Analyzing Active Compounds in Elateriospermum tapos Yogurt for Maternal Obesity: A Network Pharmacology and Molecular Docking Study
Abstract
:1. Introduction
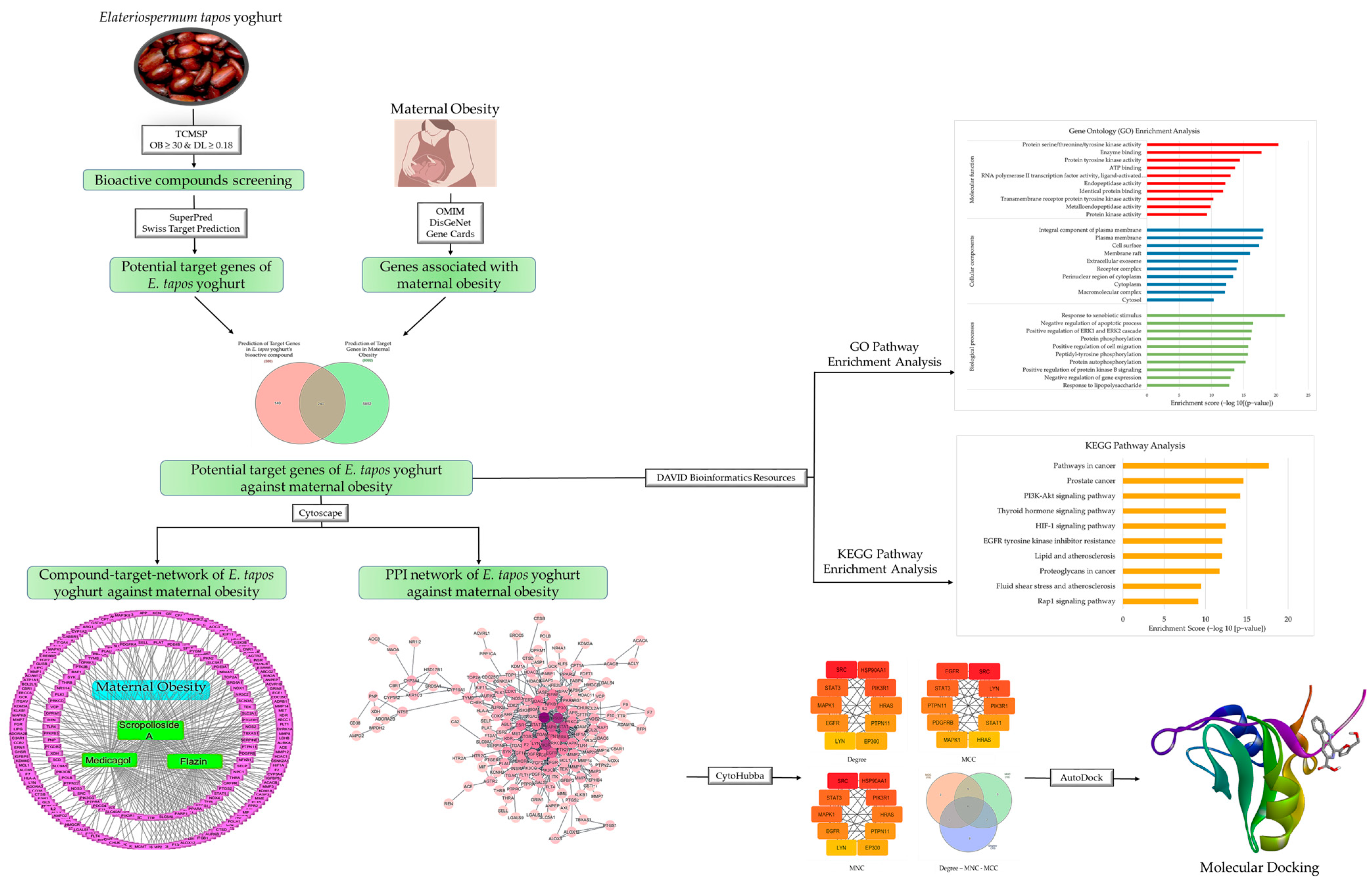
2. Materials and Methods
2.1. Screening of Bioactive Compounds of E. tapos Yogurt
2.2. Prediction of Target Genes in E. tapos Yogurt’s Bioactive Compounds
2.3. Targets of Disease-Related Compounds
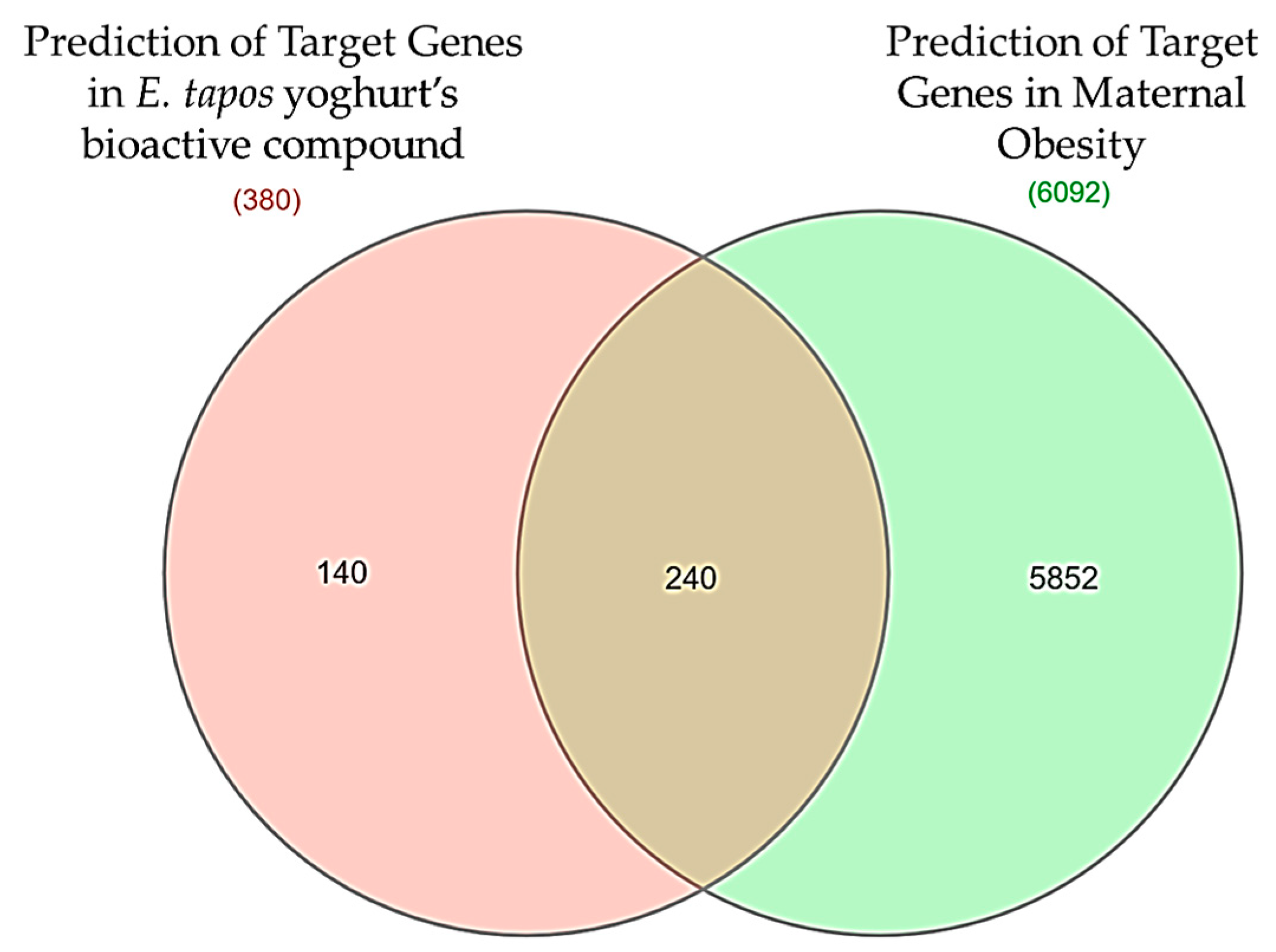
2.4. Construction of Venn Diagrams
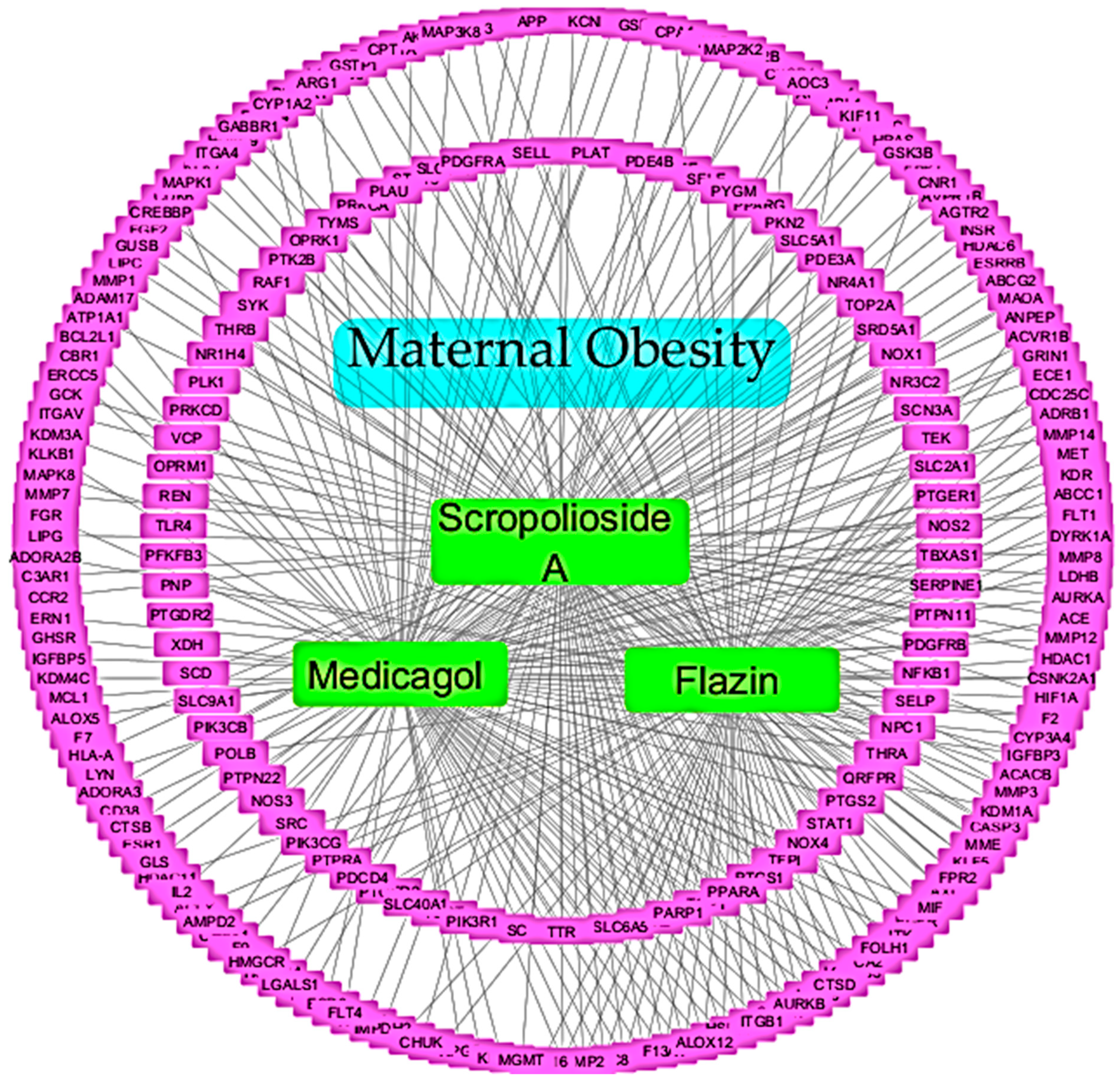
2.5. Construction of Compound–Pathway–Target Network
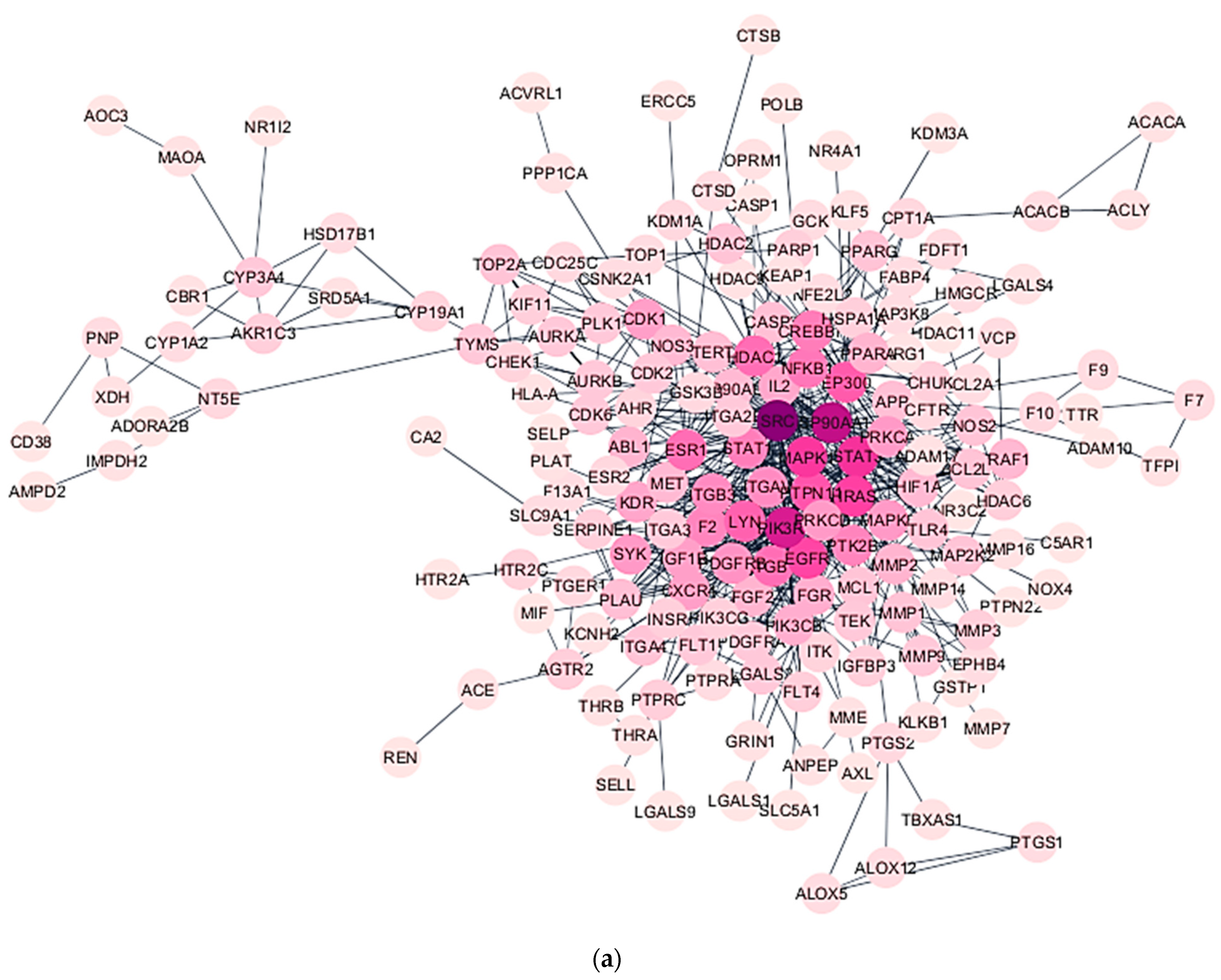
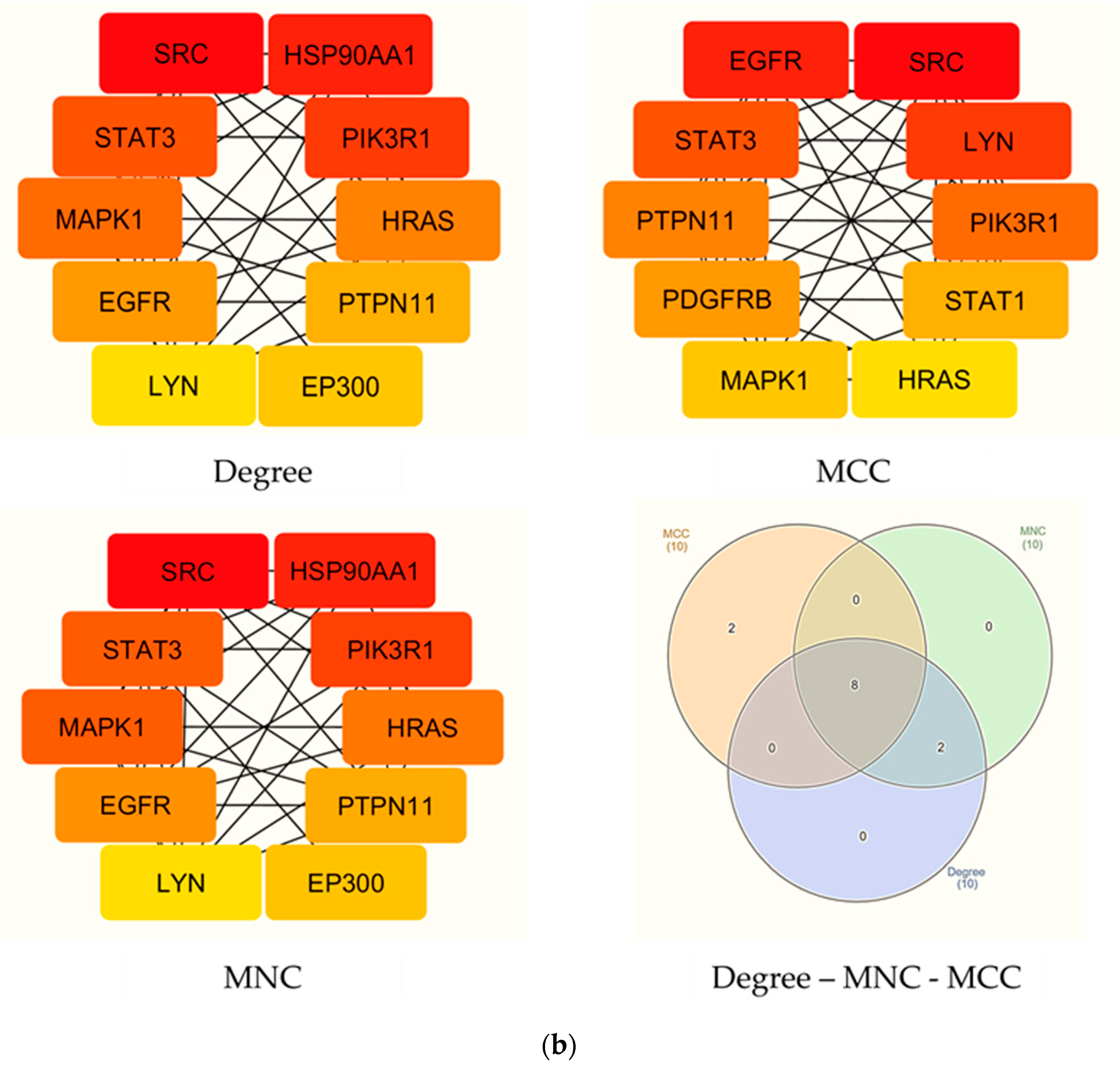
2.6. Construction of Protein–Protein Interaction (PPI) Network
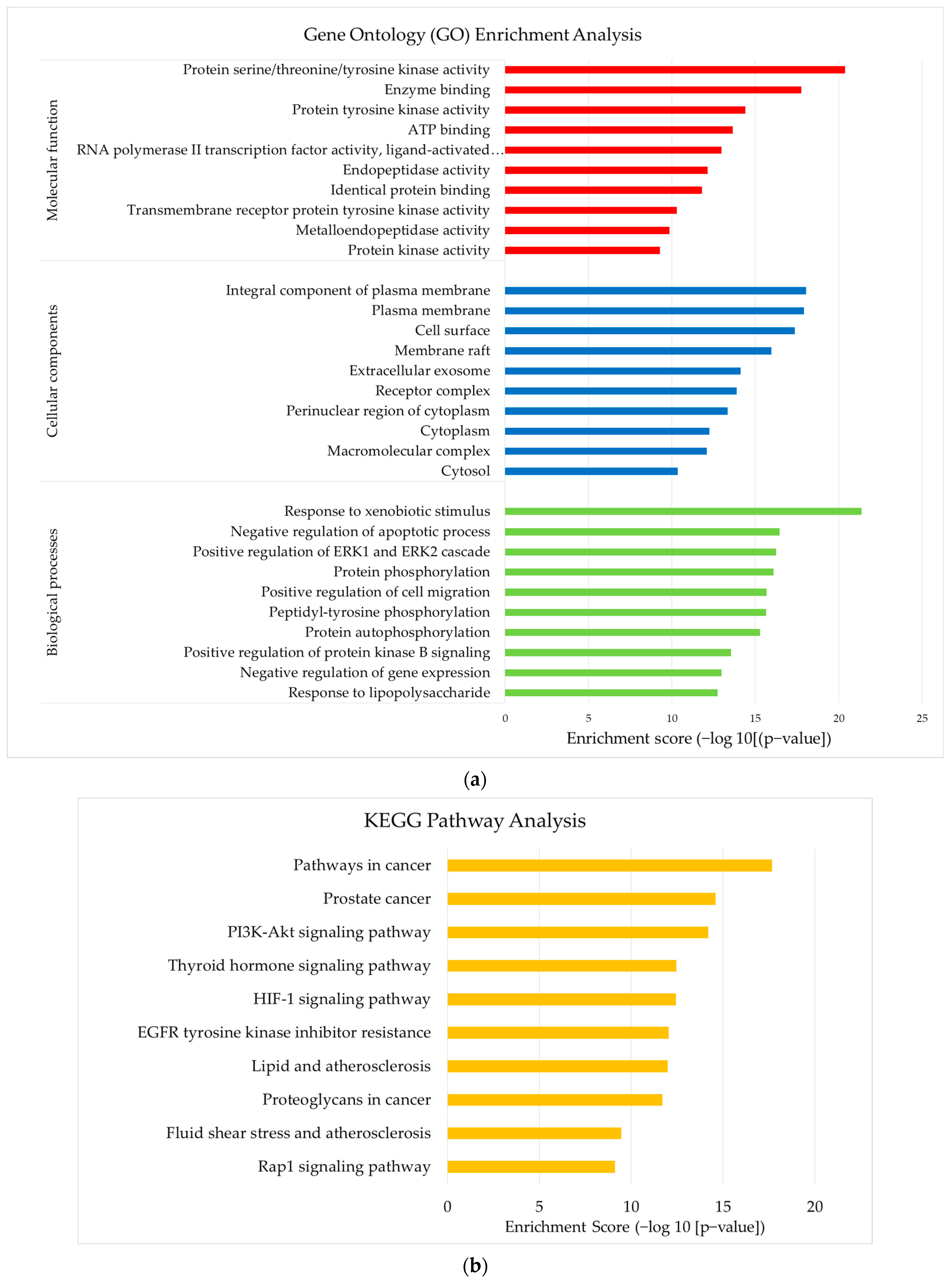
2.7. Construction of Gene Ontology and Pathway Enrichment
2.8. Molecular Docking
3. Results
3.1. Selection of Potential Bioactive Compounds
3.2. Target Gene Prediction of E. tapos Yogurt against Maternal Obesity
3.3. Construction of Compound–Pathway–Target Network
3.4. Construction of PPI Network
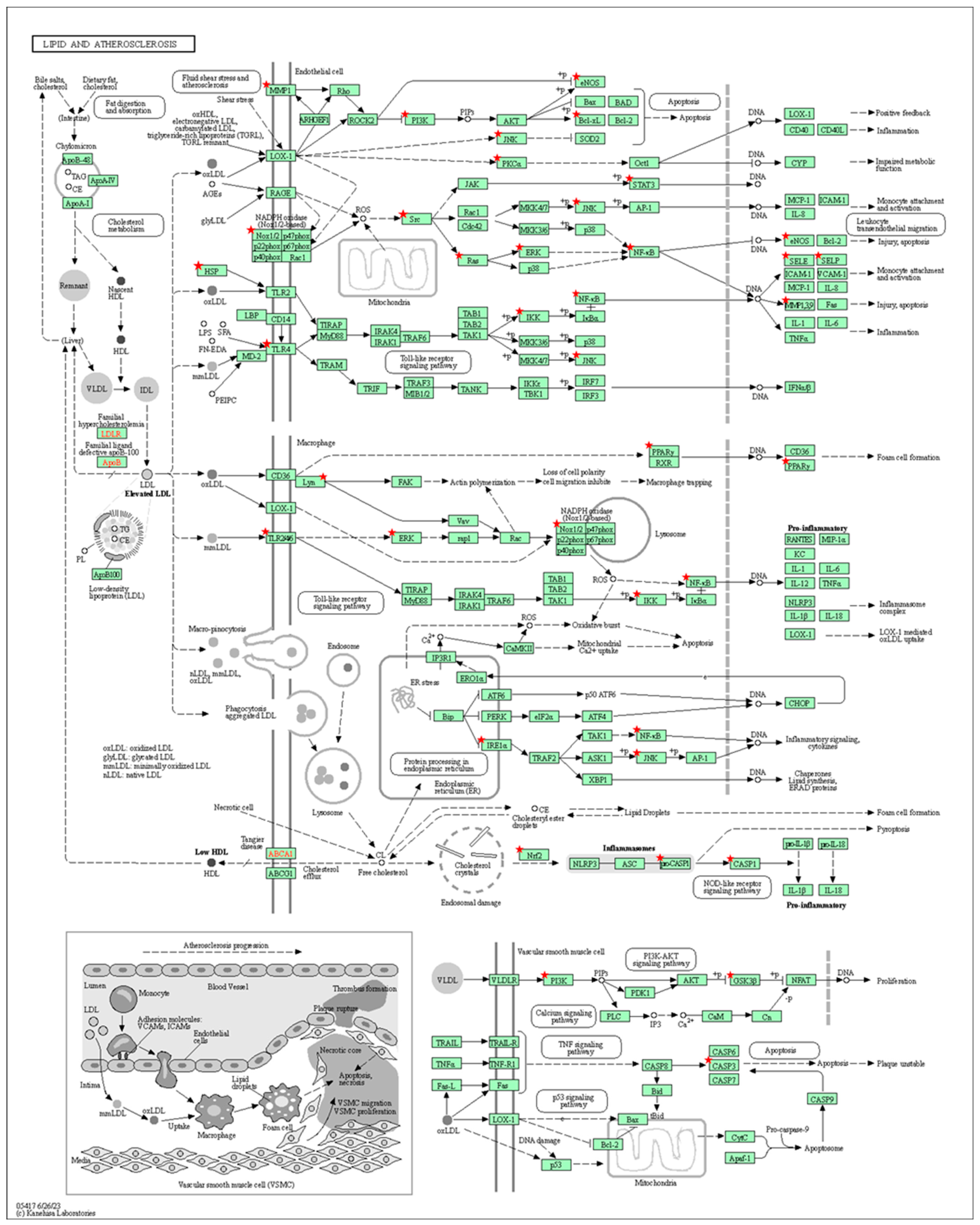
3.5. Construction of GO and KEGG Pathway
3.6. Molecular Docking
4. Discussion
5. Uses of Selected Bioactive Compounds
6. Conclusions
7. Advantages and Constraints of Ligands with Weak-Binding Affinity
8. Merits and Limitations of Similar Computation Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kong, L.; Norstedt, G.; Schalling, M.; Gissler, M.; Lavebratt, C. The risk of offspring psychiatric disorders in the setting of maternal obesity and diabetes. Pediatrics 2018, 142, e20180776. [Google Scholar] [CrossRef] [PubMed]
- King, S.E.; Skinner, M.K. Epigenetic Transgenerational Inheritance of Obesity Susceptibility. Trends Endocrinol. Metab. 2020, 31, 494. [Google Scholar] [CrossRef] [PubMed]
- Stirrat, L.I.; Reynolds, R.M. Effects of maternal obesity on early and long-term outcomes for offspring. Res. Rep. Neonatol. 2014, 4, 43–53. [Google Scholar] [CrossRef]
- Şanlı, E.; Kabaran, S. Maternal Obesity, Maternal Overnutrition and Fetal Programming: Effects of Epigenetic Mechanisms on the Development of Metabolic Disorders. Curr. Genom. 2019, 20, 427. [Google Scholar] [CrossRef] [PubMed]
- Slack, E.; Brandon, H.; Heslehurst, N. Obesity and Pregnancy. In Practical Guide to Obesity Medicine; Weaver, J.U., Ed.; Elsevier: Newcastle upon Tyne, UK, 2018; pp. 143–151. ISBN 978-0-323-48559-3. [Google Scholar]
- Shahrir, N.F.; Jalil, R.A.; Jeganathan, J.R.R.; Karalasingam, S.D.; Nordin, N.M.; Abdullah, M.F.; Sa’at, N. Maternal Obesity and Its Associated Factors and Outcomes in Klang Valley, Malaysia: Findings from National Obstetric Registry. Malays. Fam. Physician 2021, 16, 56–67. [Google Scholar] [CrossRef]
- Nurul-Farehah, S.; Rohana, A.J. Maternal obesity and its determinants: A neglected issue? Malays. Fam. Physician Off. J. Acad. Fam. Physicians Malays. 2020, 15, 42. [Google Scholar]
- Jones, K.L.; Johnson, K.A.; Dick, L.M.; Felix, R.J.; Kao, K.K.; Chambers, C.D. Pregnancy outcomes after first trimester exposure to phentermine/fenfluramine. Teratology 2002, 65, 125–130. [Google Scholar] [CrossRef]
- Ballinger, A.; Peikin, S.R. Orlistat: Its current status as an anti-obesity drug. Eur. J. Pharmacol. 2002, 440, 109–117. [Google Scholar] [CrossRef]
- Naomi, R.; Rusli, R.N.M.; Huat, T.S.; Embong, H.; Bahari, H.; Kamaruzzaman, M.A. Early Intervention of Elateriospermum tapos Yoghurt in Obese Dams Mitigates Intergenerational Cognitive Deficits and Thigmotactic Behaviour in Male Offspring via the Modulation of Metabolic Profile. Nutrients 2023, 15, 1523. [Google Scholar] [CrossRef]
- Naomi, R.; Nabila, R.; Rusli, M.; Othman, F.; Segaran Balan, S.; Abidin, A.Z.; Embong, H.; Teoh, S.H.; Jasni, A.S.; Jumidil, S.H.; et al. Elateriospermum tapos Yogurt Supplement in Maternal Obese Dams during Pregnancy Modulates the Body Composition of F1 Generation. Nutrients 2023, 15, 1258. [Google Scholar] [CrossRef]
- Liu, J.; Gong, J.; Xu, J.; Fang, M.; Su, M.; Li, W.; Wu, Y.; Hui, Y.; He, Y. Integrated Network Pharmacology and Proteomic Analyses of Targets and Mechanisms of Jianpi Tianjing Decoction in Treating Vascular Dementia. Evid. Based Complement. Altern. Med. 2023, 2023, 9021546. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Teoh, S.H.; Nabila, R.; Rusli, M.; Embong, H.; Bahari, H.; Kumar, J. Elateriospermum tapos Yoghurt as a Therapeutic Intervention for Obesity-Associated Cognitive Impairments and Anxiety-like Behaviour in a High Fat Diet Maternal Obese Rat Model. Nutrients 2023, 15, 2312. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Rusli, R.N.M.; Othman, F.; Balan, S.S.; Abidin, A.Z.; Embong, H.; Teoh, S.H.; Jasni, A.S.; Jumidil, S.H.; Bahari, H.; et al. The Role of Elateriospermum tapos yogurt in mitigating high fat dietary cause of maternal obesity -An experimental study. Front. Endocrinol. 2023, 14, 1131830. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Zhou, W.; Wang, Y.; Yang, L. Systems approaches and polypharmacology for drug discovery from herbal medicines: An example using licorice. J. Ethnopharmacol. 2013, 146, 773–793. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W3664. [Google Scholar] [CrossRef]
- Nickel, J.; Gohlke, B.O.; Erehman, J.; Banerjee, P.; Rong, W.W.; Goede, A.; Dunkel, M.; Preissner, R. SuperPred: Update on drug classification and target prediction. Nucleic Acids Res. 2014, 42, 26–31. [Google Scholar] [CrossRef]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards Version 3: The human gene integrator. Database 2010, 2010, baq020. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef]
- Piñero, J.; Queralt-Rosinach, N.; Bravo, À.; Deu-Pons, J.; Bauer-Mehren, A.; Baron, M.; Sanz, F.; Furlong, L.I. DisGeNET: A discovery platform for the dynamical exploration of human diseases and their genes. Database 2015, 2015, bav028. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, V.G.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2008, 4, 44–57. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Menting, M.D.; van de Beek, C.; Mintjens, S.; Wever, K.E.; Korosi, A.; Ozanne, S.E.; Limpens, J.; Roseboom, T.J.; Hooijmans, C.; Painter, R.C. The link between maternal obesity and offspring neurobehavior: A systematic review of animal experiments. Neurosci. Biobehav. Rev. 2018, 98, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Widen, E.M.; Nichols, A.R.; Kahn, L.G.; Factor-Litvak, P.; Insel, B.J.; Hoepner, L.; Dube, S.M.; Rauh, V.; Perera, F.; Rundle, A. Prepregnancy obesity is associated with cognitive outcomes in boys in a low-income, multiethnic birth cohort. BMC Pediatr. 2019, 19, 507. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M. An Overview of Epigenetics in Obesity: The Role of Lifestyle and Therapeutic Interventions. Int. J. Mol. Sci. 2022, 23, 1341. [Google Scholar] [CrossRef]
- Henning, R.J. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: A review of the pathophysiology and treatment of obesity. Am. J. Cardiovasc. Dis. 2021, 11, 504–529. [Google Scholar]
- Rhee, K.E.; Phelan, S.; Mccaffery, J. Early Determinants of Obesity: Genetic, Epigenetic, and In Utero Influences. Int. J. Pediatr. 2012, 2012, 463850. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Liu, Q. Brain cell type-specific cholesterol metabolism and implications for learning and memory. Trends Neurosci. 2022, 45, 401–414. [Google Scholar] [CrossRef]
- Beretta, M.; Bauer, M.; Hirsch, E. PI3K signaling in the pathogenesis of obesity: The cause and the cure. Adv. Biol. Regul. 2014, 58, 1–15. [Google Scholar] [CrossRef]
- Warbrick, I.; Rabkin, S.W. Hypoxia-inducible factor 1-alpha (HIF-1α) as a factor mediating the relationship between obesity and heart failure with preserved ejection fraction. Obes. Rev. 2019, 20, 701–712. [Google Scholar] [CrossRef]
- Cao, S.; Pan, Y.; Tang, J.; Terker, A.S.; Ornelas, J.P.A.; Jin, G.; Wang, Y.; Niu, A.; Fan, X.; Wang, S.; et al. EGFR-mediated activation of adipose tissue macrophages promotes obesity and insulin resistance. Nat. Commun. 2022, 13, 1–16. [Google Scholar] [CrossRef]
- Fuda, H.; Miyanaga, S.; Furukawa, T.; Umetsu, S.; Joko, S.; Roan, Y.; Suzuki, H.; Hui, S.P.; Watanabe, M.; Chiba, H. Flazin as a Promising Nrf2 Pathway Activator. J. Agric. Food Chem. 2019, 67, 12844–12853. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, Z.; Wu, Y.; Chen, Y.; Jia, J.; Shen, N.; Chiba, H.; Hui, S.P. Flazin as a Lipid Droplet Regulator against Lipid Disorders. Nutrients 2022, 14, 1501. [Google Scholar] [CrossRef] [PubMed]
- TheBiotek Medicagol. Available online: https://www.thebiotek.com/product/bt-334176 (accessed on 28 August 2023).
- Bas, E.; Recio, M.C.; Máñez, S.; Giner, R.M.; Escandell, J.M.; López-Ginés, C.; Ríos, J.L. New insight into the inhibition of the inflammatory response to experimental delayed-type hypersensitivity reactions in mice by scropolioside A. Eur. J. Pharmacol. 2006, 555, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, P.L.; Bonvin, A.M.J.J. On the binding affinity of macromolecular interactions: Daring to ask why proteins interact. J. R. Soc. Interface 2012, 10, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Huggins, D.J.; Sherman, W.; Tidor, B. Rational approaches to improving selectivity in drug design. J. Med. Chem. 2012, 55, 1424–1444. [Google Scholar] [CrossRef]
- Torres, P.H.M.; Sodero, A.C.R.; Jofily, P.; Silva-Jr, F.P. Key topics in molecular docking for drug design. Int. J. Mol. Sci. 2019, 20, 4574. [Google Scholar] [CrossRef]
- Talevi, A. Multi-target pharmacology: Possibilities and limitations of the “skeleton key approach” from a medicinal chemist perspective. Front. Pharmacol. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Chandran, U.; Mehendale, N.; Patil, S.; Chaguturu, R.; Patwardhan, B. Network Pharmacology. In Innovative Approaches in Drug Discovery: Ethnopharmacology, Systems Biology and Holistic Targeting; Elsevier Inc.: Pune, India, 2017; pp. 127–164. ISBN 9780128018224. [Google Scholar]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure Based Drug Discovery. Curr. Comput. Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef]
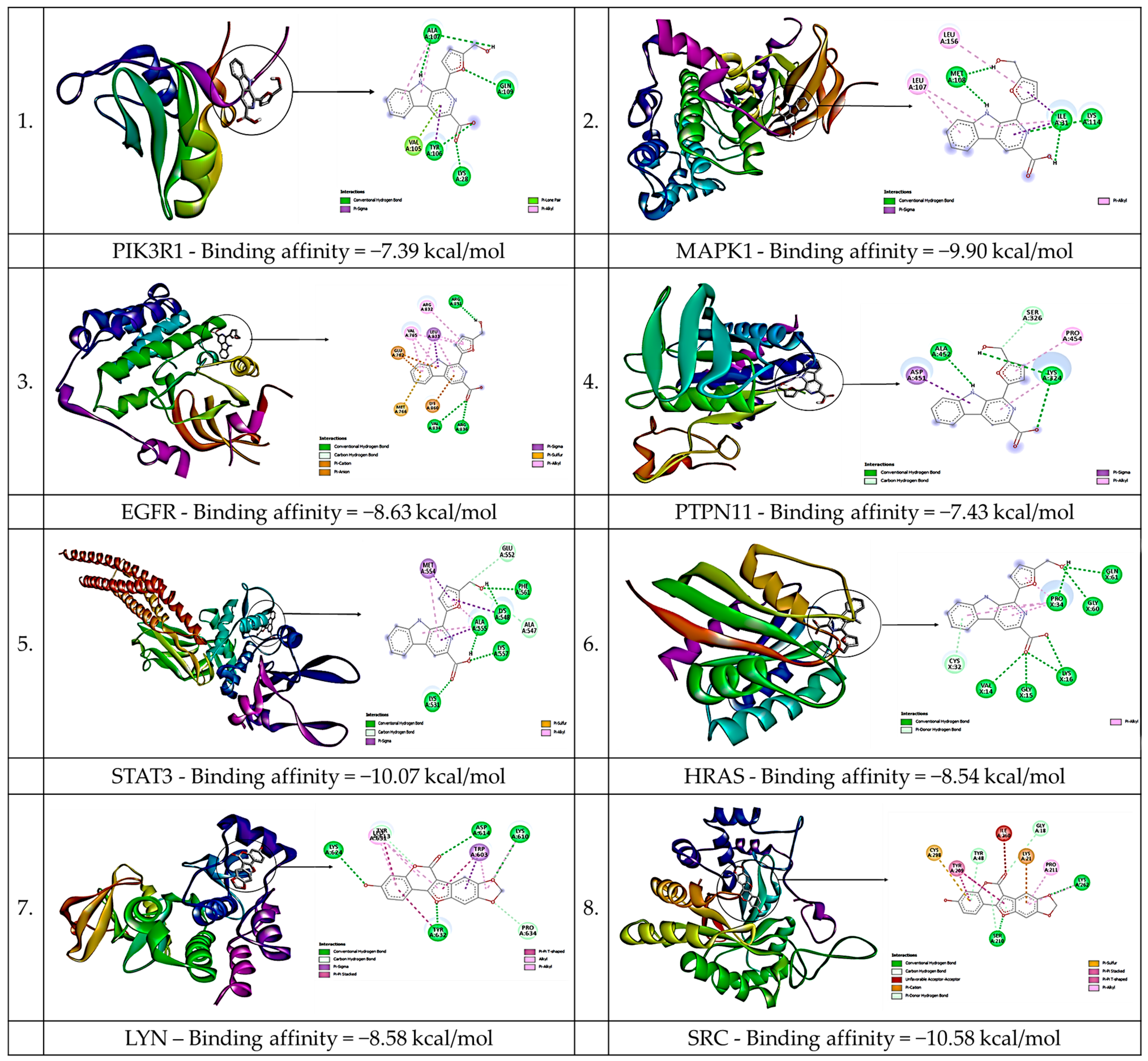
| Hub Gene | PDB ID |
|---|---|
| PIK3R1 | 1BFJ |
| MAPK1 | 2Y9Q |
| LYN | 3SXS |
| SRC | 3BCJ |
| EGFR | 3POZ |
| PTPN11 | 3O5X |
| STAT3 | 6NJS |
| HRAS | 2CE2 |
| Compound | Formula | Oral Bioavailability (OB) ≥ 30% | Drug Likeness (DL) ≥ 0.18 | |
|---|---|---|---|---|
| 1 | Scropolioside A | C35H44O18 | 38.63 | 0.77 |
| 2 | Flazin | C17H12N2O4 | 94.28 | 0.39 |
| 3 | Medicagol | C16H8O6 | 57.49 | 0.60 |
| Binding Energy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compounds | PIK3R1 | MAPK1 | LYN | SRC | EGFR | PTPN11 | STAT3 | HRAS |
| Flazin | −7.39 | −9.90 | −7.78 | −10.4 | −8.63 | −7.43 | −10.1 | −8.54 |
| Medicagol | −6.86 | −7.85 | −8.58 | −10.6 | −8.07 | −7.32 | −7.15 | −7.87 |
| Scropolioside A | −3.54 | −5.05 | −4.51 | −8.70 | −5.59 | −4.37 | −3.22 | −4.43 |
| Compound | Uses |
|---|---|
| Flazin [43] | -Lipid Droplet Regulator -Reduce triglyceride levels -Upregulates mRNA expression in adipose triglyceride lipase -Suppressive effect on lipogenesis |
| Medicagol [44] | -Induce apoptosis -Modulate the activity of various enzymes and transcription factors involved in cell signaling pathways |
| Scropolioside A [45] | -Anti-inflammatory |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naomi, R.; Teoh, S.H.; Embong, H.; Balan, S.S.; Othman, F.; Mamat-Hamidi, K.; Bahari, H.; Yazid, M.D. Analyzing Active Compounds in Elateriospermum tapos Yogurt for Maternal Obesity: A Network Pharmacology and Molecular Docking Study. Foods 2023, 12, 3575. https://doi.org/10.3390/foods12193575
Naomi R, Teoh SH, Embong H, Balan SS, Othman F, Mamat-Hamidi K, Bahari H, Yazid MD. Analyzing Active Compounds in Elateriospermum tapos Yogurt for Maternal Obesity: A Network Pharmacology and Molecular Docking Study. Foods. 2023; 12(19):3575. https://doi.org/10.3390/foods12193575
Chicago/Turabian StyleNaomi, Ruth, Soo Huat Teoh, Hashim Embong, Santhra Segaran Balan, Fezah Othman, Kamalludin Mamat-Hamidi, Hasnah Bahari, and Muhammad Dain Yazid. 2023. "Analyzing Active Compounds in Elateriospermum tapos Yogurt for Maternal Obesity: A Network Pharmacology and Molecular Docking Study" Foods 12, no. 19: 3575. https://doi.org/10.3390/foods12193575








