Kombucha Fermentation of Olympus Mountain Tea (Sideritis scardica) Sweetened with Thyme Honey: Physicochemical Analysis and Evaluation of Functional Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Kombucha Production—Fermentation Conditions
2.3. Sampling
2.4. Color Measurement
2.5. Determination of pH and Titratable Acidity
2.6. Enumeration of Yeasts and Acetic Acid Bacteria
2.7. Determination of Ethanol, Sugars, Organic Acid, and Minerals
2.8. Vitamin C
2.9. Vitamins of the B-Complex
2.10. Total Phenolic Content
2.11. Total Flavonoid Content
2.12. Analysis of Antioxidant Activity
2.13. α-Amylase and α-Glucosidase Inhibition Assays
2.13.1. α-Glycosidase Inhibition Assay
2.13.2. α-Amylase Inhibition Assay
2.14. Anticholinesterase Assays: AChe and BChe Inhibition
2.15. Statistical Analysis
3. Results and Discussion
3.1. Macroscopic Changes during Fermentation. Mat Formation, Color Changes, and Clarity of the Beverages
3.2. pH and TA
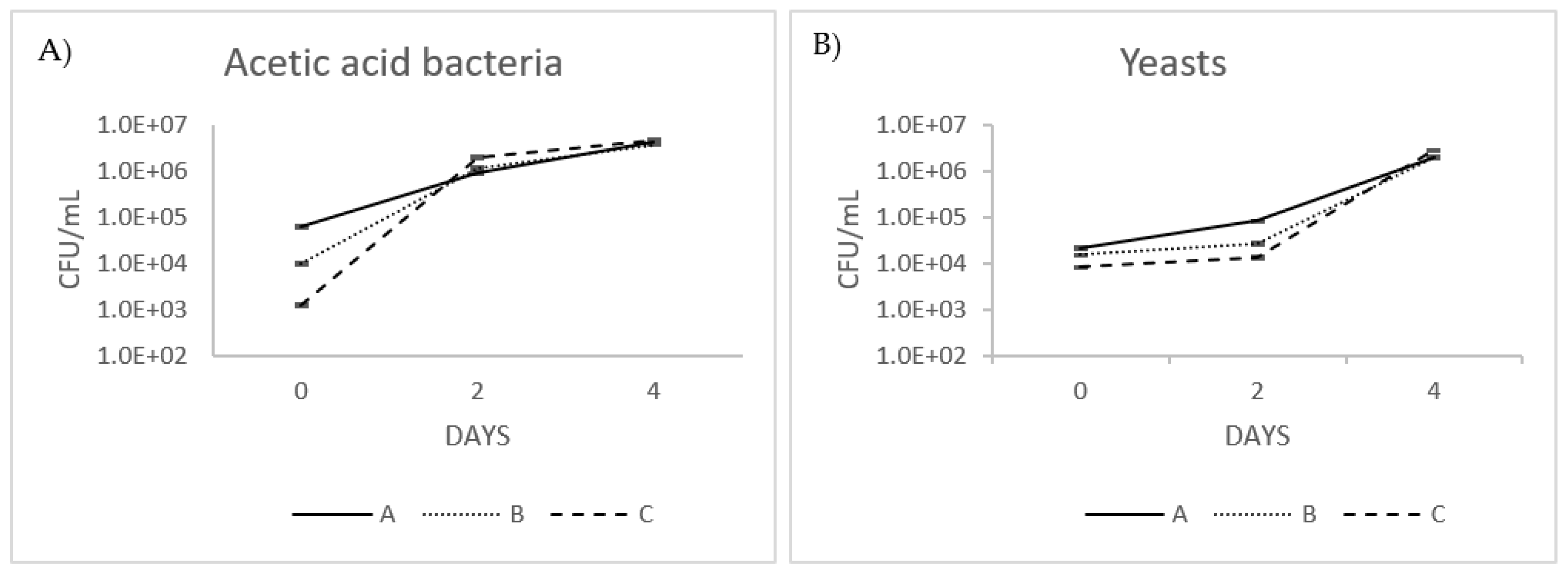
3.3. Yeast and Acetic Acid Bacteria Count
3.4. Changes in Sugars, Ethanol, Organic Acids, Minerals
3.4.1. Sugars
3.4.2. Acids
3.4.3. Ethanol
3.4.4. Minerals
3.5. Vitamin C
3.6. Vitamin B-Complex
3.7. Changes in Total Phenolic and Flavonoid Compounds
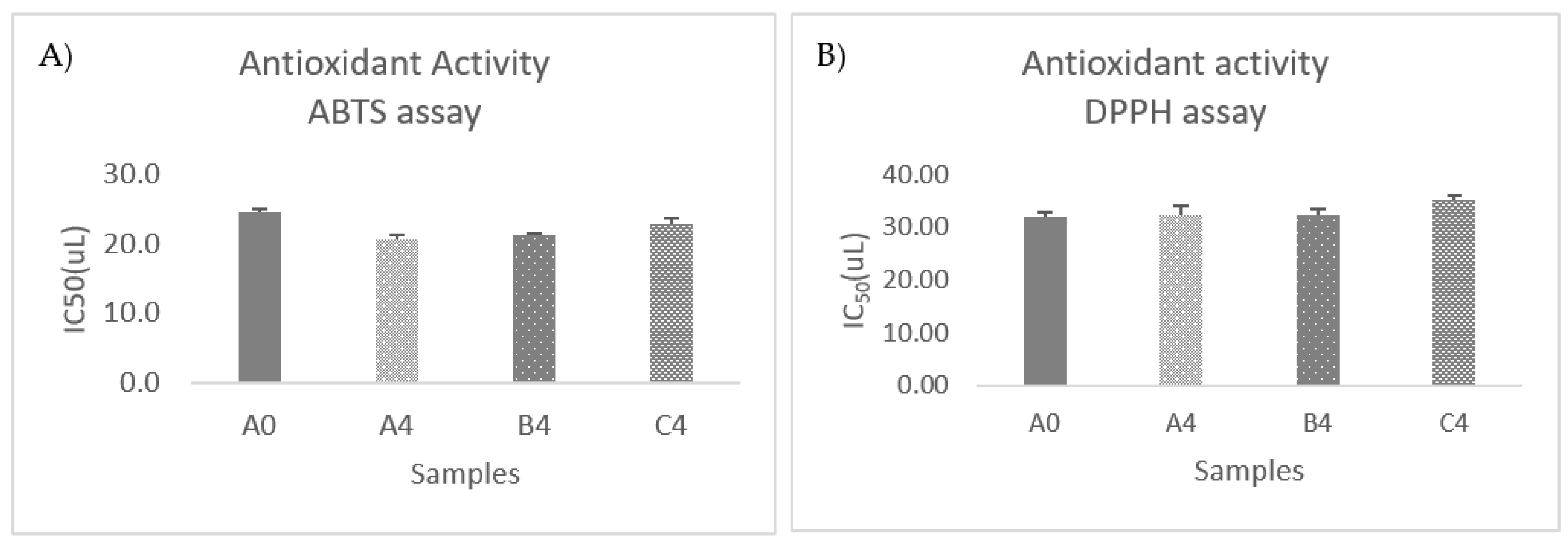
3.8. Antioxidant Activity Evaluation
3.9. α-Amylase and α-Glucosidase Inhibition
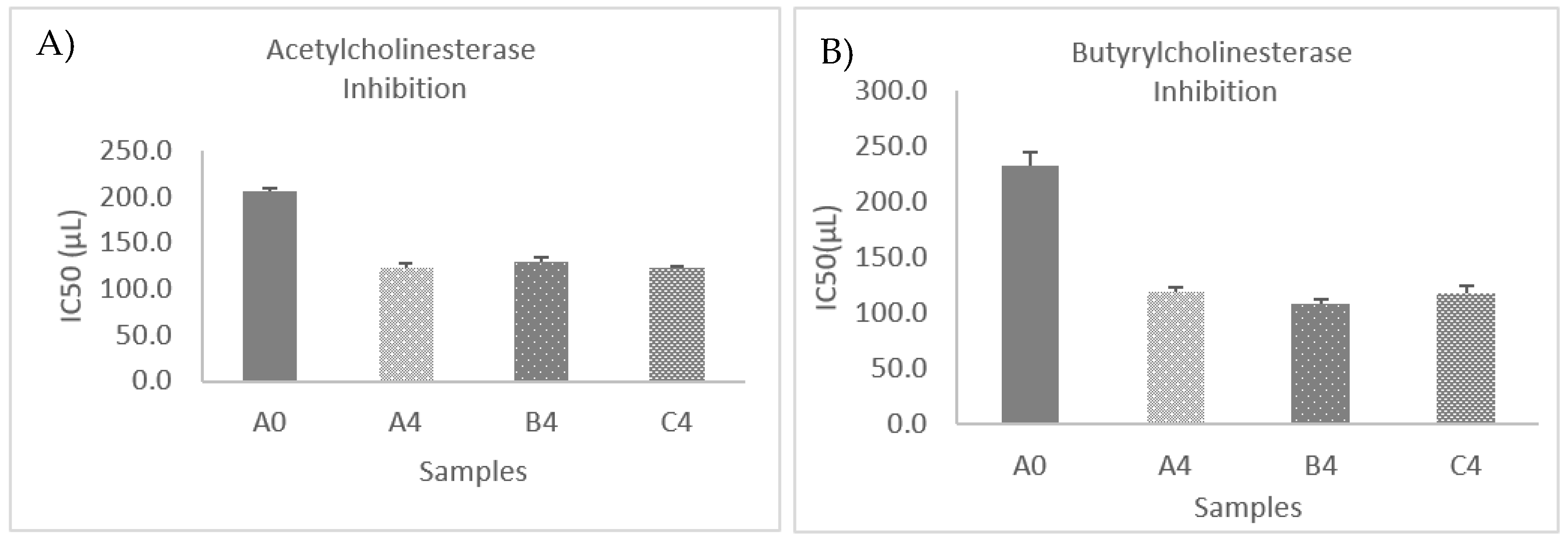
3.10. Cholinesterase Inhibition Assays
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pop, O.L.; Salanță, L.-C.; Pop, C.R.; Coldea, T.; Socaci, S.A.; Suharoschi, R.; Vodnar, D.C. Prebiotics and Dairy Applications. In Dietary Fiber: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–277. [Google Scholar]
- Peyer, L.C.; Zannini, E.; Arendt, E.K. Lactic Acid Bacteria as Sensory Biomodulators for Fermented Cereal-Based Beverages. Trends Food Sci. Technol. 2016, 54, 1F7–25. [Google Scholar] [CrossRef]
- Salanță, L.C.; Uifălean, A.; Iuga, C.-A.; Tofană, M.; Cropotova, J.; Pop, O.L.; Pop, C.R.; Rotar, M.A.; Bautista-Ávila, M.; González, C.V. Valuable Food Molecules with Potential Benefits for Human Health. In The Health Benefits of Foods-Current Knowledge and Further Development; IntechOpen: London, UK, 2020; pp. 1–45. [Google Scholar]
- Fernandesa, C.G.; Sonawaneb, S.K.; Arya, S.S. Cereal Based Functional Beverages: A Review. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 914–919. [Google Scholar] [CrossRef]
- Uifălean, A.; Schneider, S.; Ionescu, C.; Lalk, M.; Iuga, C. Soy Isoflavones and Breast Cancer Cell Lines: Molecular Mechanisms and Future Perspectives. Molecules 2015, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Uifălean, A.; Schneider, S.; Gierok, P.; Ionescu, C.; Iuga, C.; Lalk, M. The Impact of Soy Isoflavones on MCF-7 and MDA-MB-231 Breast Cancer Cells Using a Global Metabolomic Approach. Int. J. Mol. Sci. 2016, 17, 1443. [Google Scholar] [CrossRef] [PubMed]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional Beverages: The Emerging Side of Functional Foods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Ghoshal, G.; Kansal, S.K. The Emerging Trends in Functional and Medicinal Beverage Research and Its Health Implication. In Functional and Medicinal Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–71. [Google Scholar]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-Based Milk Alternatives an Emerging Segment of Functional Beverages: A Review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Aloulou, A.; Hamden, K.; Elloumi, D.; Ali, M.B.; Hargafi, K.; Jaouadi, B.; Ayadi, F.; Elfeki, A.; Ammar, E. Hypoglycemic and Antilipidemic Properties of Kombucha Tea in Alloxan-Induced Diabetic Rats. BMC Complement. Altern. Med. 2012, 12, 63. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; Singh, M.; Rao, P.V.L.; Bhattacharya, R.; Kumar, P.; Sugendran, K.; Kumar, O.; Pant, S.C.; Singh, R. Subacute (90 Days) Oral Toxicity Studies of Kombucha Tea. Biomed. Environ. Sci. 2000, 13, 293–299. [Google Scholar]
- Velićanski, A.S.; Cvetković, D.D.; Markov, S.L.; Tumbas Šaponjac, V.T.; Vulić, J.J. Antioxidant and Antibacterial Activity of the Beverage Obtained by Fermentation of Sweetened Lemon Balm (Melissa offi cinalis L.) Tea with Symbiotic Consortium of Bacteria and Yeasts. Food Technol. Biotechnol. 2014, 52, 420–429. [Google Scholar] [CrossRef]
- Rinihapsari, E.; Richter, C.A. Fermentasi Kombucha Dan Potensinya Sebagai Minuman Kesehatan. Media Farm. Indones. 2008, 3, 149855. [Google Scholar]
- Baschali, A.; Tsakalidou, E.; Kyriacou, A.; Karavasiloglou, N.; Matalas, A.-L. Traditional Low-Alcoholic and Non-Alcoholic Fermented Beverages Consumed in European Countries: A Neglected Food Group. Nutr. Res. Rev. 2017, 30, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black and Red Tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Choo, C.; Waisundara, V.Y. RETRACTED: Application of the Kombucha ‘Tea Fungus’ for the Enhancement of Antioxidant and Starch Hydrolase Inhibitory Properties of Ten Herbal Teas. Food Chem. 2016, 194, 304–311. [Google Scholar] [CrossRef]
- Vitas, J.; Malbaša, R.; Jokić, A.; Lončar, E.; Milanović, S. Modeliranje Antioksidativnih Svojstava Kombuha Fermentiranih Mliječnih Napitaka s Mentom Primjenom ANNs i RSM Metode. Mljekarstvo Časopis Za Unaprjeđenje Proizv. I Prerade Mlijeka 2018, 68, 116–125. [Google Scholar]
- Tu, C.; Tang, S.; Azi, F.; Hu, W.; Dong, M. Use of Kombucha Consortium to Transform Soy Whey into a Novel Functional Beverage. J. Funct. Foods 2019, 52, 81–89. [Google Scholar] [CrossRef]
- Danesi, F.; Saha, S.; Kroon, P.A.; Glibetić, M.; Konić-Ristić, A.; D’Antuono, L.F.; Bordoni, A. Bioactive-Rich Sideritis scardica Tea (Mountain Tea) Is as Potent as Camellia sinensis Tea at Inducing Cellular Antioxidant Defences and Preventing Oxidative Stress. J. Sci. Food Agric. 2013, 93, 3558–3564. [Google Scholar] [CrossRef]
- Kratchanova, M.; Denev, P.; Ciz, M.; Lojek, A.; Mihailov, A. Evaluation of Antioxidant Activity of Medicinal Plants Containing Polyphenol Compounds. Comparison of Two Extraction Systems. Acta Biochim. Pol. 2010, 57, 233. [Google Scholar] [CrossRef]
- Tadić, V.; Bojović, D.; Arsić, I.; Đorđević, S.; Aksentijevic, K.; Stamenić, M.; Janković, S. Chemical and Antimicrobial Evaluation of Supercritical and Conventional Sideritis scardica Griseb., Lamiaceae Extracts. Molecules 2012, 17, 2683–2703. [Google Scholar] [CrossRef]
- Tadić, V.; Jeremic, I.; Dobric, S.; Isakovic, A.; Markovic, I.; Trajkovic, V.; Bojovic, D.; Arsic, I. Anti-Inflammatory, Gastroprotective, and Cytotoxic Effects of Sideritis scardica Extracts. Planta Med. 2012, 78, 415–427. [Google Scholar] [CrossRef]
- Heiner, F.; Feistel, B.; Wink, M. Sideritis scardica Extracts Inhibit Aggregation and Toxicity of Amyloid-β in Caenorhabditis Elegans Used as a Model for Alzheimer’s Disease. PeerJ 2018, 6, e4683. [Google Scholar] [CrossRef]
- Crane, E. Honey a Comprehensive Survey; Maurizio, A., White, J.W., Eds.; Morrison and Gibb Ltd.: New York, NY, USA, 1975; pp. 194–206. [Google Scholar]
- Khan, R.U.; Naz, S.; Abudabos, A.M. Towards a Better Understanding of the Therapeutic Applications and Corresponding Mechanisms of Action of Honey. Environ. Sci. Pollut. Res. 2017, 24, 27755–27766. [Google Scholar] [CrossRef]
- Cvetković, D.; Markov, S.; Djurić, M.; Savić, D.; Velićanski, A. Specific Interfacial Area as a Key Variable in Scaling-up Kombucha Fermentation. J. Food Eng. 2008, 85, 387–392. [Google Scholar] [CrossRef]
- Zou, C.; Li, R.-Y.; Chen, J.-X.; Wang, F.; Gao, Y.; Fu, Y.-Q.; Xu, Y.-Q.; Yin, J.-F. Zijuan Tea-Based Kombucha: Physicochemical, Sensorial, and Antioxidant Profile. Food Chem. 2021, 363, 130322. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha Tea Fermentation: Microbial and Biochemical Dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Kim, D.-H.; Chon, J.-W.; Kim, H.; Seo, K.-H. Development of a Novel Selective Medium for the Isolation and Enumeration of Acetic Acid Bacteria from Various Foods. Food Control 2019, 106, 106717. [Google Scholar] [CrossRef]
- Bajaj, K.L.; Kaur, G. Spectrophotometric Determination of L-Ascorbic Acid in Vegetables and Fruits. Analyst 1981, 106, 117. [Google Scholar] [CrossRef] [PubMed]
- Ciulu, M.; Solinas, S.; Floris, I.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Spano, N.; Sanna, G. RP-HPLC Determination of Water-Soluble Vitamins in Honey. Talanta 2011, 83, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Elsevie: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. ISBN 0076-6879. [Google Scholar]
- Markham, K.R. Flavones, Flavonols and Their Glycosides. In Methods in Plant Biochemistry; Academic Press: Cambridge, MA, USA, 1989; pp. 197–235. [Google Scholar]
- Kwon, Y.-I.; Apostolidis, E.; Kim, Y.-C.; Shetty, K. Health Benefits of Traditional Corn, Beans, and Pumpkin: In Vitro Studies for Hyperglycemia and Hypertension Management. J. Med. Food 2007, 10, 266–275. [Google Scholar] [CrossRef]
- Xia, X.; Dai, Y.; Wu, H.; Liu, X.; Wang, Y.; Yin, L.; Wang, Z.; Li, X.; Zhou, J. Kombucha Fermentation Enhances the Health-Promoting Properties of Soymilk Beverage. J. Funct. Foods 2019, 62, 103549. [Google Scholar] [CrossRef]
- Yang, X.-W.; Huang, M.-Z.; Jin, Y.-S.; Sun, L.-N.; Song, Y.; Chen, H.-S. Phenolics from Bidens Bipinnata and Their Amylase Inhibitory Properties. Fitoterapia 2012, 83, 1169–1175. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea-Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in Content of Organic Acids and Tea Polyphenols during Kombucha Tea Fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Dufresne, C.; Farnworth, E. Tea, Kombucha, and Health: A Review. Food Res. Int. 2000, 33, 409–421. [Google Scholar] [CrossRef]
- Nummer, B.A. Kombucha Brewing under the Food and Drug Administration Model Food Code: Risk Analysis and Processing Guidance. J. Environ. Health 2013, 76, 8–11. [Google Scholar]
- Belloso-Morales, G.; Hernández-Sánchez, H. Manufacture of a Beverage from Cheese Whey Using a” Tea Fungus” Fermentation. Rev. Latinoam. Microbiol. Mex. 2003, 45, 5. [Google Scholar]
- Sreeramulu, G.; Zhu, Y.; Knol, W. Kombucha Fermentation and Its Antimicrobial Activity. J. Agric. Food Chem. 2000, 48, 2589–2594. [Google Scholar] [CrossRef]
- Sievers, M.; Lanini, C.; Weber, A.; Schuler-Schmid, U.; Teuber, M. Microbiology and Fermentation Balance in a Kombucha Beverage Obtained from a Tea Fungus Fermentation. Syst. Appl. Microbiol. 1995, 18, 590–594. [Google Scholar] [CrossRef]
- Teoh, A.L.; Heard, G.; Cox, J. Yeast Ecology of Kombucha Fermentation. Int. J. Food Microbiol. 2004, 95, 119–126. [Google Scholar] [CrossRef]
- Zhao, Z.-J.; Sui, Y.-C.; Wu, H.-W.; Zhou, C.-B.; Hu, X.-C.; Zhang, J. Flavour Chemical Dynamics during Fermentation of Kombucha Tea. Emir. J. Food Agric. 2018, 30, 732–741. [Google Scholar] [CrossRef]
- Goh, W.N.; Rosma, A.; Kaur, B.; Fazilah, A.; Karim, A.A.; Bhat, R. Fermentation of Black Tea Broth (Kombucha): I. Effects of Sucrose Concentration and Fermentation Time on the Yield of Microbial Cellulose. Int. Food Res. J. 2012, 19, 109–117. [Google Scholar]
- Chen, C.; Liu, B.Y. Changes in Major Components of Tea Fungus Metabolites during Prolonged Fermentation. J. Appl. Microbiol. 2000, 89, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-C.; Chen, C. Effects of Origins and Fermentation Time on the Antioxidant Activities of Kombucha. Food Chem. 2006, 98, 502–507. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Steinkraus, K.H.; Ledford, R.A. Kombucha, the Fermented Tea: Microbiology, Composition, and Claimed Health Effects. J. Food Prot. 2000, 63, 976–981. [Google Scholar] [CrossRef]
- Frank, G.W. Kombucha; Wilhelm Ennsthaler: Upper Austria, Austria, 1995; ISBN 9783850683371. [Google Scholar]
- Bai, F.W.; Zhao, X.Q. High Gravity Ethanol Fermentations and Yeast Tolerance. In Microbial Stress Tolerance for Biofuels: Systems Biology; Liu, Z.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 117–135. ISBN 978-3-642-21467-7. [Google Scholar]
- Raspor, P.; Goranovič, D. Biotechnological Applications of Acetic Acid Bacteria. Crit. Rev. Biotechnol. 2008, 28, 101–124. [Google Scholar] [CrossRef]
- Liu, C.-H.; Hsu, W.-H.; Lee, F.-L.; Liao, C.-C. The Isolation and Identification of Microbes from a Fermented Tea Beverage, Haipao, and Their Interactions during Haipao Fermentation. Food Microbiol. 1996, 13, 407–415. [Google Scholar] [CrossRef]
- Fu, C.; Yan, F.; Cao, Z.; Xie, F.; Lin, J. Antioxidant Activities of Kombucha Prepared from Three Different Substrates and Changes in Content of Probiotics during Storage. Food Sci. Technol. 2014, 34, 123–126. [Google Scholar] [CrossRef]
- Crane, E. A Book of Honey; Northern Bee Books: Mytholmroyd, UK, 2019; ISBN 9780860982883. [Google Scholar]
- Val, A.; Huidobro, J.F.; Sánchez, M.P.; Muniategui, S.; Fernández-Muiño, M.A.; Sancho, M.T. Enzymatic Determination of Galactose and Lactose in Honey. J. Agric. Food Chem. 1998, 46, 1381–1385. [Google Scholar] [CrossRef]
- White, J.W., Jr. Composition of Honey. Honey. A Comprehensive Survey; Heinemann, C., Ed.; Heinemann: London, UK, 1979; pp. 157–207. [Google Scholar]
- Lazaridou, A.; Biliaderis, C.G.; Bacandritsos, N.; Sabatini, A.G. Composition, Thermal and Rheological Behaviour of Selected Greek Honeys. J. Food Eng. 2004, 64, 9–21. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Schweitzer, P.; Bachir Bey, M.; Djoudad-Kadji, H.; Louaileche, H. HPLC Sugar Profiles of Algerian Honeys. Food Chem. 2010, 121, 561–568. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Brokl, M.; Soria, A.C.; Sanz, M.L.; Martínez-Castro, I. Gas Chromatographic–Mass Spectrometric Characterisation of Tri- and Tetrasaccharides in Honey. Food Chem. 2010, 120, 637–642. [Google Scholar] [CrossRef]
- Shin, H.-S.; Ustunol, Z. Carbohydrate Composition of Honey from Different Floral Sources and Their Influence on Growth of Selected Intestinal Bacteria: An in Vitro Comparison. Food Res. Int. 2005, 38, 721–728. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid Contents and the Effect of Fermentation Condition of Kombucha Tea Beverages on Physicochemical, Microbiological and Sensory Properties. CyTA–J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Talawat, S.; Ahantharik, P.; Laohawiwattanakul, S.; Premsuk, A.; Ratanapo, S. Efficacy of Fermented Teas in Antibacterial Activity. Agric. Nat. Resour. 2006, 40, 925–933. [Google Scholar]
- Kallel, L.; Desseaux, V.; Hamdi, M.; Stocker, P.; Ajandouz, E.H. Insights into the Fermentation Biochemistry of Kombucha Teas and Potential Impacts of Kombucha Drinking on Starch Digestion. Food Res. Int. 2012, 49, 226–232. [Google Scholar] [CrossRef]
- Mo, H.; Zhu, Y.; Chen, Z. Microbial Fermented Tea—A Potential Source of Natural Food Preservatives. Trends Food Sci. Technol. 2008, 19, 124–130. [Google Scholar] [CrossRef]
- Seto, A.; Kojima, Y.; Tonouchi, N.; Tsuchida, T.; Yoshinaga, F. Screening of Bacterial Cellulose-Producing Acetobacter Strains Suitable for Sucrose as a Carbon Source. Biosci. Biotechnol. Biochem. 1997, 61, 735–736. [Google Scholar] [CrossRef]
- Gama, M.; Gatenholm, P.; Klemm, D. Bacterial Nanocellulose: A Sophisticated Multifunctional Material; CRC Press: Boca Raton, FL, USA, 2012; ISBN 143986991X. [Google Scholar]
- Zubaidah, E.; Dewantari, F.J.; Novitasari, F.R.; Srianta, I.; Blanc, P.J. Potential of Snake Fruit (Salacca zalacca (Gaerth.) Voss) for the Development of a Beverage through Fermentation with the Kombucha Consortium. Biocatal. Agric. Biotechnol. 2018, 13, 198–203. [Google Scholar] [CrossRef]
- Gomes, S.; Dias, L.G.; Moreira, L.L.; Rodrigues, P.; Estevinho, L. Physicochemical, Microbiological and Antimicrobial Properties of Commercial Honeys from Portugal. Food Chem. Toxicol. 2010, 48, 544–548. [Google Scholar] [CrossRef]
- Valbuena, A.O.; Maeso, M.C.F.; de la Torre, E.S.M. Principales Características de La Miel de La Alcarria. Investigación Agraria en Castilla la Mancha Consejería de Agricultura y medio Ambiente: Madrid, Spain, 1996. [Google Scholar]
- Sancho, M.T.; Mato, I.; Huidobro, J.F.; Fernández-Muiño, M.A.; Pascual-Maté, A. Nonaromatic Organic Acids of Honeys. In Pot-Honey; Springer: New York, NY, USA, 2013; pp. 447–458. [Google Scholar]
- Cavia, M.M.; Fernández-Muiño, M.A.; Alonso-Torre, S.R.; Huidobro, J.F.; Sancho, M.T. Evolution of Acidity of Honeys from Continental Climates: Influence of Induced Granulation. Food Chem. 2007, 100, 1728–1733. [Google Scholar] [CrossRef]
- Mato, I.; Huidobro, J.F.; Simal-Lozano, J.; Sancho, M.T. Significance of Nonaromatic Organic Acids in Honey. J. Food Prot. 2003, 66, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. The Honey Book. Bee Prod. Sci. 2011, 23, 12. [Google Scholar]
- Anklam, E. A Review of the Analytical Methods to Determine the Geographical and Botanical Origin of Honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Cherchi, A.; Spanedda, L.; Tuberoso, C.; Cabras, P. Solid-Phase Extraction and High-Performance Liquid Chromatographic Determination of Organic Acids in Honey. J. Chromatogr. A 1994, 669, 59–64. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Venskutonis, P.R. Floral Markers in Honey of Various Botanical and Geographic Origins: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Keke, A.; Cinkmanis, I. Determination of Organic Acids in Honey Samples from Latvian Market by High-Performance Liquid Chromatography. Res. Rural. Dev. 2019, 1, 229–233. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Almeida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; Barros, F.A.R. de Kombuchas from Green and Black Teas Have Different Phenolic Profile, Which Impacts Their Antioxidant Capacities, Antibacterial and Antiproliferative Activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Khosravi, S.; Safari, M.; Emam-Djomeh, Z.; Golmakani, M.-T. Development of Fermented Date Syrup Using Kombucha Starter Culture. J. Food Process Preserv. 2019, 43, e13872. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbiological and Technological Parameters Impacting the Chemical Composition and Sensory Quality of Kombucha. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2050–2070. [Google Scholar] [CrossRef]
- Li, R.; Xu, Y.; Chen, J.; Wang, F.; Zou, C.; Yin, J. Enhancing the Proportion of Gluconic Acid with a Microbial Community Reconstruction Method to Improve the Taste Quality of Kombucha. LWT 2022, 155, 112937. [Google Scholar] [CrossRef]
- Tu, Y.; Xia, H. Antimicrobial Activity of Fermented Green Tea Liquid. Int. J. Tea Sci. 2008, 6, 1–7. [Google Scholar]
- Ye, M.; Yue, T.; Yuan, Y. Evolution of Polyphenols and Organic Acids during the Fermentation of Apple Cider. J. Sci. Food Agric. 2014, 94, 2951–2957. [Google Scholar] [CrossRef]
- Srihari, T.; Satyanarayana, U. Changes in Free Radical Scavenging Activity of Kombucha during Fermentation. J. Pharm. Sci. Res. 2012, 4, 1978–1981. [Google Scholar]
- Talebi, M.; Frink, L.A.; Patil, R.A.; Armstrong, D.W. Examination of the Varied and Changing Ethanol Content of Commercial Kombucha Products. Food Anal. Methods 2017, 10, 4062–4067. [Google Scholar] [CrossRef]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Harangozo, Ľ.; Kántor, A.; Kačániová, M. The Evaluation of Chemical, Antioxidant, Antimicrobial and Sensory Properties of Kombucha Tea Beverage. J. Food Sci. Technol. 2020, 57, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Doner, L.W. Honey. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 3125–3130. [Google Scholar]
- Ortiz, V.; Fernández, M.; Sabra, M. de la T. Principal Characteristics of Honey from La Alcarria [Spain]. Princ. Charact. Honey Alcarria [Spain] 1996, 1, 94. [Google Scholar]
- Lonǎr, E.; Djurić, M.; Malbaša, R.; Kolarov, L.J.; Klašnja, M. Influence of Working Conditions upon Kombucha Conducted Fermentation of Black Tea. Food Bioprod. Process. 2006, 84, 186–192. [Google Scholar] [CrossRef]
- Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Čanadanović-Brunet, J.M. Influence of Starter Cultures on the Antioxidant Activity of Kombucha Beverage. Food Chem. 2011, 127, 1727–1731. [Google Scholar] [CrossRef]
- Bauer-Petrovska, B.; Petrushevska-Tozi, L. Mineral and Water Soluble Vitamin Content in the Kombucha Drink. Int. J. Food Sci. Technol. 2000, 35, 201–205. [Google Scholar] [CrossRef]
- León-Ruiz, V.; Vera, S.; González-Porto, A.V.; San Andrés, M.P. Analysis of Water-Soluble Vitamins in Honey by Isocratic RP-HPLC. Food Anal. Methods 2013, 6, 488–496. [Google Scholar] [CrossRef]
- León-Ruiz, V.; Vera, S.; González-Porto, A.V.; Andrés, M.P.S. Vitamin C and Sugar Levels as Simple Markers for Discriminating Spanish Honey Sources. J. Food Sci. 2011, 76, C356–C361. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, N.; Wang, X.-H.; Engeseth, N.J. Identification and Quantification of Antioxidant Components of Honeys from Various Floral Sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Katsumata, N.; Hirano, M.; Yoshikawa, K.; Ogata, T.; Tanaka, T.; Nagamine, T. Determination of Biotin (Vitamin H) by the High-Performance Affinity Chromatography with a Trypsin-Treated Avidin-Bound Column. J. Chromatogr. B 2008, 869, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. A Comparative Study on Phenolic Profile, Vitamin C Content and Antioxidant Activity of Italian Honeys of Different Botanical Origin. Int. J. Food Sci. Technol. 2013, 48, 1899–1908. [Google Scholar] [CrossRef]
- Vassilevska-Ivanova, R.; Shtereva, L.; Stancheva, I.; Geneva, M.; Hristozkova, M. Determination of the Antioxidant Capacity of Sideritis scardica Specimens Collected at Different Regions in Bulgaria. Comptes Rendus L’académie Bulg. Sci. 2016, 69, 1307–1314. [Google Scholar]
- Dutton, G.J. Glucuronidation of Drugs and Other Compounds; Chapman and Hall/CRC: Boca Raton, FL, USA, 2019; ISBN 9780429264177. [Google Scholar]
- Candra, A.; Prasetyo, B.E.; Tarigan, J.B. Study of Vitamin C Level of Soursop Leaves (Annona muricata L.) and Galactomannan Utilization in Kombucha during Fermentation. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2021; p. 100007. [Google Scholar]
- Sengun, I.Y.; Karabiyikli, S. Importance of Acetic Acid Bacteria in Food Industry. Food Control 2011, 22, 647–656. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Kruk, M.; Ścibisz, I.; Zielińska, D. The Novel Strain of Gluconobacter oxydans H32 Isolated from Kombucha as a Proposition of a Starter Culture for Sour Ale Craft Beer Production. Appl. Sci. 2022, 12, 3047. [Google Scholar] [CrossRef]
- Prust, C.; Hoffmeister, M.; Liesegang, H.; Wiezer, A.; Fricke, W.F.; Ehrenreich, A.; Gottschalk, G.; Deppenmeier, U. Complete Genome Sequence of the Acetic Acid Bacterium Gluconobacter oxydans. Nat. Biotechnol. 2005, 23, 195–200. [Google Scholar] [CrossRef]
- Jang, K.-I.; Hyeon, G.L. Influence of Acetic Acid Solution on Heat Stability of L-Ascorbic Acid. Food Sci. Biotechnol. 2008, 17, 637–641. [Google Scholar]
- Rodriguez, M.; Sadler, G.D.; Sims, C.A.; Braddock, R.J. Chemical Changes during Storage of an Alcoholic Orange Juice Beverage. J. Food Sci. 1991, 56, 475–479. [Google Scholar] [CrossRef]
- Farah, H.S.; Alhmoud, J.F.; Al-Othman, A.; Alqaisi, K.M.; Atoom, A.M.; Shadid, K.; Shakya, A.; AlQaisi, T. Effect of pH, Temperature and Metal Salts in Different Storage Conditions on the Stability of Vitamin C Content of Yellow Bell Pepper Extracted in Aqueous Media. Syst. Rev. Pharm. 2020, 11, 661–667. [Google Scholar]
- van der Riet, W.B.; Wight, A.W.; Cilliers, J.J.L.; Datel, J.M. Food Chemical Analysis of Tempeh Prepared from South African-Grown Soybeans. Food Chem. 1987, 25, 197–206. [Google Scholar] [CrossRef]
- Kneifel, W.; Holub, S.; Wirthmann, M. Monitoring of B-Complex Vitamins in Yogurt during Fermentation. J. Dairy. Res. 1989, 56, 651–656. [Google Scholar] [CrossRef]
- Shahani, K.M.; Chandan, R.C. Nutritional and Healthful Aspects of Cultured and Culture-Containing Dairy Foods. J. Dairy. Sci. 1979, 62, 1685–1694. [Google Scholar] [CrossRef]
- Djurtoft, R.; Nielsen, J.P. Increase In Some B Vitamins, Including B12, during Fermentation of Tempeh, Produced from Cowpeas Or Soy Beans. J. Plant Foods 1983, 5, 135–141. [Google Scholar] [CrossRef]
- Aparna, A.R.; Rajalakshmi, D. Honey—Its Characteristics, Sensory Aspects, and Applications. Food Rev. Int. 1999, 15, 455–471. [Google Scholar] [CrossRef]
- El-Hawiet, A.; Elessawy, F.M.; El Demellawy, M.A.; El-Yazbi, A.F. Green Fast and Simple UPLC-ESI-MRM/MS Method for Determination of Trace Water-Soluble Vitamins in Honey: Greenness Assessment Using GAPI and Analytical Eco-Scale. Microchem. J. 2022, 181, 107625. [Google Scholar] [CrossRef]
- Farag, M.A.; Sheashea, M.; Zhao, C.; Maamoun, A.A. UV Fingerprinting Approaches for Quality Control Analyses of Food and Functional Food Coupled to Chemometrics: A Comprehensive Analysis of Novel Trends and Applications. Foods 2022, 11, 2867. [Google Scholar] [CrossRef]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of Honey in Modern Medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef]
- Taha, A.; Balabel, N.; Elshishtawy, H. Physicochemical Characterization and Antimicrobial Activity of Sidr Honey Produced by Dwarf Honey Bees (Apis florea F.). J. Plant Prot. Pathol. 2019, 10, 613–619. [Google Scholar] [CrossRef]
- Behrendt, I.; Schneider, I.; Schuchardt, J.P.; Bitterlich, N.; Hahn, A. Effect of an Herbal Extract of Sideritis scardica and B-Vitamins on Cognitive Performance under Stress: A Pilot Study. Int. J. Phytomed. 2016, 8, 95–103. [Google Scholar] [CrossRef]
- Jayabalan, R.; Subathradevi, P.; Marimuthu, S.; Sathishkumar, M.; Swaminathan, K. Changes in Free-Radical Scavenging Ability of Kombucha Tea during Fermentation. Food Chem. 2008, 109, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Godočíková, L.; Árvay, J.; Kačániová, M. Kombucha Tea Beverage: Microbiological Characteristic, Antioxidant Activity, and Phytochemical Composition. Acta Aliment. 2019, 48, 324–331. [Google Scholar] [CrossRef]
- Blanc, P.J. Characterization of the Tea Fungus Metabolites. Biotechnol. Lett. 1996, 18, 139–142. [Google Scholar] [CrossRef]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha Beverage from Green, Black and Rooibos Teas: A Comparative Study Looking at Microbiology, Chemistry and Antioxidant Activity. Nutrients 2019, 11, 1. [Google Scholar] [CrossRef]
- Rechner, A. Colonic Metabolism of Dietary Polyphenols: Influence of Structure on Microbial Fermentation Products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of Phenolic Compounds and Their Antioxidant Activity in Fruits and Cereals. Talanta 2007, 71, 1741–1751. [Google Scholar] [CrossRef]
- Gamboa-Gómez, C.I.; González-Laredo, R.F.; Gallegos-Infante, J.A.; Pérez, M.Ş.d.M.L.; Moreno-Jiménez, M.R.; Flores-Rueda, A.G.; Rocha-Guzmán, N.E. Antioxidant and Angiotensin-Converting Enzyme Inhibitory Activity of Eucalyptus Camaldulensis and Litsea Glaucescens Infusions Fermented with Kombucha Consortium. Food Technol. Biotechnol. 2016, 54, 367. [Google Scholar] [CrossRef]
- Barbosa, E.L.; Netto, M.C.; Bendel Junior, L.; de Moura, L.F.; Brasil, G.A.; Bertolazi, A.A.; de Lima, E.M.; Vasconcelos, C.M. Kombucha Fermentation in Blueberry (Vaccinium myrtillus) Beverage and Its in Vivo Gastroprotective Effect: Preliminary Study. Future Foods 2022, 5, 100129. [Google Scholar] [CrossRef]
- Morán Vieyra, F.E.; Boggetti, H.J.; Zampini, I.C.; Ordoñez, R.M.; Isla, M.I.; Alvarez, R.M.S.; De Rosso, V.; Mercadante, A.Z.; Borsarelli, C.D. Singlet Oxygen Quenching and Radical Scavenging Capacities of Structurally-Related Flavonoids Present in Zuccagnia punctata Cav. Free Radic. Res. 2009, 43, 553–564. [Google Scholar] [CrossRef]
- Faria, A.F.; Marques, M.C.; Mercadante, A.Z. Identification of Bioactive Compounds from Jambolão (Syzygium cumini) and Antioxidant Capacity Evaluation in Different PH Conditions. Food Chem. 2011, 126, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, H.; Weerakkody, N.S.; Waisundara, V.Y. Evaluation of Physicochemical Properties and Antioxidant Activities of Kombucha “Tea Fungus” during Extended Periods of Fermentation. Food Sci. Nutr. 2018, 6, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of Fermentation Conditions on the Production of Bioactive Compounds with Anticancer, Anti-Inflammatory and Antioxidant Properties in Kombucha Tea Extracts. Process Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef]
- Lemańska, K.; Szymusiak, H.; Tyrakowska, B.; Zieliński, R.; Soffers, A.E.M.F.; Rietjens, I.M.C.M. The Influence of PH on Antioxidant Properties and the Mechanism of Antioxidant Action of Hydroxyflavones. Free Radic. Biol. Med. 2001, 31, 869–881. [Google Scholar] [CrossRef]
- Tyrakowska, B.; Soffers, A.E.M.F.; Szymusiak, H.; Boeren, S.; Boersma, M.G.; Lemańska, K.; Vervoort, J.; Rietjens, I.M.C.M. TEAC Antioxidant Activity of 4-Hydroxybenzoates. Free Radic. Biol. Med. 1999, 27, 1427–1436. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; Geraci, C. Electron-Transfer Reaction of Cinnamic Acids and Their Methyl Esters with the DPPH • Radical in Alcoholic Solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Lee, W.K.; Wong, L.L.; Loo, Y.Y.; Kasapis, S.; Huang, D. Evaluation of Different Teas against Starch Digestibility by Mammalian Glycosidases. J. Agric. Food Chem. 2010, 58, 148–154. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. Enhancement of the Functional Properties of Coffee Through Fermentation by “Tea Fungus” (Kombucha). J. Food Process Preserv. 2015, 39, 2596–2603. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. Value-Added Tea (Camellia sinesis) as a Functional Food Using the Kombucha ‘Tea Fungus’. Warasan Khana Witthayasat Maha Witthayalai Chiang Mai 2018, 45, 136–146. [Google Scholar]
- Bei, Q.; Chen, G.; Liu, Y.; Zhang, Y.; Wu, Z. Improving Phenolic Compositions and Bioactivity of Oats by Enzymatic Hydrolysis and Microbial Fermentation. J. Funct. Foods 2018, 47, 512–520. [Google Scholar] [CrossRef]
- Custódio, L.; Patarra, J.; Alberício, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. In Vitro Antioxidant and Inhibitory Activity of Water Decoctions of Carob Tree (Ceratonia siliqua L.) on Cholinesterases, α-Amylase and α-Glucosidase. Nat. Prod. Res. 2015, 29, 2155–2159. [Google Scholar] [CrossRef] [PubMed]
- Mata, R.; Cristians, S.; Escandón-Rivera, S.; Juárez-Reyes, K.; Rivero-Cruz, I. Mexican Antidiabetic Herbs: Valuable Sources of Inhibitors of α-Glucosidases. J. Nat. Prod. 2013, 76, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Nagappan, H.; Pee, P.P.; Kee, S.H.Y.; Ow, J.T.; Yan, S.W.; Chew, L.Y.; Kong, K.W. Malaysian Brown Seaweeds Sargassum Siliquosum and Sargassum Polycystum: Low Density Lipoprotein (LDL) Oxidation, Angiotensin Converting Enzyme (ACE), α-Amylase, and α-Glucosidase Inhibition Activities. Food Res. Int. 2017, 99, 950–958. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and Hydrophilic Antioxidant Capacities of Common Foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Rahmani, R.; Beaufort, S.; Villarreal-Soto, S.A.; Taillandier, P.; Bouajila, J.; Debouba, M. Kombucha Fermentation of African Mustard (Brassica tournefortii) Leaves: Chemical Composition and Bioactivity. Food Biosci. 2019, 30, 100414. [Google Scholar] [CrossRef]
- Deveci, E.; Tel-Çayan, G.; Duru, M.E. Essential Oil Composition, Antioxidant, Anticholinesterase and Anti-Tyrosinase Activities of Two Turkish Plant Species: Ferula Elaeochytris and Sideritis Stricta. Nat. Prod. Commun. 2018, 13, 1934578X1801300130. [Google Scholar] [CrossRef]
- Tel-Çayan, G.; Yildirim, H.; Duru, M.E.; Deveci, E. Chemical Composition, Antioxidant, Anticholinesterase and Anti-Urease Activities of Sideritis pisidica Boiss. Heldr. Endemic to Turkey. Marmara Pharm. J. 2017, 21, 898–905. [Google Scholar] [CrossRef]
- Deveci, E.; Tel-Çayan, G.; Usluer, Ö.; Emin Duru, M. Chemical Composition, Antioxidant, Anticholinesterase and Anti-Tyrosinase Activities of Essential Oils of Two Sideritis Species from Turkey. Iran. J. Pharm. Res. 2019, 18, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R. Sideritis galatica Bornm: A Source of Multifunctional Agents for the Management of Oxidative Damage, Alzheimer’s’s and Diabetes Mellitus. J. Funct. Foods 2014, 11, 538–547. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E.; Winiarska-Mieczan, A.; Gajowniczek-Ałasa, D. Honeys as Possible Sources of Cholinesterase Inhibitors. Nutrients 2022, 14, 2969. [Google Scholar] [CrossRef] [PubMed]
- Amessis-Ouchemoukh, N.; Madani, K.; Falé, P.L.V.; Serralheiro, M.L.; Araújo, M.E.M. Antioxidant Capacity and Phenolic Contents of Some Mediterranean Medicinal Plants and Their Potential Role in the Inhibition of Cyclooxygenase-1 and Acetylcholinesterase Activities. Ind. Crops Prod. 2014, 53, 6–15. [Google Scholar] [CrossRef]
- Papandreou, M.A.; Dimakopoulou, A.; Linardaki, Z.I.; Cordopatis, P.; Klimis-Zacas, D.; Margarity, M.; Lamari, F.N. Effect of a Polyphenol-Rich Wild Blueberry Extract on Cognitive Performance of Mice, Brain Antioxidant Markers and Acetylcholinesterase Activity. Behav. Brain Res. 2009, 198, 352–358. [Google Scholar] [CrossRef]
- Ali Reza, A.S.M.; Hossain, M.S.; Akhter, S.; Rahman, M.R.; Nasrin, M.S.; Uddin, M.J.; Sadik, G.; Khurshid Alam, A.H.M. In Vitro Antioxidant and Cholinesterase Inhibitory Activities of Elatostema papillosum Leaves and Correlation with Their Phytochemical Profiles: A Study Relevant to the Treatment of Alzheimer’s Disease. BMC Complement. Altern. Med. 2018, 18, 123. [Google Scholar] [CrossRef]
- Lim, Y.J.; Oh, C.-S.; Park, Y.-D.; Eom, S.H.; Kim, D.-O.; Kim, U.-J.; Cho, Y.-S. Physiological Components of Kiwifruits with in Vitro Antioxidant and Acetylcholinesterase Inhibitory Activities. Food Sci. Biotechnol. 2014, 23, 943–949. [Google Scholar] [CrossRef]
- Philip, Y.; Fadzelly, A.B.M. Antioxidative and Acetylcholinesterase Inhibitor Potential of Selected Honey of Sabah, Malaysian Borneo. Int. Food Res. J. 2015, 22, 1953. [Google Scholar] [CrossRef]
- Zaidi, H.; Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Debbache, N.; Pacheco, R.; Serralheiro, M.L.; Araujo, M.E. Biological Properties of Phenolic Compound Extracts in Selected Algerian Honeys—The Inhibition of Acetylcholinesterase and α-Glucosidase Activities. Eur. J. Integr. Med. 2019, 25, 77–84. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Winiarska-Mieczan, A. Honey as the Potential Natural Source of Cholinesterase Inhibitors in Alzheimer’s Disease. Plant Foods Human. Nutr. 2020, 75, 30–32. [Google Scholar] [CrossRef] [PubMed]




| A0 | A4 | B4 | C4 | |
|---|---|---|---|---|
| L* | 60.6 ± 0.59 a,1 | 66.1 ± 0.51 b | 66.4 ± 0.49 b | 67.1 ± 0.53 b |
| a* | 0.4 ± 0.02 c | −4.1 ± 0.24 d | −4.3± 0.25 d | −3.8 ± 0.21 d |
| b* | 29.3 ± 0.64 e | 22.3 ± 0.53 f | 22.4 ± 0.56 f | 21.4 ± 0.61 f |
| ΔΕ* | - | 10.0 ± 0.1 g | 10.2 ± 0.1 g | 11.1 ± 0.1 g |
| Samples | Vitamins (μg/mL) 1 | ||||
|---|---|---|---|---|---|
| B1 | B6 | B12 | B2 | B7 | |
| A0 | 0.15 ± 0.01 a | 0.59 ± 0.02 a | 1.78 ± 0.04 a | 4.37 ± 0.09 a | 2.79 ± 0.09 a |
| A4 | 1.74 ± 0.04 b | 2.71 ± 0.08 b | 2.18 ± 0.06 b | 5.47 ± 0.16 b | 3.42 ± 0.11 b |
| B4 | 2.64 ± 0.08 c | 3.12 ± 0.09 c | 3.30 ± 0.08 c | 5.39 ± 0.13 b | 3.27 ± 0.10 b |
| C4 | 2.59 ± 0.06 c | 2.99 ± 0.09 c | 3.46 ± 0.10 c | 5.34 ± 0.12 b | 3.16 ± 0.12 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geraris Kartelias, I.; Karantonis, H.C.; Giaouris, E.; Panagiotakopoulos, I.; Nasopoulou, C. Kombucha Fermentation of Olympus Mountain Tea (Sideritis scardica) Sweetened with Thyme Honey: Physicochemical Analysis and Evaluation of Functional Properties. Foods 2023, 12, 3496. https://doi.org/10.3390/foods12183496
Geraris Kartelias I, Karantonis HC, Giaouris E, Panagiotakopoulos I, Nasopoulou C. Kombucha Fermentation of Olympus Mountain Tea (Sideritis scardica) Sweetened with Thyme Honey: Physicochemical Analysis and Evaluation of Functional Properties. Foods. 2023; 12(18):3496. https://doi.org/10.3390/foods12183496
Chicago/Turabian StyleGeraris Kartelias, Ioannis, Haralabos Christos Karantonis, Efstathios Giaouris, Ioannis Panagiotakopoulos, and Constantina Nasopoulou. 2023. "Kombucha Fermentation of Olympus Mountain Tea (Sideritis scardica) Sweetened with Thyme Honey: Physicochemical Analysis and Evaluation of Functional Properties" Foods 12, no. 18: 3496. https://doi.org/10.3390/foods12183496
APA StyleGeraris Kartelias, I., Karantonis, H. C., Giaouris, E., Panagiotakopoulos, I., & Nasopoulou, C. (2023). Kombucha Fermentation of Olympus Mountain Tea (Sideritis scardica) Sweetened with Thyme Honey: Physicochemical Analysis and Evaluation of Functional Properties. Foods, 12(18), 3496. https://doi.org/10.3390/foods12183496








