Fate of Planktonic and Biofilm-Derived Listeria monocytogenes on Unwaxed Apples during Air and Controlled Atmosphere Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apples and Storage Conditions
2.2. Bacterial Strains and Growth Conditions
2.3. Preparation of Planktonic and Biofilm-Derived L. monocytogenes Inoculum
2.4. Apple Inoculation and Storage
2.5. Sampling and L. monocytogenes Enumeration
2.6. Statistical Analysis
3. Results
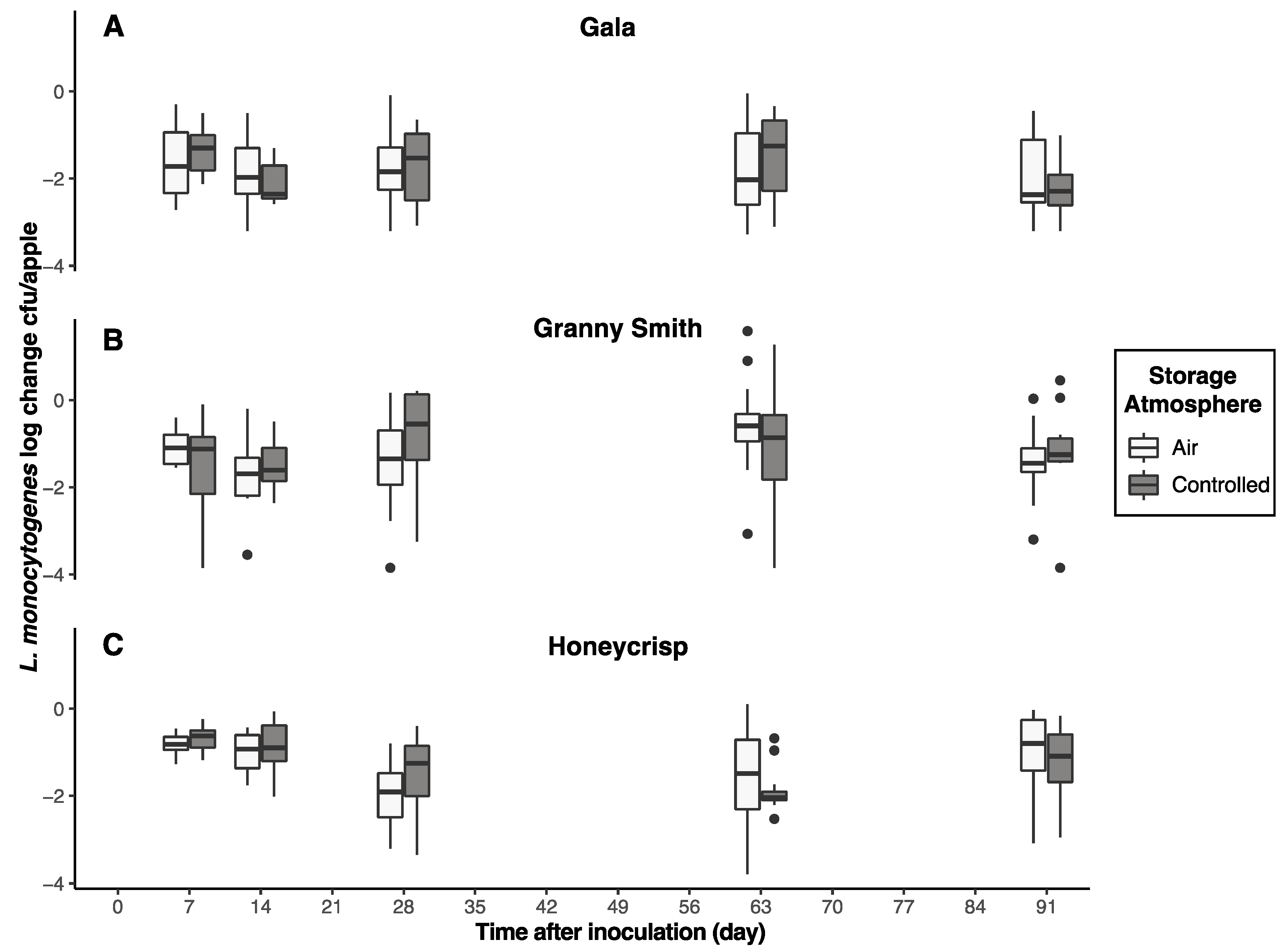
3.1. Storage Atmosphere
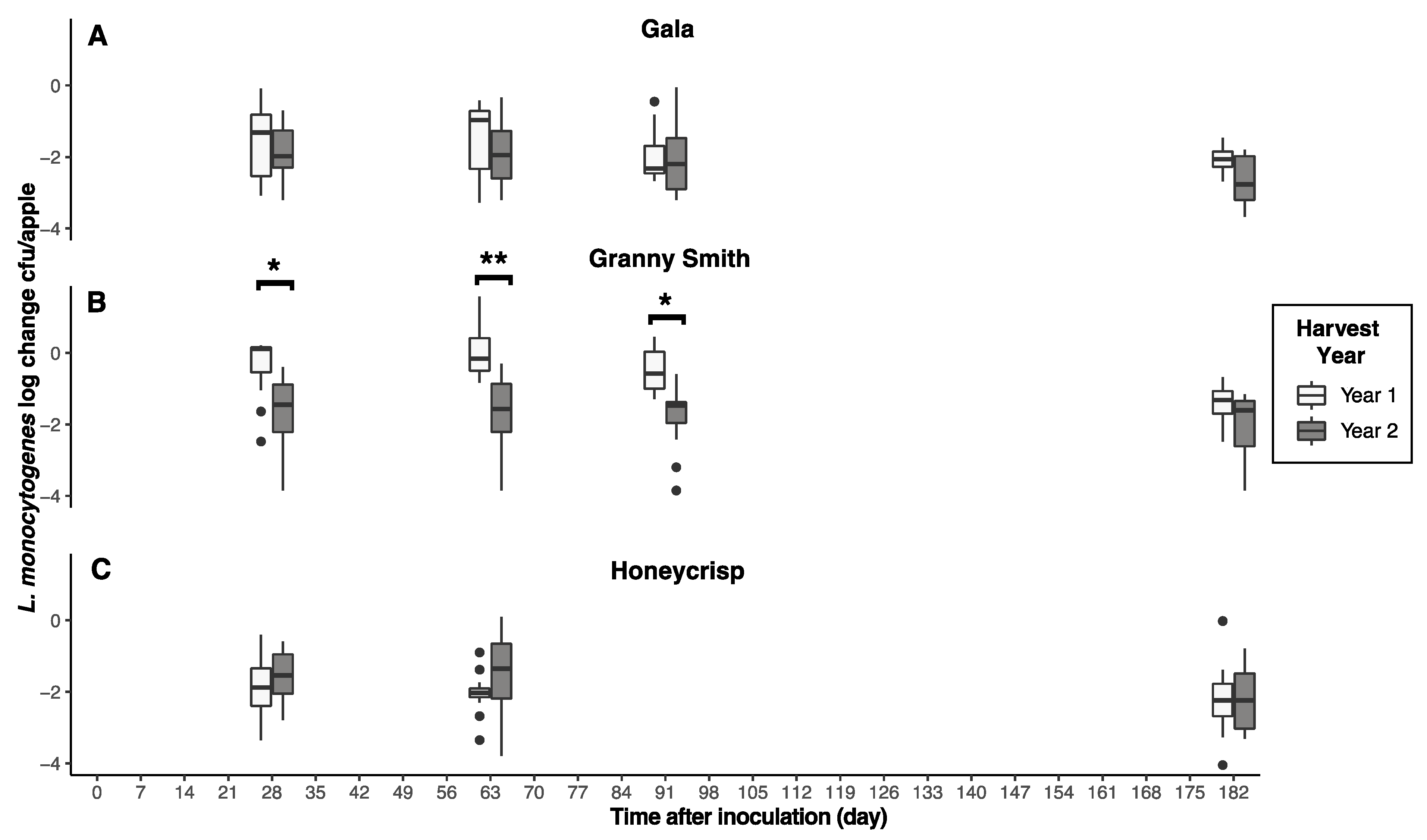
3.2. Harvest Year
3.3. Apple Cultivar
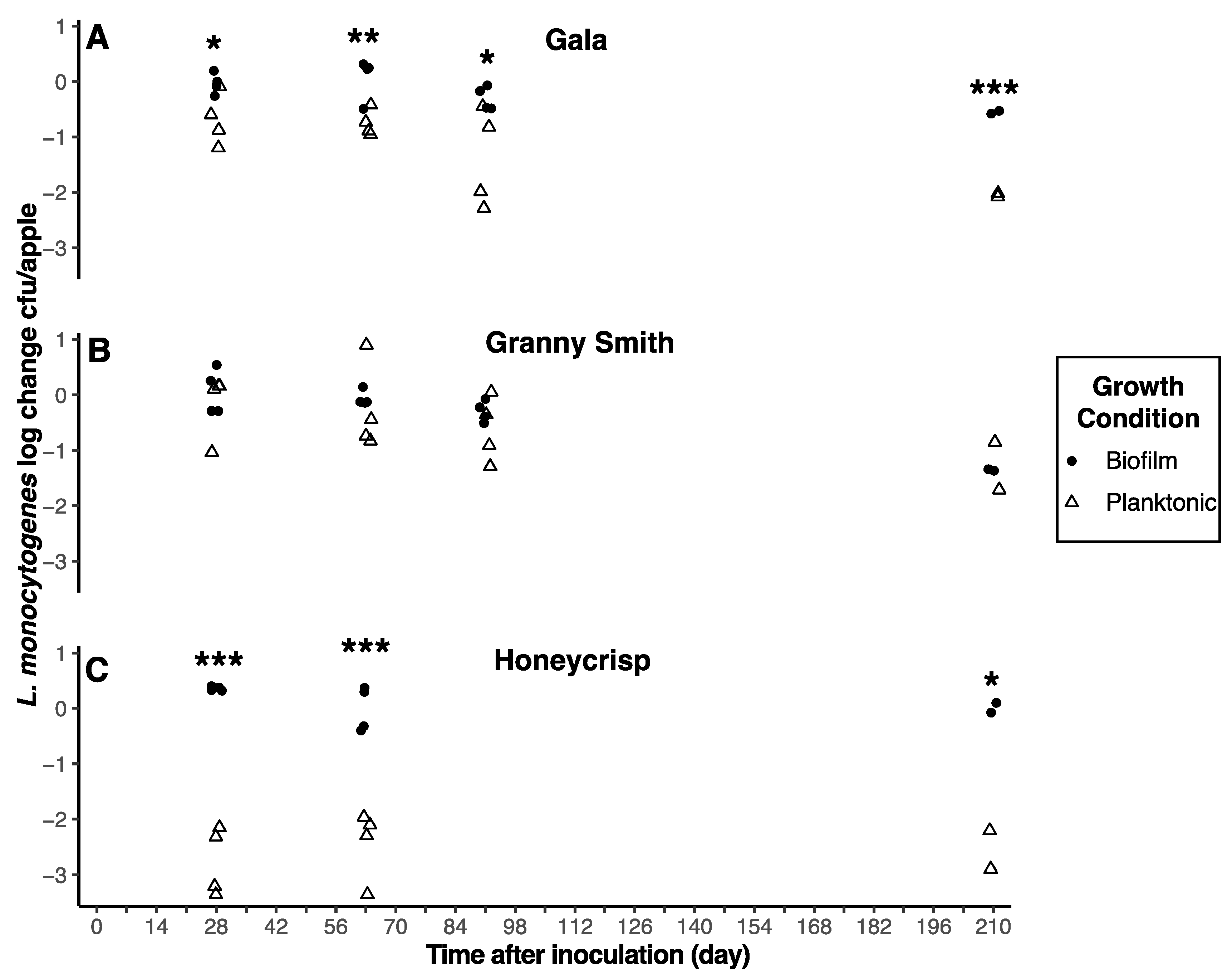
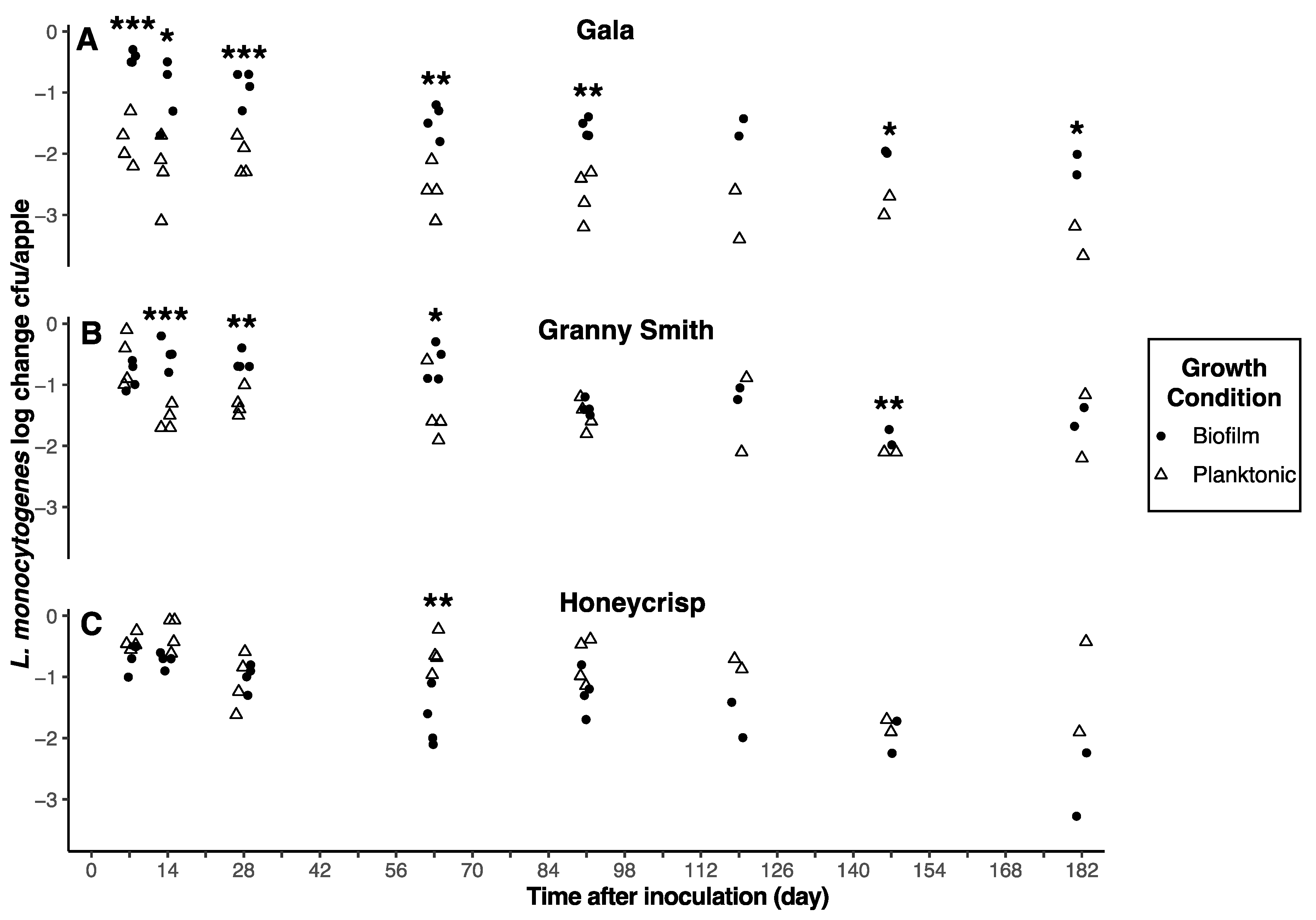
3.4. Inoculum Type
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Listeria (Listeriosis). Available online: https://www.cdc.gov/listeria/index.html (accessed on 1 October 2022).
- Datta, A.R.; Burall, L.S. Serotype to genotype: The changing landscape of listeriosis outbreak investigations. Food Microbiol. 2018, 75, 18–27. [Google Scholar] [CrossRef]
- Desai, A.N.; Anyoha, A.; Madoff, L.C.; Lassmann, B. Changing epidemiology of Listeria monocytogenes outbreaks, sporadic cases, and recalls globally: A review of ProMED reports from 1996 to 2018. Int. J. Infect. Dis. 2019, 84, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2016, 75, 1–13. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Outbreaks of Escherichia coli O157:H7 infection and cryptosporidiosis associated with drinking unpasteurized apple cider—Connecticut and New York, October 1996. Morb. Mortal. Wkly. Rep. 1997, 46, 4–8. [Google Scholar]
- Buchanan, R.L.; Edelson, S.G.; Miller, R.L.; Sapers, G.M. Contamination of Intact Apples after Immersion in an Aqueous Environment Containing Escherichia coli O157:H7. J. Food Prot. 1999, 5, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Conway, W.S.; Levere, B.; Saftner, R.A.; Janisiewicz, W.J.; Sams, C.E.; Leblanc, E. Survival and growth of Listeria monocytogenes on fresh-cut apple slices and its interaction with Glomerella cingulate and Penicillium expansum. Plant Dis. 2000, 84, 177–181. [Google Scholar] [CrossRef]
- Angelo, K.M.; Conrad, A.R.; Saupe, A.; Gragoo, H.; West, N.; Sorenson, A.; Barnes, A.; Doyle, M.; Beal, J.; Jackson, K.A.; et al. Multistate outbreak of Listeria monocytogenes infections linked to whole apples used in commercially produced, prepackaged caramel apples: United States, 2014–2015. Epidemiol. Infect. 2017, 145, 848–856. [Google Scholar] [CrossRef]
- Colagiorgi, A.; Bruini, L.; Di Ciccio, P.A.; Zanardi, E.; Ghidini, S.; Ianieri, A. Listeria monocytogenes biofilms in the wonderland of food industry. Pathogens 2017, 6, 41. [Google Scholar] [CrossRef]
- Food and Drug Administration. Recalls, Market Withdrawals, & Safety Alerts. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts (accessed on 1 October 2022).
- Garner, D.; Kathariou, S. Fresh produce-associated listeriosis outbreaks, sources of concern, teachable moments, and insights. J. Food Prot. 2016, 79, 337–344. [Google Scholar]
- Marus, J.R.; Bidol, S.; Altman, S.M.; Oni, O.; Parker-Strobe, N.; Otto, M.; Pereira, E.; Buchholz, A.; Huffman, J.; Conrad, A.R.; et al. Notes from the field: Outbreak of listeriosis likely associated with prepackaged caramel apples—United States, 2017. Morbid. Mortal. Wkly. Rep. 2019, 68, 76–77. [Google Scholar] [CrossRef]
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M. Listeria monocytogenes persistence in food-associated environments: Epidemiology, strain characteristics, and implications for public health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [PubMed]
- Kaminski, C.N.; Davidson, G.R.; Ryser, E.T. Listeria monocytogenes transfer during mechanical dicing of celery and growth during subsequent storage. J. Food Prot. 2014, 77, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Macarisin, D.; Wooten, A.; De Jesus, A.; Hur, M.; Bae, S.; Patel, P.; Evans, P.; Brown, E.; Hammack, T.; Chen, Y. Internalization of Listeria monocytogenes in cantaloupes during dump tank washing and hydrocooling. Int. J. Food Microbiol. 2017, 257, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Pietrysiak, E.; Smith, S.; Ganjyal, G.M. Food safety interventions to control Listeria monocytogenes in the fresh apple packing industry: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1705–1726. [Google Scholar] [PubMed]
- Ruiz-Llacsahuanga, B.; Hamilton, A.; Zaches, R.; Hanrahan, I.; Critzer, F. Prevalence of Listeria species on food contact surfaces in Washington State apple packinghouses. Appl. Environ. Microbiol. 2021, 87, e02932-20. [Google Scholar] [CrossRef]
- Scollon, A.M.; Wang, H.; Ryser, E.T. Transfer of Listeria monocytogenes during mechanical slicing of onions. Food Control 2016, 65, 160–167. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.L.; Ha, S. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585. [Google Scholar]
- Tan, X.; Chung, T.; Chen, Y.; Macarisin, D.; LaBorde, L.; Kovac, J. The occurrence of Listeria monocytogenes is associated with built environment microbiota in three tree fruit processing facilities. Microbiome 2019, 7, 115. [Google Scholar] [CrossRef]
- Zhu, Q.; Gooneratne, R.; Hussain, M.A. Listeria monocytogenes in fresh produce: Outbreaks, prevalence and contamination levels. Foods 2017, 6, 21. [Google Scholar] [CrossRef]
- Botticella, G.; Russo, P.; Capozzi, V.; Amodio, M.L.; Massa, S.; Spano, G.; Beneduce, L. Listeria monocytogenes, biofilm formation and fresh cut produce; Formatex Research Center: Badajoz, Spain, 2013; pp. 114–123. [Google Scholar]
- Rodriguez-Lopez, P.; Rodriguez-Herrera, J.J.; Vazquez-Sanchez, D.; Lopez Cabo, M. Current knowledge on Listeria monocytogenes biofilms in food-related environments: Incidence, resistance to biocides, ecology and biocontrol. Foods 2018, 7, 85. [Google Scholar] [CrossRef]
- Yu, T.; Chen, Y. Effects of elevated carbon dioxide on environmental microbes and its mechanisms: A review. Sci. Total Environ. 2019, 655, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Edwards, K.; Tsai, H.C.; Hanrahan, I.; Zhu, M.J. Fate of Listeria monocytogenes on fresh apples under different storage temperatures. Front. Microbiol. 2017, 8, 1396. [Google Scholar] [CrossRef] [PubMed]
- Macarisin, D.; Sheth, I.; Hur, M.; Wooten, A.; Kwon, H.J.; Gao, Z.; De Jesus, A.; Jurick, W.; Chen, Y. Survival of outbreak, food, and environmental strains of Listeria monocytogenes on whole apples as affected by cultivar and wax coating. Sci. Rep. 2019, 9, 12170. [Google Scholar] [CrossRef] [PubMed]
- Nangul, A.; Bozkurt, H.; Gupta, S.; Woolf, A.; Phan-Thien, K.Y.; McConchie, R.; Fletcher, G.C. Decline of Listeria monocytogenes on fresh apples during long-term, low-temperature simulated international sea-freight transport. Int. J. Food Microbiol. 2021, 341, 109069. [Google Scholar] [CrossRef]
- Zhang, T.; Abel, S.; Abel Zur Wiesch, P.; Sasabe, J.; Davis, B.M.; Higgins, D.E.; Waldor, M.K. Deciphering the landscape of host barriers to Listeria monocytogenes infection. Proc. Natl. Acad. Sci. USA 2017, 114, 6334–6339. [Google Scholar] [CrossRef]
- Lauer, P.; Chow, M.Y.N.; Loessner, M.J.; Portnoy, D.A.; Calendar, R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 2002, 184, 4177–4186. [Google Scholar] [CrossRef]
- Li, G. Molecular Mechanisms Underlying the Involvement of the ltrB Locus in Cold Tolerance of Listeria monocytogenes. Ph.D. Dissertation, University of Hawaii, Honolulu, HI, USA, 2001. [Google Scholar]
- Parsons, C.; Lee, S.; Jayeola, V.; Kathariou, S. Novel cadmium resistance determinant in Listeria monocytogenes. Appl. Environ. Microbiol. 2017, 83, e02580-16. [Google Scholar] [CrossRef]
- Scollard, J.; Francis, G.A.; O’Beirne, D. Effects of essential oil treatment, gas atmosphere, and storage temperature on Listeria monocytogenes in a model vegetable system. J. Food Prot. 2009, 72, 1209–1215. [Google Scholar] [CrossRef]
- Wright, A.H.; Delong, J.M.; Arul, J.; Prange, R.K. The trend toward lower oxygen levels during apple (Malus x domestica Borkh) storage. J. Hort. Sci. Biotechnol. 2015, 90, 1–13. [Google Scholar] [CrossRef]
- Jydegaard-Axelsen, A.M.; Høiby, P.E.; Holmstrom, K.; Russell, N.; Knøchel, S. CO2- and anaerobiosis-induced changes in physiology and gene expression of different Listeria monocytogenes strains. Appl. Environ. Microbiol. 2004, 70, 4111–4117. [Google Scholar] [CrossRef]
- Lungu, B.; Ricke, S.C.; Johnson, M.G. Growth, survival, proliferation and pathogenesis of Listeria monocytogenes under low oxygen or anaerobic conditions: A review. Anaerobe 2009, 15, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.N.; Chakravarty, D.; Gardner, J.C.; Ricke, S.C.; Donaldson, J.R. Listeria monocytogenes response to anaerobic environments. Pathogens 2020, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Bösch, Y.; Britt, E.; Perren, S.; Naef, A.; Frey, J.E.; Buhlmann, A. Dynamics of the apple fruit microbiome after harvest and implications for fruit quality. Microorganisms 2021, 9, 272. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef] [PubMed]
- National Centers for Envirinmental Information–National Oceanic and Atmospheric Administration. Available online: www.ncei.noaa.gov/access/monitoring/monthly-report/national/2022 (accessed on 1 October 2022).
- Pietrysiak, E.; Ganjyal, G.M. Apple peel morphology and attachment of Listeria innocua through aqueous environment as shown by scanning electron microscopy. Food Control 2018, 92, 362–369. [Google Scholar] [CrossRef]
- Burnett, S.L.; Chen, J.; Beuchat, L.R. Attachment of Escherichia coli O157:H7 to the surfaces and internal structures of apples as detected by confocal scanning laser microscopy. Appl. Environ. Microbiol. 2000, 66, 4679–4687. [Google Scholar] [CrossRef]
- Nybom, H.; Ahmadi-Afzadi, M.; Rumpunen, K.; Tahir, I. Review of the impact of apple fruit ripening, texture and chemical contents on genetically determined susceptibility to storage rots. Plants 2020, 9, 831. [Google Scholar] [CrossRef]
- Jelodarian, S.; Haghir Ebrahimabadi, A.; Jookar Kashi, F. Evaluation of antimicrobial activity of Malus domestica fruit extract from Kashan area. Avicenna J. Phytomed. 2013, 3, 1–6. [Google Scholar]
- Alberto, M.R.; Canavosio, M.A.R.; de Nadra, M.C.M. Antimicrobial effect of polyphenols from apple skins on human bacterial pathogens. Elec. J. Biotechnol. 2006, 9. [Google Scholar] [CrossRef]
- Lata, B.; Przeradzka, M.; Binkowska, M. Great differences in antioxidant properties exist between 56 apple cultivars and vegetation seasons. J. Agric. Food Chem. 2005, 53, 8970–8978. [Google Scholar] [CrossRef]
- Finn, L.; Onyeaka, H.; O’Neill, S. Listeria monocytogenes Biofilms in Food Associated Environments: A Persistent Enigma. Foods 2023, 12, 3339. [Google Scholar] [CrossRef] [PubMed]
- Boruck, M.K.; Peppin, J.D.; White, D.; Loge, F.; Call, D.R. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2003, 69, 7336–7342. [Google Scholar] [CrossRef] [PubMed]
- Pouillet, R.; Klontz, K.C.; Chen, Y.; Burall, L.S.; Macarisin, D.; Doyle, M.; Bally, K.M.; Strain, E.; Datta, A.R.; Hammack, T.S.; et al. Infectious dose of Listeria monocytogenes outbreak linked to ice cream, United States, 2015. Emerg. Infect. Dis. 2016, 22, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Atis, L.; Siddiq, M.; Bourquin, L.; Marks, B.; Dolan, K. Assessment of apple packers’ training needs and attitudes on food safety and the food safety modernization act (FSMA). Food Prot. Trends 2020, 40, 39. [Google Scholar]
| Strain | Serotype | Genotype | Outbreak |
|---|---|---|---|
| 4b1-GFP | 4b | ST2 | Clinical isolate,1962 |
| F2365-2 | 4b | ST1 | California cheese outbreak, 1985 |
| H7858-1 | 4b | ST6 | Hot dog outbreak, 1998–99 |
| 2010L-1723-4 | 1/2a | ST378 | Celery outbreak, 2010 |
| CFSAN023957-A10 | 4bv-1 | ST554 | Mung bean sprouts outbreak, 2014 |
| 2014L-6680-7 | 4b | ST1 | Caramel Apple outbreak, 2014–2015 |
| 2014L-6695-5 | 4b | ST382 | Caramel Apple outbreak, 2014–2015 |
| CFSAN073872-6 | 1/2b | ST581 | Apples, 2017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sloniker, N.; Raftopoulou, O.; Chen, Y.; Ryser, E.T.; Beaudry, R. Fate of Planktonic and Biofilm-Derived Listeria monocytogenes on Unwaxed Apples during Air and Controlled Atmosphere Storage. Foods 2023, 12, 3673. https://doi.org/10.3390/foods12193673
Sloniker N, Raftopoulou O, Chen Y, Ryser ET, Beaudry R. Fate of Planktonic and Biofilm-Derived Listeria monocytogenes on Unwaxed Apples during Air and Controlled Atmosphere Storage. Foods. 2023; 12(19):3673. https://doi.org/10.3390/foods12193673
Chicago/Turabian StyleSloniker, Natasha, Ourania Raftopoulou, Yi Chen, Elliot T. Ryser, and Randy Beaudry. 2023. "Fate of Planktonic and Biofilm-Derived Listeria monocytogenes on Unwaxed Apples during Air and Controlled Atmosphere Storage" Foods 12, no. 19: 3673. https://doi.org/10.3390/foods12193673





