | −0.345 | 0.767 | 0.520 |
| Fructose | 0.913 | 0.339 | −0.174 |
| Glucose | 0.914 | 0.366 | −0.118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Zhou, Y.; Zhu, W.; Yin, Y. Assessment of the Fruit Chemical Characteristics and Antioxidant Activity of Different Mulberry Cultivars (Morus spp.) in Semi-Arid, Sandy Regions of China. Foods 2023, 12, 3495. https://doi.org/10.3390/foods12183495
Sun Z, Zhou Y, Zhu W, Yin Y. Assessment of the Fruit Chemical Characteristics and Antioxidant Activity of Different Mulberry Cultivars (Morus spp.) in Semi-Arid, Sandy Regions of China. Foods. 2023; 12(18):3495. https://doi.org/10.3390/foods12183495
Chicago/Turabian StyleSun, Zhiyu, Yongbin Zhou, Wenxu Zhu, and You Yin. 2023. "Assessment of the Fruit Chemical Characteristics and Antioxidant Activity of Different Mulberry Cultivars (Morus spp.) in Semi-Arid, Sandy Regions of China" Foods 12, no. 18: 3495. https://doi.org/10.3390/foods12183495
APA StyleSun, Z., Zhou, Y., Zhu, W., & Yin, Y. (2023). Assessment of the Fruit Chemical Characteristics and Antioxidant Activity of Different Mulberry Cultivars (Morus spp.) in Semi-Arid, Sandy Regions of China. Foods, 12(18), 3495. https://doi.org/10.3390/foods12183495






