Investigating the Water Relations in Aqueous Extract Powders of Mango (Mangifera indica) Peel and Seed Waste for Their Use in Food Matrices as a Value-Added By-Product
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Chemical Reagents
2.3. Extraction Process
2.4. Sample Composition
2.5. Water Sorption Isotherms
2.6. Calorimetric Analyses
2.6.1. Thermogravimetric (TGA) Analysis
2.6.2. Differential Scanning Calorimetry (DSC) Analysis
Statistical Analysis
3. Results and Discussion
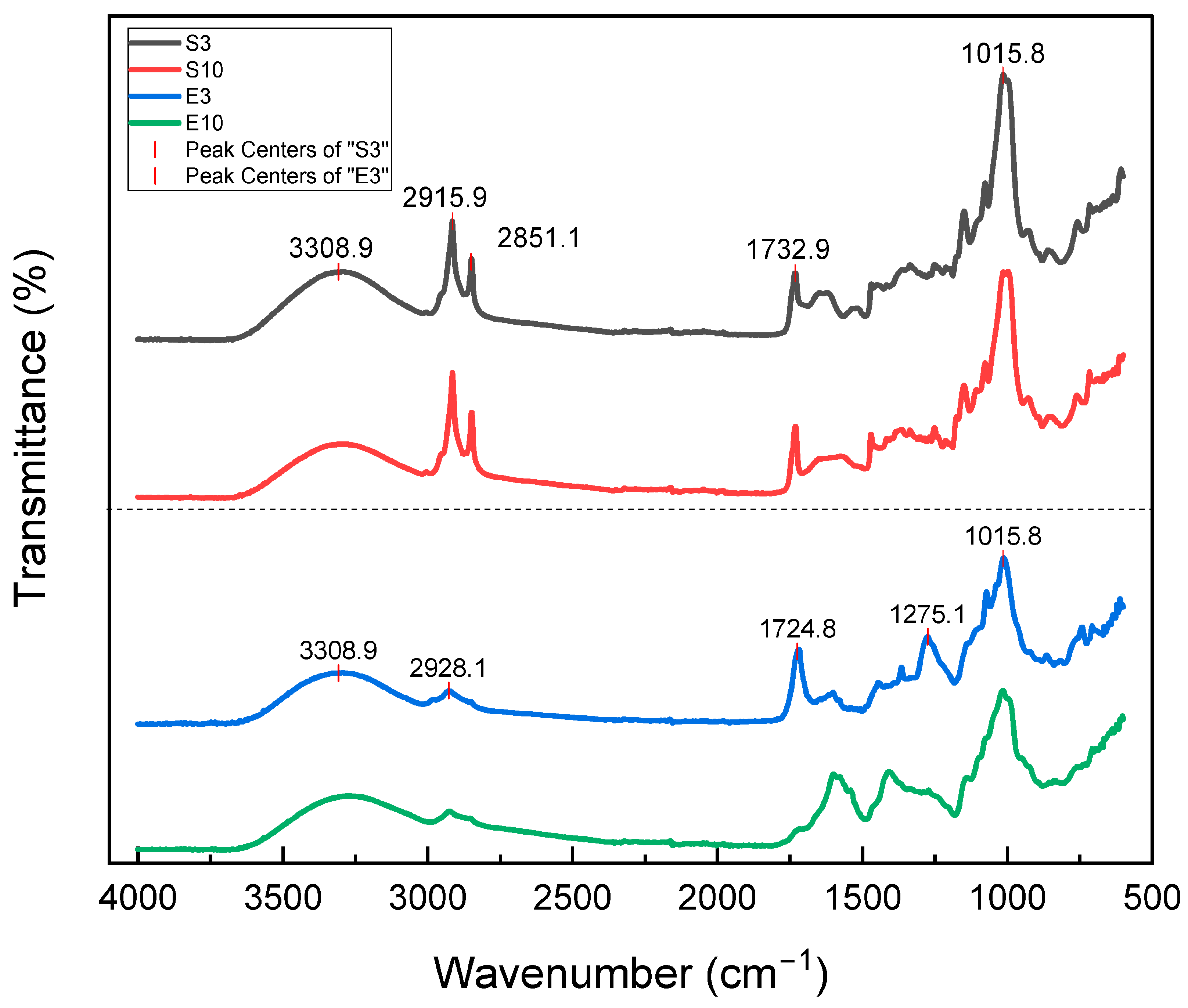
3.1. Sample Composition
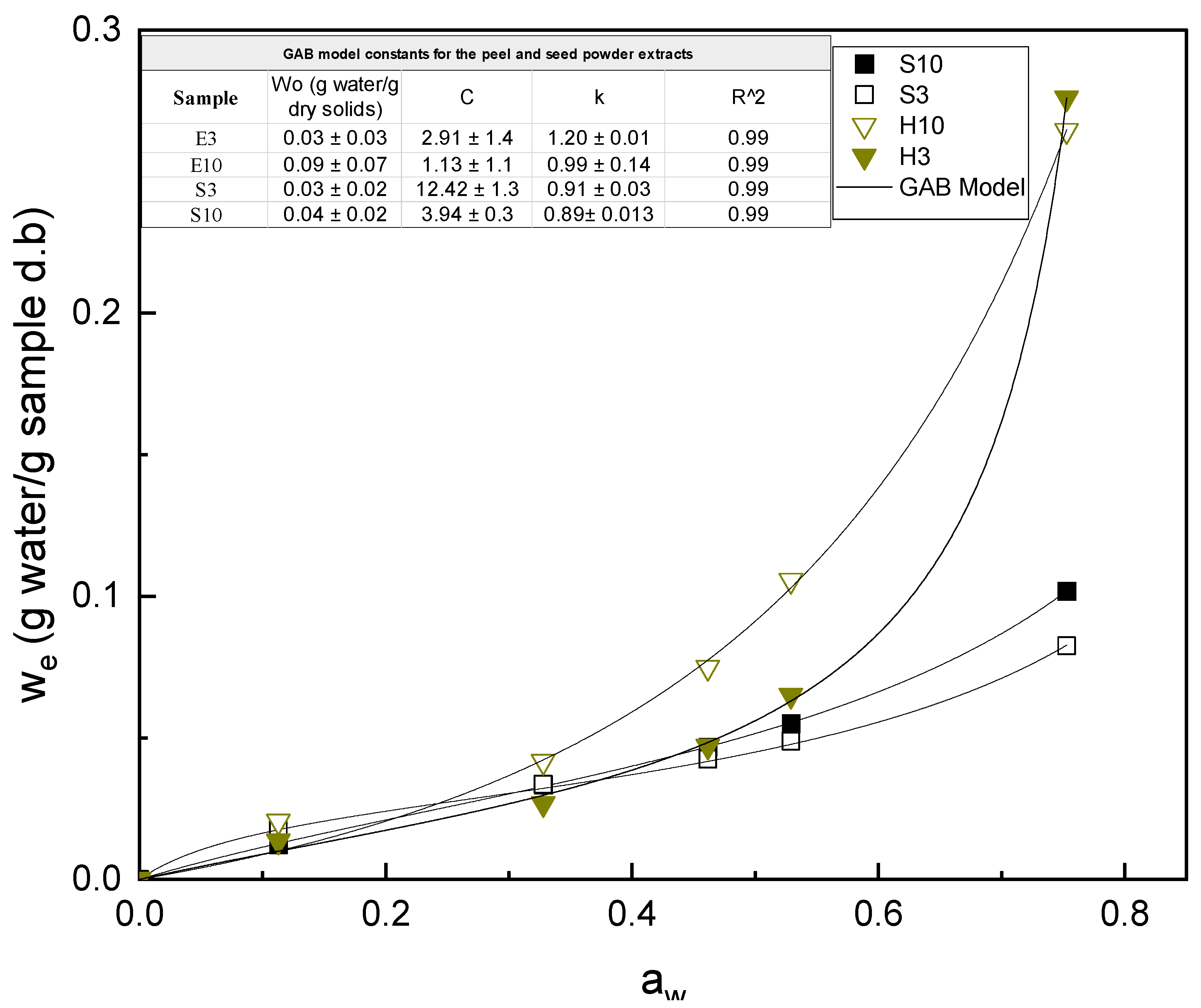
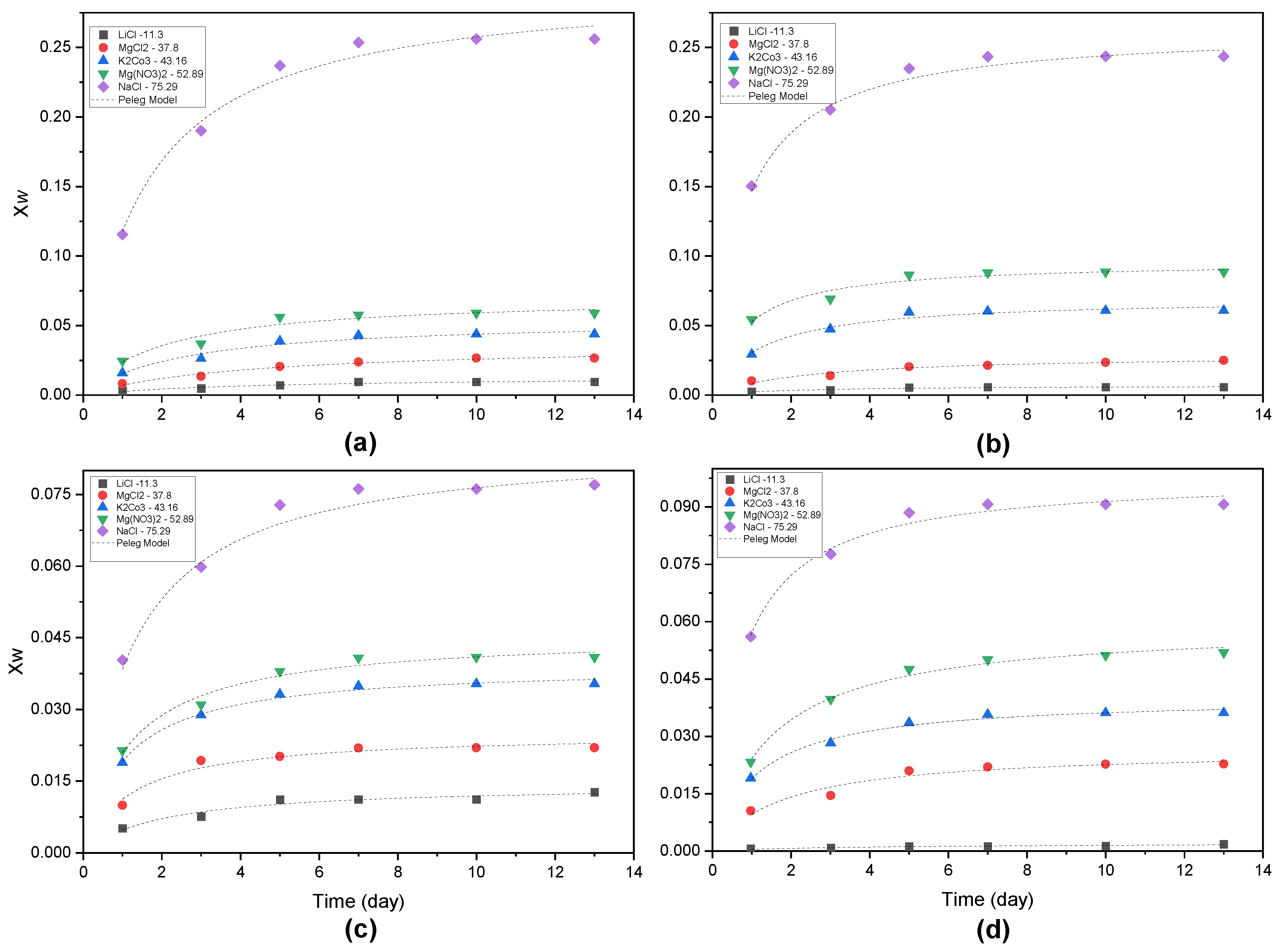
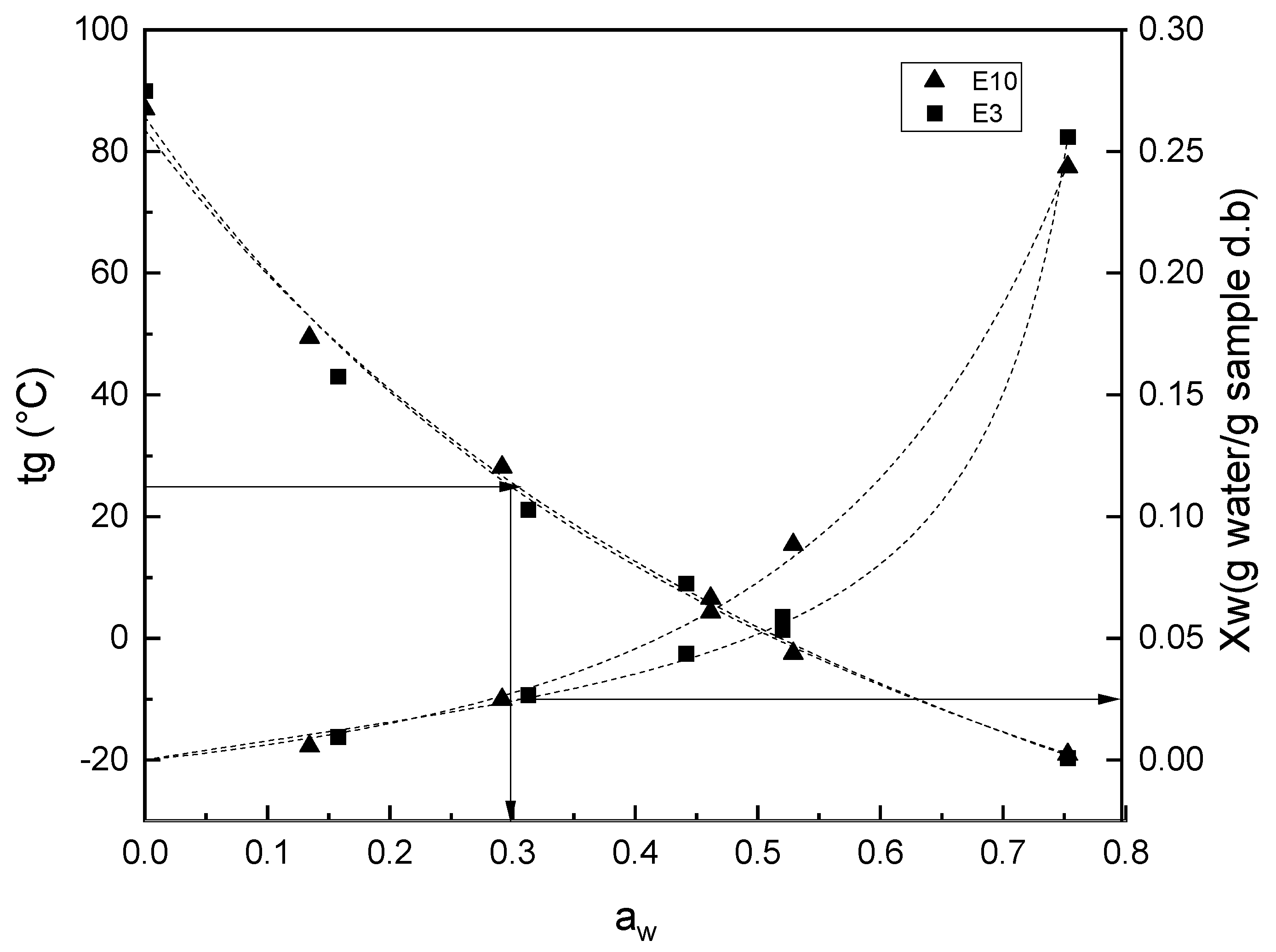
3.2. Water Sorption Isotherms
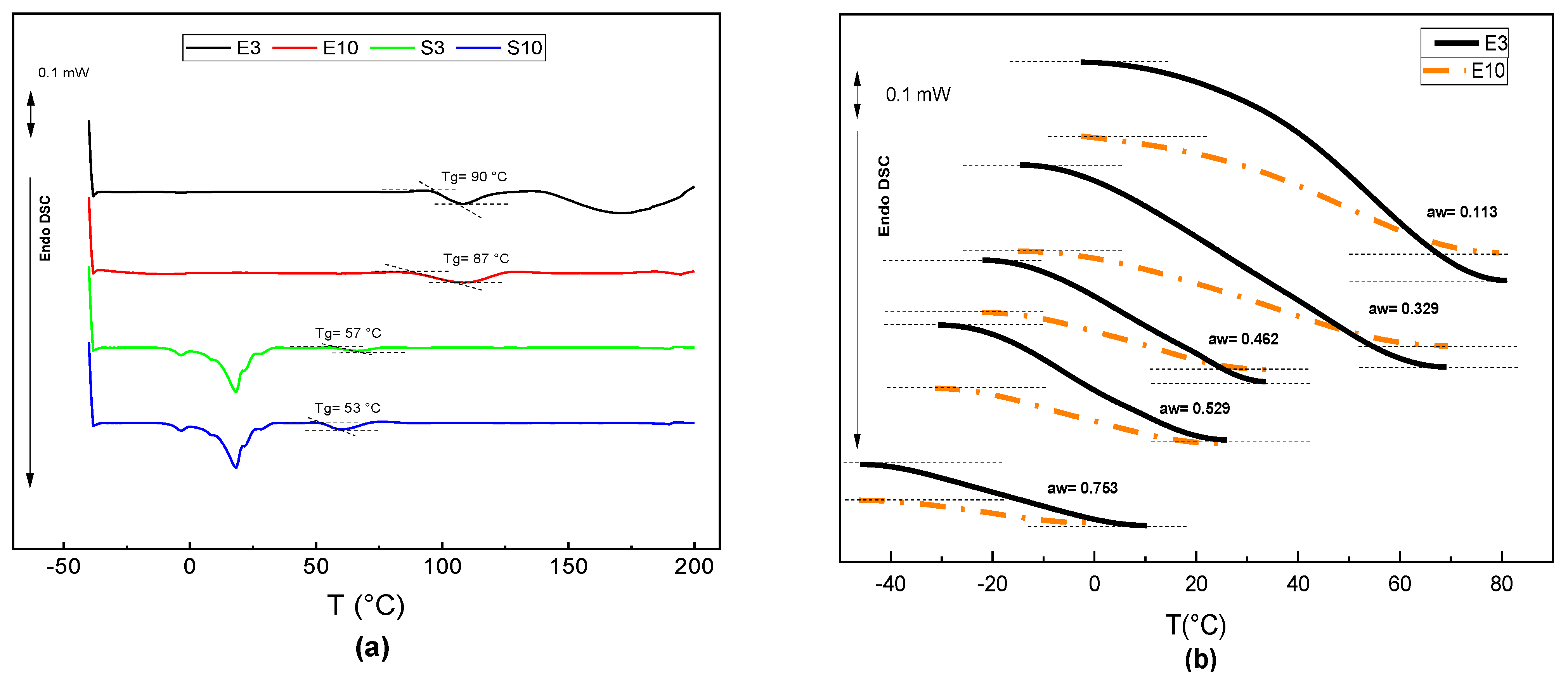
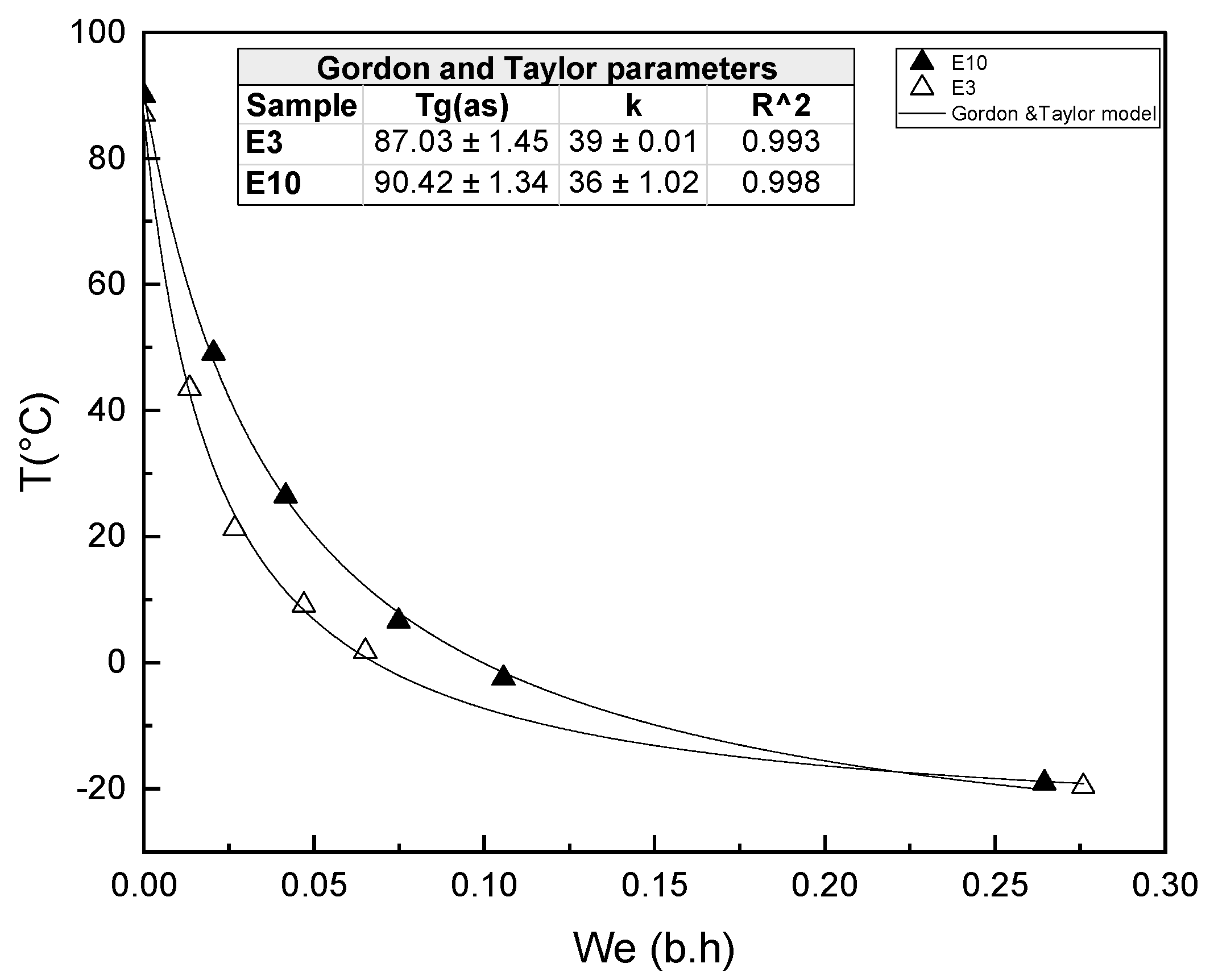
3.3. Phase Transitions
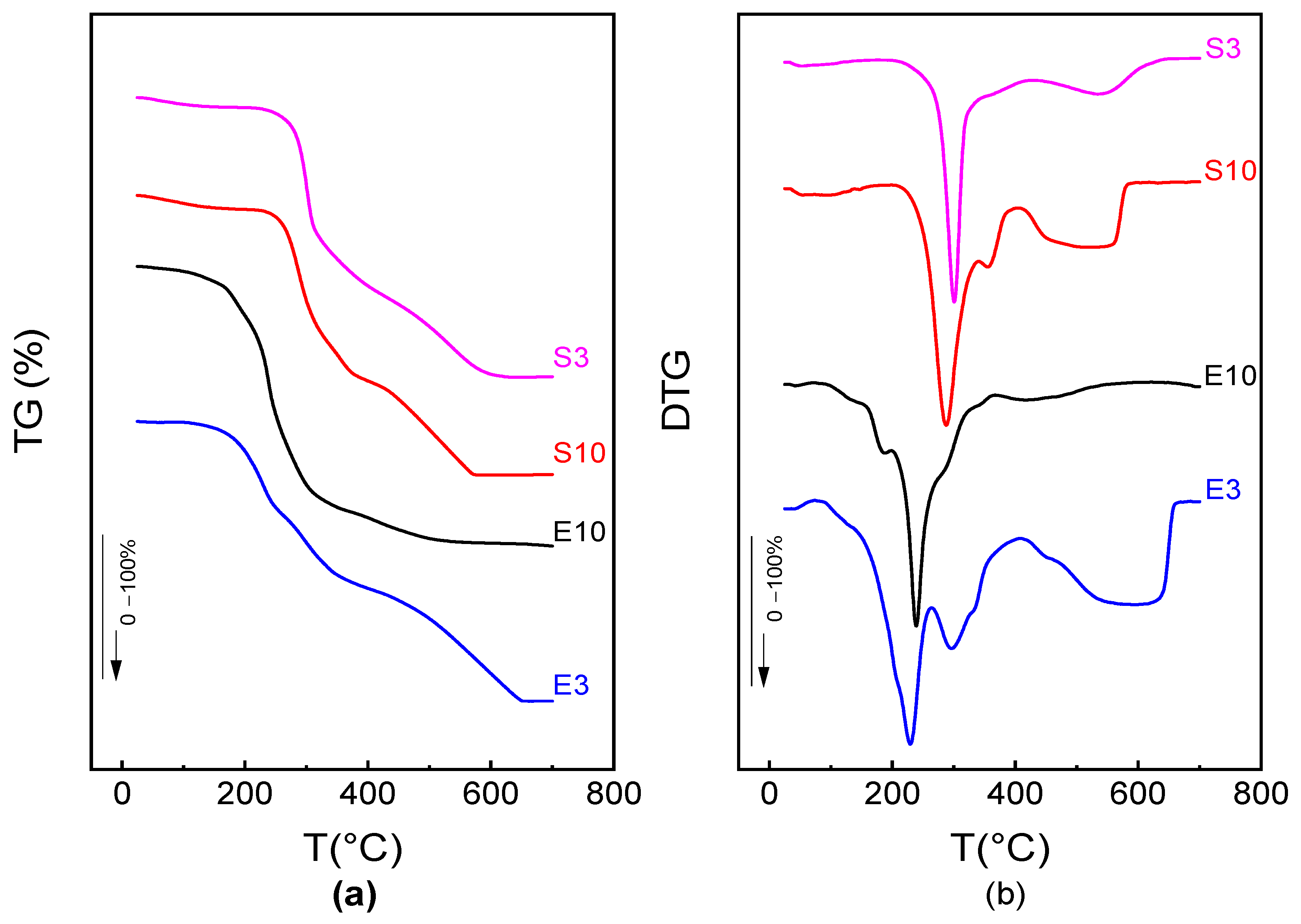
3.4. Thermal Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Otles, S.; Kartal, C. Food Waste Valorization. In Sustainable Food Systems from Agriculture to Industry; Academic Press: Cambridge, MA, USA, 2018; pp. 371–399. [Google Scholar] [CrossRef]
- Del Sánchez-Camargo, A.P.; Gutiérrez, L.F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.E. Valorisation of Mango Peel: Proximate Composition, Supercritical Fluid Extraction of Carotenoids, and Application as an Antioxidant Additive for an Edible Oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food By-Products and Food Wastes: Are They Safe Enough for Their Valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- SOFA. 2019. Available online: https://www.fao.org/state-of-food-agriculture/2019/es/ (accessed on 14 September 2023).
- Thiviya, P.; Gamage, A.; Kapilan, R.; Merah, O.; Madhujith, T. Production of Single-Cell Protein from Fruit Peel Wastes Using Palmyrah Toddy Yeast. Fermentation 2022, 8, 355. [Google Scholar] [CrossRef]
- Seviour, R.J.; Harvey, L.M.; Fazenda, M.; McNeil, B. Production of Foods and Food Components by Microbial Fermentation: An Introduction. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; Woodhead Publishing: Cambridge, UK, 2013; pp. 97–124. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid Waste Issue: Sources, Composition, Disposal, Recycling, and Valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of Apple Processing Wastes as Low-Cost Substrates for Bioproduction of High Value Products: A Review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Legesse, M.B.; Emire, S.A. Functional and Physicochemical Properties of Mango Seed Kernels and Wheat Flour and Their Blends for Biscuit Production. Afr. J. Food Sci. Technol. 2012, 3, 193–203. [Google Scholar]
- Siol, M.; Sadowska, A.; Król, K.; Najman, K. Bioactive and Physicochemical Properties of Exotic Fruit Seed Powders: Mango (Mangefiera indica L.) and Rambutan (Nephelium lappaceum L.) Obtained by Various Drying Methods. Appl. Sci. 2022, 12, 4995. [Google Scholar] [CrossRef]
- Lauricella, M.; Galbo, V.L.; Cernigliaro, C.; Maggio, A.; Piccionello, A.P.; Calvaruso, G.; Carlisi, D.; Emanuele, S.; Giuliano, M.; D’Anneo, A. The Anti-Cancer Effect of Mangifera indica L. Peel Extract Is Associated to ΓH2AX-Mediated Apoptosis in Colon Cancer Cells. Antioxidants 2019, 8, 422. [Google Scholar] [CrossRef]
- García-Rivera, D.; Delgado, R.; Bougarne, N.; Haegeman, G.; Vanden Berghe, W. Gallic Acid Indanone and Mangiferin Xanthone Are Strong Determinants of Immunosuppressive Anti-Tumour Effects of Mangifera indica L. Bark in MDA-MB231 Breast Cancer Cells. Cancer Lett. 2011, 305, 21–31. [Google Scholar] [CrossRef]
- Mohan, C.G.; Deepak, M.; Viswanatha, G.L.; Savinay, G.; Hanumantharaju, V.; Rajendra, C.E.; Halemani, P.D. Anti-Oxidant and Anti-Inflammatory Activity of Leaf Extracts and Fractions of Mangifera indica. Asian Pac. J. Trop. Med. 2013, 6, 311–314. [Google Scholar] [CrossRef]
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D’Anneo, A. Multifaceted Health Benefits of Mangifera indica L. (Mango): The Inestimable Value of Orchards Recently Planted in Sicilian Rural Areas. Nutrients 2017, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.L.; Norulaini, N.A.N.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-Products and Their Valuable Components: A Review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, R.; Nema, P.K.; Kaur, B.P. Quality Evaluation and Drying Characteristics of Mango Cubes Dried Using Low-Pressure Superheated Steam, Vacuum and Hot Air Drying Methods. LWT 2018, 92, 548–555. [Google Scholar] [CrossRef]
- Manhongo, T.T.; Chimphango, A.; Thornley, P.; Röder, M. Techno-Economic and Environmental Evaluation of Integrated Mango Waste Biorefineries. J. Clean. Prod. 2021, 325, 129335. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. A Hydrocolloid Based Biorefinery Approach to the Valorisation of Mango Peel Waste. Food Hydrocoll. 2018, 77, 142–151. [Google Scholar] [CrossRef]
- Madhusudhan, M.C.; Amrutha, N.; Rani, A.; Raghavarao, K. Aqueous Two-Phase Extraction in Downstream Processing. Curr. Biochem. Eng. 2015, 2, 39–48. [Google Scholar] [CrossRef]
- Saha, N.; Sarkar, B.; Sen, K. Aqueous Biphasic Systems: A Robust Platform for Green Extraction of Biomolecules. J. Mol. Liq. 2022, 363, 119882. [Google Scholar] [CrossRef]
- Rogers, R.D.; Willauer, H.D.; Griffin, S.T.; Huddleston, J.G. Partitioning of Small Organic Molecules in Aqueous Biphasic Systems. J. Chromatogr. B Biomed. Sci. Appl. 1998, 711, 255–263. [Google Scholar] [CrossRef]
- Hamta, A.; Dehghani, M.R. Application of Polyethylene Glycol Based Aqueous Two-Phase Systems for Extraction of Heavy Metals. J. Mol. Liq. 2017, 231, 20–24. [Google Scholar] [CrossRef]
- Malika, A.; Mohammed, A.; Boukhlifi, F. Kinetic and Energy Study of Thermal Degradation of Biomass Materials under Oxidative Atmosphere Using TGA, DTA and DSC. J. Multidiscip. Eng. Sci. Technol. 2014, 1, 3159–3199. [Google Scholar]
- Pires, C.; de Motta, L.A.C.; dos Ferreira, R.A.R.; de Caixeta, C.O.; Savastano, H. Thermomechanical and Thermo-Hydro-Mechanical Treatments of Luffa Cylindrical Fibers. Nat. Fibers 2020, 18, 2351–2363. [Google Scholar] [CrossRef]
- Sahu, S.; Sahu, S.B.B.P.J.; Nayak, S.; Roul, M.K. Mechanical, Thermal and Microstructural Studies of Acrylonitrile Butadiene Styrene Reinforced with Rattan (Calamus beccarii) Fiber Composites. Polym. Compos. 2022, 43, 5582–5591. [Google Scholar] [CrossRef]
- Jaiswal, D.; Devnani, G.L.; Rajeshkumar, G.; Sanjay, M.R.; Siengchin, S. Review on Extraction, Characterization, Surface Treatment and Thermal Degradation Analysis of New Cellulosic Fibers as Sustainable Reinforcement in Polymer Composites. Curr. Res. Green Sustain. Chem. 2022, 5, 100271. [Google Scholar] [CrossRef]
- Nayak, S.; Khuntia, S.K. Development and Study of Properties of Moringa Oleifera Fruit Fibers/ Polyethylene Terephthalate Composites for Packaging Applications. Compos. Commun. 2019, 15, 113–119. [Google Scholar] [CrossRef]
- Menczel, J.D.; Kohl, W.S. Differential Scanning Calorimetry (DSC) in Fiber Research. In Thermal Analysis of Textiles and Fibers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–69. [Google Scholar] [CrossRef]
- Sai Revanth, J.; Sai Madhav, V.; Kalyan Sai, Y.; Vineeth Krishna, D.; Srividya, K.; Mohan Sumanth, C.H. TGA and DSC Analysis of Vinyl Ester Reinforced by Vetiveria Zizanioides, Jute and Glass Fiber. Mater. Today Proc. 2020, 26, 460–465. [Google Scholar] [CrossRef]
- Zug, J.P.; Razzitte, A. Monografía de Termodinámica Estadística Isotermas de Adsorción: Bases Mecánico-Estadísticas; University of Buenos Aires: Buenos Aires, Argentina, 2002. [Google Scholar]
- Lopez-Serrano, F.; Mendizábal, E.; Ortega-Gudiño, P. Introducción a La Ciencia de Los Polímeros. In Manuales Básicos (MANUBAS); Universidad de Guadalajara: Guadalajara, Mexico, 2015; Volume 338. [Google Scholar]
- Mosquera, L.H.; Moraga, G.; Martínez-Navarrete, N. Critical Water Activity and Critical Water Content of Freeze-Dried Strawberry Powder as Affected by Maltodextrin and Arabic Gum. Food Res. Int. 2012, 47, 201–206. [Google Scholar] [CrossRef]
- Fabra, M.J.; Márquez, E.; Castro, D.; Chiralt, A. Effect of Maltodextrins in the Water-Content–Water Activity–Glass Transition Relationships of Noni (Morinda citrifolia L.) Pulp Powder. J. Food Eng. 2011, 103, 47–51. [Google Scholar] [CrossRef]
- Roos, Y.H. Phase Transitions in Foods; Academic Press: Cambridge, MA, USA, 1995; p. 360. [Google Scholar]
- Wan, Z.; Tang, J.; Marsiglia-Fuentes, R.; Quintana, S.E.; Zapateiro, L.A.G. Novel Hydrocolloids Obtained from Mango (Mangifera indica) Var. Hilaza: Chemical, Physicochemical, Techno-Functional, and Structural Characteristics. Gels 2022, 8, 354. [Google Scholar] [CrossRef]
- López-Barraza, D.; Ortega-Ramos, A.; Torregroza-Fuentes, E.; Quintana, S.E.; García-Zapateiro, L.A. Rheological and Functional Properties of Hydrocolloids from Pereskia bleo Leaves. Fluids 2021, 6, 349. [Google Scholar] [CrossRef]
- Martínez, S.E.Q.; Fuentes, E.E.T.; Zapateiro, L.A.G. Food Hydrocolloids from Butternut Squash (Cucurbita moschata) Peel: Rheological Properties and Their Use in Carica Papaya Jam. ACS Omega 2021, 6, 12114–12123. [Google Scholar] [CrossRef]
- Standard, I.; Carbon, S.; Ratio, I.; Principle, A.; Apparatus, B. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs/Edited by William Horwitz. Computer 2000, 10, 44–46. [Google Scholar]
- López-Vidaña, E.C.; Castillo Téllez, M.; Pilatowsky Figueroa, I.; Santis Espinosa, L.F.; Castillo-Téllez, B. Moisture Sorption Isotherms, Isosteric Heat, and Gibbs Free Energy of Stevia Leaves. J. Food Process. Preserv. 2021, 45, e15016. [Google Scholar] [CrossRef]
- Peleg, M. An Empirical Model for the Description of Moisture Sorption Curves. J. Food Sci. 1988, 53, 1216–1217. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Applying Ultrasound-Assisted Processing to Obtain Cellulose Fibres from Rice Straw to Be Used as Reinforcing Agents. Innov. Food Sci. Emerg. Technol. 2022, 76, 102932. [Google Scholar] [CrossRef]
- Andrade, J.; González-Martínez, C.; Chiralt, A. Effect of Phenolic Acids on the Properties of Films from Poly (Vinyl Alcohol) of Different Molecular Characteristics. Food Packag. Shelf. Life 2021, 29, 100711. [Google Scholar] [CrossRef]
- Gannasin, S.P.; Adzahan, N.M.; Hamzah, M.Y.; Mustafa, S.; Muhammad, K. Physicochemical Properties of Tamarillo (Solanum betaceum Cav.) Hydrocolloid Fractions. Food Chem. 2015, 182, 292–301. [Google Scholar] [CrossRef]
- Bertuzzi, M.A.; Slavutsky, A.M.; Armada, M. Physicochemical Characterisation of the Hydrocolloid from Brea Tree (Cercidium praecox). Int. J. Food Sci. Technol. 2012, 47, 768–775. [Google Scholar] [CrossRef]
- Chaudhari, B.B.; Annapure, U.S. Rheological, Physicochemical, and Spectroscopic Characterizations of Limonia acidissima L. Gum Exudate with an Application in Extrusion Processing. Carbohydr. Polym. Technol. Appl. 2021, 2, 100020. [Google Scholar] [CrossRef]
- Nikolic, G.S.; Cakic, M.D. Physical Investigation of the Colloidal Iron-Inulin Complex. Colloid J. 2007, 69, 464–473. [Google Scholar] [CrossRef]
- Eitex, T.T. Valorisation of Mango Fruit By-Products: Physicochemical Characterisation and Future Prospect. Chem. Process. Eng. Res. 2017, 50, 22–34. [Google Scholar]
- Razavi, S.M.A.; Cui, S.W.; Guo, Q.; Ding, H. Some Physicochemical Properties of Sage (Salvia macrosiphon) Seedgum. Food Hydrocoll. 2014, 35, 453–462. [Google Scholar] [CrossRef]
- Pal, S.; Mal, D.; Singh, R.P. Cationic Starch: An Effective Flocculating Agent. Carbohydr. Polym. 2005, 59, 417–423. [Google Scholar] [CrossRef]
- Bostan, A.; Razavi, S.M.A.; Farhoosh, R. Optimization of Hydrocolloid Extraction from Wild Sage Seed (Salvia macrosiphon) Using Response Surface. Int. J. Food Prop. 2010, 13, 1380–1392. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Yousefi, A.; Ako, K. Steady/Dynamic Rheological Characterization and FTIR Study on Wheat Starch-Sage Seed Gum Blends. Food Hydrocoll. 2021, 111, 106380. [Google Scholar] [CrossRef]
- Lijian, Q. Preliminary Study on Antifungal Activities of Different Extracts of the Peel of Mango (Mangifera indica linnaeus). Chin. J. Trop. Agric. 2007, 30, 2598–2604. [Google Scholar]
- Cuba-Chiem, L.T.; Huynh, L.; Ralston, J.; Beattie, D.A. In Situ Particle Film ATR FTIR Spectroscopy of Carboxymethyl Cellulose Adsorption on Talc: Binding Mechanism, PH Effects, and Adsorption Kinetics. Langmuir 2008, 24, 8036–8044. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van Der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Turhan, M.; Sayar, S.; Gunasekaran, S. Application of Peleg Model to Study Water Absorption in Chickpea during Soaking. J. Food Eng. 2002, 53, 153–159. [Google Scholar] [CrossRef]
- Giner, S.A.; Gely, M.C. Sorptional Parameters of Sunflower Seeds of Use in Drying and Storage Stability Studies. Biosyst. Eng. 2005, 92, 217–227. [Google Scholar] [CrossRef]
- Zhou, Y.; Roos, Y.H. Stability and Plasticizing and Crystallization Effects of Vitamins in Amorphous Sugar Systems. J. Agric. Food Chem. 2012, 60, 1075–1083. [Google Scholar] [CrossRef]
- Lazouk, M.A.; Savoire, R.; Kaddour, A.; Castello, J.; Lanoisellé, J.L.; Van Hecke, E.; Thomasset, B. Oilseeds Sorption Isoterms, Mechanical Properties and Pressing: Global View of Water Impact. J. Food Eng. 2015, 153, 73–80. [Google Scholar] [CrossRef]
- Vignolles, M.L.; Jeantet, R.; Lopez, C.; Schuck, P. Free Fat, Surface Fat and Dairy Powders: Interactions between Process and Product. A Review. Lait 2007, 87, 187–236. [Google Scholar] [CrossRef]
- Alvino Granados, A.E.; Kawai, K. Effect of Cellulose Powder Content on the Water Sorption, Glass Transition, Mechanical Relaxation, and Caking of Freeze-Dried Carbohydrate Blend and Food Powders. LWT 2021, 148, 111798. [Google Scholar] [CrossRef]
- SOPADE, P.A.; OBEKPA, J.A. Modelling Water Absorption in Soybean, Cowpea and Peanuts at Three Temperatures Using Peleg’s Equation. J. Food Sci. 1990, 55, 1084–1087. [Google Scholar] [CrossRef]
- Yildirim, A.; Bulut, Y. Adsorption Behaviors of Malachite Green by Using Crosslinked Chitosan/Polyacrylic Acid/Bentonite Composites with Different Ratios. Environ. Technol. Innov. 2020, 17, 100560. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Ariff, N.F.M.; Hanafiah, M.A.K.M. Preparation, Characterization, and Environmental Application of Crosslinked Chitosan-Coated Bentonite for Tartrazine Adsorption from Aqueous Solutions. Water Air Soil Pollut. 2010, 206, 225–236. [Google Scholar] [CrossRef]
- Rinaudo, M.; Milas, M. Gellan Gum, a Bacterial Gelling Polymer. Dev. Food Sci. 2000, 41, 239–263. [Google Scholar] [CrossRef]
- Silalai, N.; Roos, Y.H. Mechanical Relaxation Times as Indicators of Stickiness in Skim Milk–Maltodextrin Solids Systems. J. Food Eng. 2011, 106, 306–317. [Google Scholar] [CrossRef]
- Maidannyk, V.A.; Roos, Y.H. Modification of the WLF Model for Characterization of the Relaxation Time-Temperature Relationship in Trehalose-Whey Protein Isolate Systems. J. Food Eng. 2016, 188, 21–31. [Google Scholar] [CrossRef]
- Maidannyk, V.A.; Lim, A.S.L.; Auty, M.A.E.; Roos, Y.H. Effects of Lipids on the Water Sorption, Glass Transition and Structural Strength of Carbohydrate-Protein Systems. Food Res. Int. 2019, 116, 1212–1222. [Google Scholar] [CrossRef]
- Lieb, V.M.; Schuster, L.K.; Kronmüller, A.; Schmarr, H.G.; Carle, R.; Steingass, C.B. Fatty Acids, Triacylglycerols, and Thermal Behaviour of Various Mango (Mangifera indica L.) Kernel Fats. Food Res. Int. 2019, 116, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Jaya, S.; Das, H. Glass Transition and Sticky Point Temperatures and Stability/Mobility Diagram of Fruit Powders. Food Bioproc. Tech. 2009, 2, 89–95. [Google Scholar] [CrossRef]
- Roos, Y.H. Water Activity and Physical State Effects on Amorphous Food Stability. J. Food Process. Preserv. 1993, 16, 433–447. [Google Scholar] [CrossRef]
- Fongin, S.; Alvino Granados, A.E.; Harnkarnsujarit, N.; Hagura, Y.; Kawai, K. Effects of Maltodextrin and Pulp on the Water Sorption, Glass Transition, and Caking Properties of Freeze-Dried Mango Powder. J. Food Eng. 2019, 247, 95–103. [Google Scholar] [CrossRef]
- Palzer, S. The Effect of Glass Transition on the Desired and Undesired Agglomeration of Amorphous Food Powders. Chem. Eng. Sci. 2005, 60, 3959–3968. [Google Scholar] [CrossRef]
- Fongin, S.; Kawai, K.; Harnkarnsujarit, N.; Hagura, Y. Effects of Water and Maltodextrin on the Glass Transition Temperature of Freeze-Dried Mango Pulp and an Empirical Model to Predict Plasticizing Effect of Water on Dried Fruits. J. Food Eng. 2017, 210, 91–97. [Google Scholar] [CrossRef]
- Reinoso, D.; Martín-Alfonso, M.J.; Luckham, P.F.; Martínez-Boza, F.J. Flow Behavior and Thermal Resistance of Xanthan Gum in Formate Brine. J. Pet Sci. Eng. 2020, 188, 106881. [Google Scholar] [CrossRef]
- Nuvoli, L.; Conte, P.; Fadda, C.; Reglero Ruiz, J.A.; García, J.M.; Baldino, S.; Mannu, A. Structural, Thermal, and Mechanical Properties of Gelatin-Based Films Integrated with Tara Gum. Polymer 2021, 214, 123244. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Harwood, J.L. The Lipid Handbook with CD-ROM—Google Libros. Available online: https://books.google.com.co/books?hl=es&lr=&id=INZa6WmqDA8C&oi=fnd&pg=PP1&ots=Sv9x2c9ys-&sig=EelrtS8tgDtqJFaI8scanyTAzGs&redir_esc=y#v=onepage&q&f=false (accessed on 24 May 2023).
- Motta, M.V.L.; de Castro, E.V.R.; Muri, E.J.B.; Loureiro, B.V.; Costalonga, M.L.; Filgueiras, P.R. Thermal and Spectroscopic Analyses of Guar Gum Degradation Submitted to Turbulent Flow. Int. J. Biol. Macromol. 2019, 131, 43–49. [Google Scholar] [CrossRef]
| Sample Code | Moisture % | * Ash % | * Lipids % | * Carbohydrate % | * Proteins % |
|---|---|---|---|---|---|
| E3 | 13.16 ± 0.99 a | 9.30 ± 0.61 a | 2.93 ± 0.01 a | 73.44 ± 1.20 b | 0.35 ± 0.03 b |
| E10 | 11.24 ± 0.54 d | 9.10 ± 0.71 a | 3.18 ± 0.31 a | 75.95 ± 0.54 a | 0.37 ± 0.03 b |
| S3 | 12.55 ± 0.23 c | 8.47 ± 0.46 a | 18.56 ± 0.10 b | 28.05 ± 0.30 c | 32.07 ± 0.2 a |
| S10 | 12.96 ± 0.83 b | 9.65 ± 0.53 a | 17.32 ± 0.58 b | 28.16 ± 0.90 c | 31.25 ± 0.4 a |
| Samples | Sales | RH (%) | K1 (min(g/g db))−1 | K1 (g/g db)−1 | R2 | 1/K1 | 1/K2 |
|---|---|---|---|---|---|---|---|
| E3 | LiCl | 11.3 | 322.13 ± 7.49 b | 75.03 ± 1.18 c | 0.92 | 0.003 a | 0.013 n |
| Mgcl2 | 32.78 | 114.30 ± 7.12 d | 27.39 ± 2.34 h | 0.97 | 0.009 i | 0.037 k | |
| K2Co3 | 46.16 | 46.69 ± 8.59 h | 18.22 ± 1.35 k | 0.95 | 0.021 f | 0.055 h | |
| Mg(NO3)l2 | 52.89 | 28.26 ± 6.47 i | 14.09 ± 1.10 m | 0.91 | 0.035 c | 0.071 e | |
| NaCl | 75.28 | 5.13 ± 0.60 l | 3.37 ± 0.11 o | 0.97 | 0.195 k | 0.296 a | |
| E10 | LiCl | 11.3 | 328.61 ± 16.24 a | 144.68 ± 14.12 b | 0.89 | 0.003 a | 0.007 o |
| Mgcl2 | 32.78 | 85.42 ± 5.16 e | 34.41 ± 2.41 g | 0.94 | 0.012 i | 0.029 l | |
| K2Co3 | 46.16 | 28.50 ± 2.86 i | 14.38 ± 0.57 m | 0.95 | 0.035 f | 0.070 f | |
| Mg(NO3)l2 | 52.89 | 18.59 ± 1.53 k | 10.45 ± 0.36 m | 0.92 | 0.054 c | 0.096 c | |
| NaCl | 75.28 | 6.01 ± 0.41 l | 3.80 ± 0.10 o | 0.95 | 0.166 k | 0.263 a | |
| S3 | LiCl | 11.3 | 173.20 ± 0.95 c | 70.43 ± 5.09 d | 0.92 | 0.006 b | 0.014 n |
| Mgcl2 | 32.78 | 59.93 ± 0.15 g | 40.05 ± 1.83 e | 0.94 | 0.017 d | 0.025 m | |
| K2Co3 | 46.16 | 28.93 ± 2.05 i | 25.55 ± 0.45 i | 0.99 | 0.035 f | 0.039 j | |
| Mg(NO3)l2 | 52.89 | 26.05 ± 3.18 i | 21.85 ± 0.66 n | 0.97 | 0.037 g | 0.046 i | |
| NaCl | 75.28 | 16.41 ± 2.81 k | 11.67 ± 0.57 n | 0.92 | 0.061 j | 0.086 d | |
| S10 | LiCl | 11.3 | 172.11 ± 4.17 c | 487.25 ± 3.34 a | 0.87 | 0.006 b | 0.002 p |
| Mgcl2 | 32.78 | 66.89 ± 13.87 f | 37.67 ± 2.47 f | 0.92 | 0.015 h | 0.027 m | |
| K2Co3 | 46.16 | 28.13 ± 2.60 i | 24.89 ± 0.55 o | 0.98 | 0.036 ef | 0.040 j | |
| Mg(NO3)l2 | 52.89 | 21.58 ± 2.05 j | 16.92 ± 0.39 l | 0.99 | 0.041 e | 0.059 g | |
| NaCl | 75.28 | 16.35± 0.71 k | 10.22 ± 0.18 n | 0.98 | 0.061 j | 0.098 b |
| Degradation Range | Sample | |||
|---|---|---|---|---|
| E3 | E10 | S3 | S10 | |
| T Peak 1 (°C) | 228–299 c | 200–225 b | 301 a | 293 a |
| T Peak 2 (°C) | 591 b | 424 c | 613 a | 615 a |
| Initiation of degradation (°C) | 175 ± 3 d | 201 ± 0 c | 225 ± 0 b | 269 ± 0 a |
| End of degradation (°C) | 641 ± 16 b | 635 ± 4 b | 650 ± 0 a | 650 ± 19 a |
| Water mass loss (%) | 2.4 ± 0.1 c | 4.5 ± 0.1 a | 3.8 ± 0.1 b | 4.5 ± 0.0 a |
| Peak 1 Mass loss (%) | 81 ± 7 b | 87 ± 5 a | 86.2 ± 0.4 a | 79.9 ± 0.2 b |
| Peak 2 Mass loss (%) | 16.4 ± 0.14 b | 9.6 ± 0.62 a | 9.4 ± 0.11 a | 14.6 ± 0.8 b |
| Residue (%) | 2.4 ± 0.8 c | 3.2 ± 1.1 bc | 4.5 ± 0.2 b | 7.0 ± 0.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsiglia-Fuentes, R.; Chiralt, A.; García-Zapateiro, L.A. Investigating the Water Relations in Aqueous Extract Powders of Mango (Mangifera indica) Peel and Seed Waste for Their Use in Food Matrices as a Value-Added By-Product. Foods 2023, 12, 3497. https://doi.org/10.3390/foods12183497
Marsiglia-Fuentes R, Chiralt A, García-Zapateiro LA. Investigating the Water Relations in Aqueous Extract Powders of Mango (Mangifera indica) Peel and Seed Waste for Their Use in Food Matrices as a Value-Added By-Product. Foods. 2023; 12(18):3497. https://doi.org/10.3390/foods12183497
Chicago/Turabian StyleMarsiglia-Fuentes, Ronald, Amparo Chiralt, and Luis A. García-Zapateiro. 2023. "Investigating the Water Relations in Aqueous Extract Powders of Mango (Mangifera indica) Peel and Seed Waste for Their Use in Food Matrices as a Value-Added By-Product" Foods 12, no. 18: 3497. https://doi.org/10.3390/foods12183497






