Effect of Edible Coating Made from Arrowroot Flour and Kaffir Lime Leaf Essential Oil on the Quality Changes of Pork Sausage under Prolonged Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of ATF
2.3. Preparation of ATF-KEO Coating Emulsion
2.4. Coating Pork Sausage and Storage
2.5. Quality Analysis
2.5.1. Determination of Color Characteristics
2.5.2. Determination of pH
2.5.3. Determination of Moisture Content
2.5.4. Determination of Water Activity
2.5.5. Determination of Textural Profile
2.5.6. Determination of Lipid Oxidation
Extraction of Lipids
Determination of Peroxide Value (PV)
Determination of Thiobarbituric acid Reactive Substances (TBARS)
2.6. Microbiological Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. ATF Properties
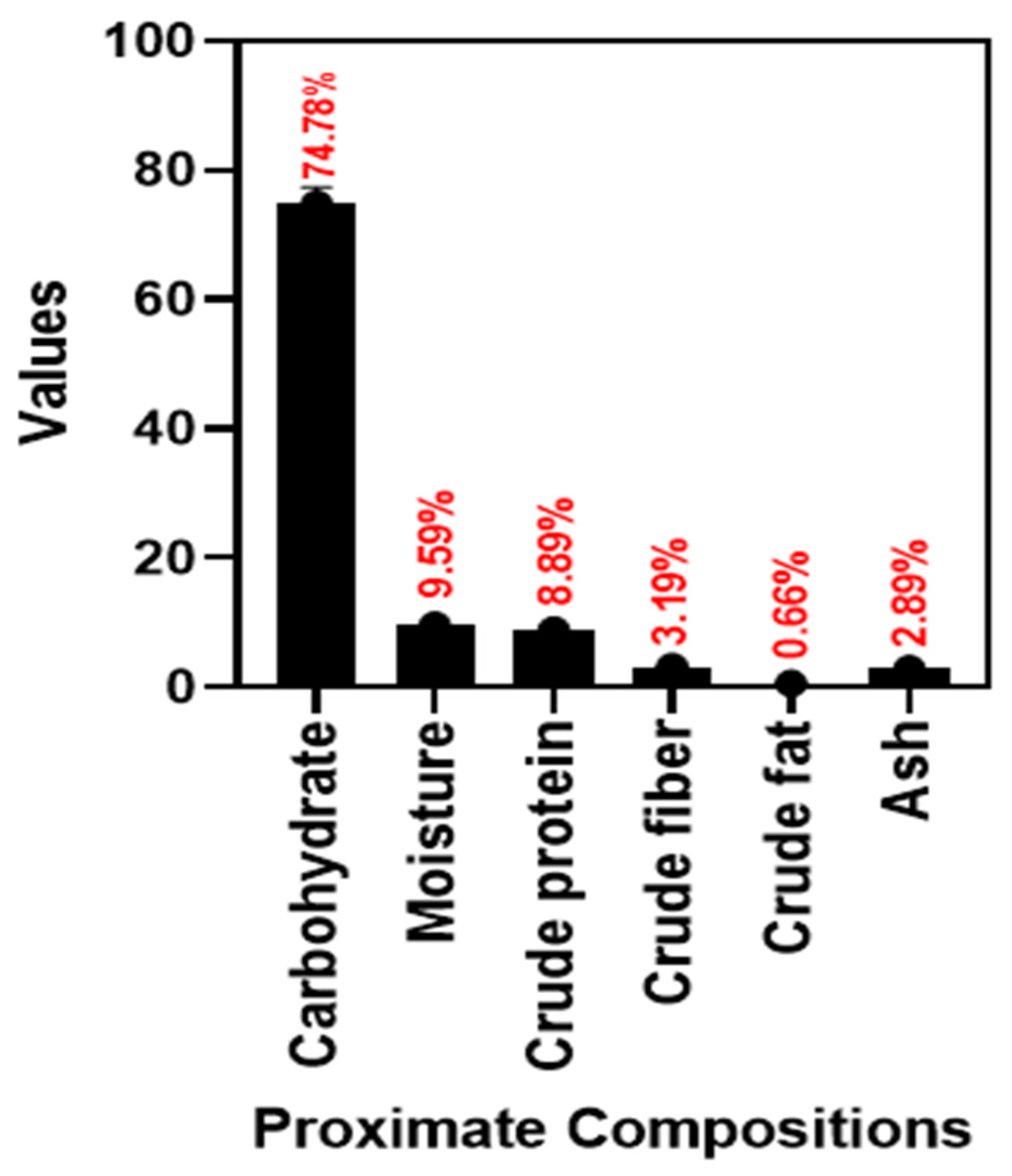
3.1.1. Proximate Composition
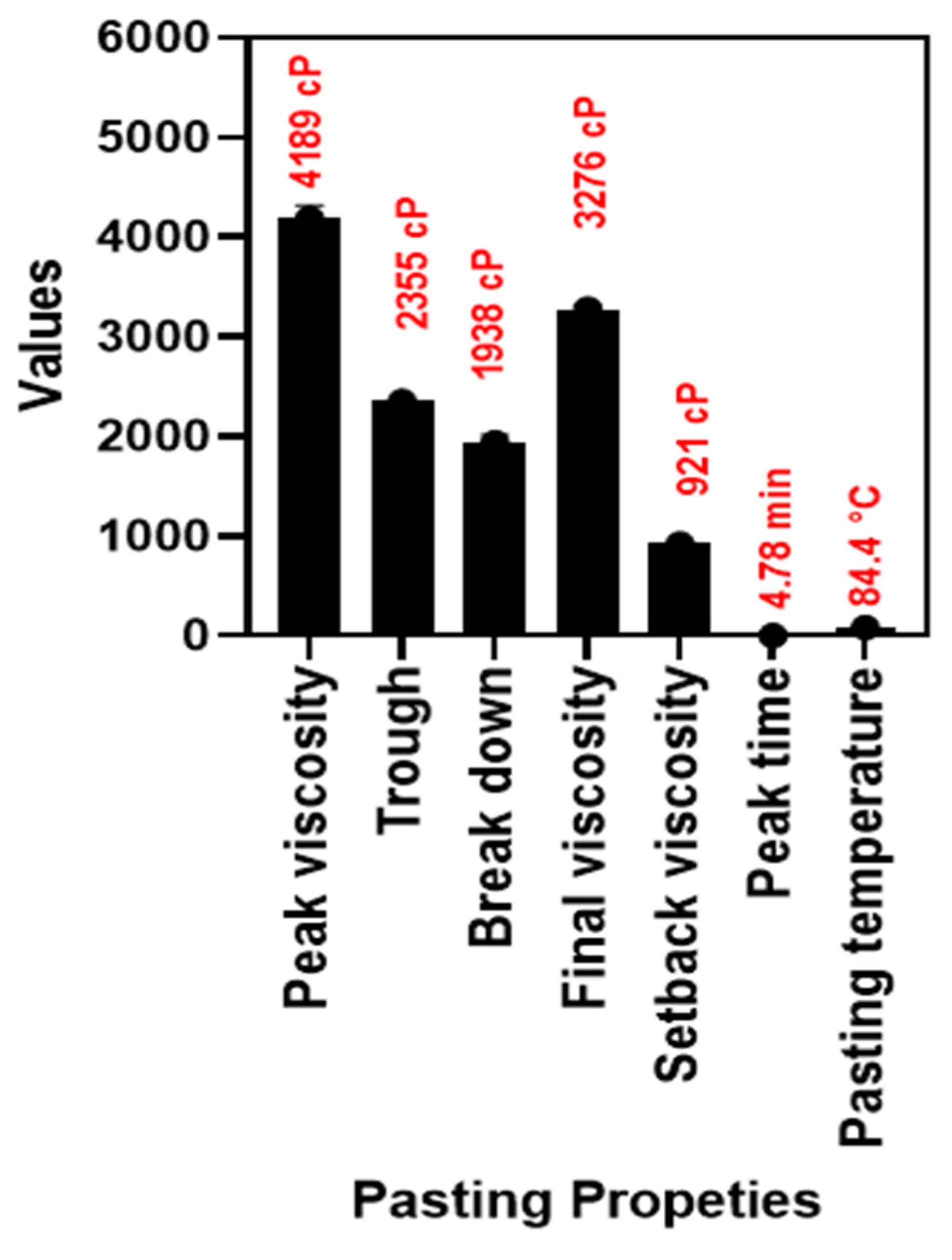
3.1.2. Pasting Properties
3.2. Structural Characterization of ATF-KEO Coating
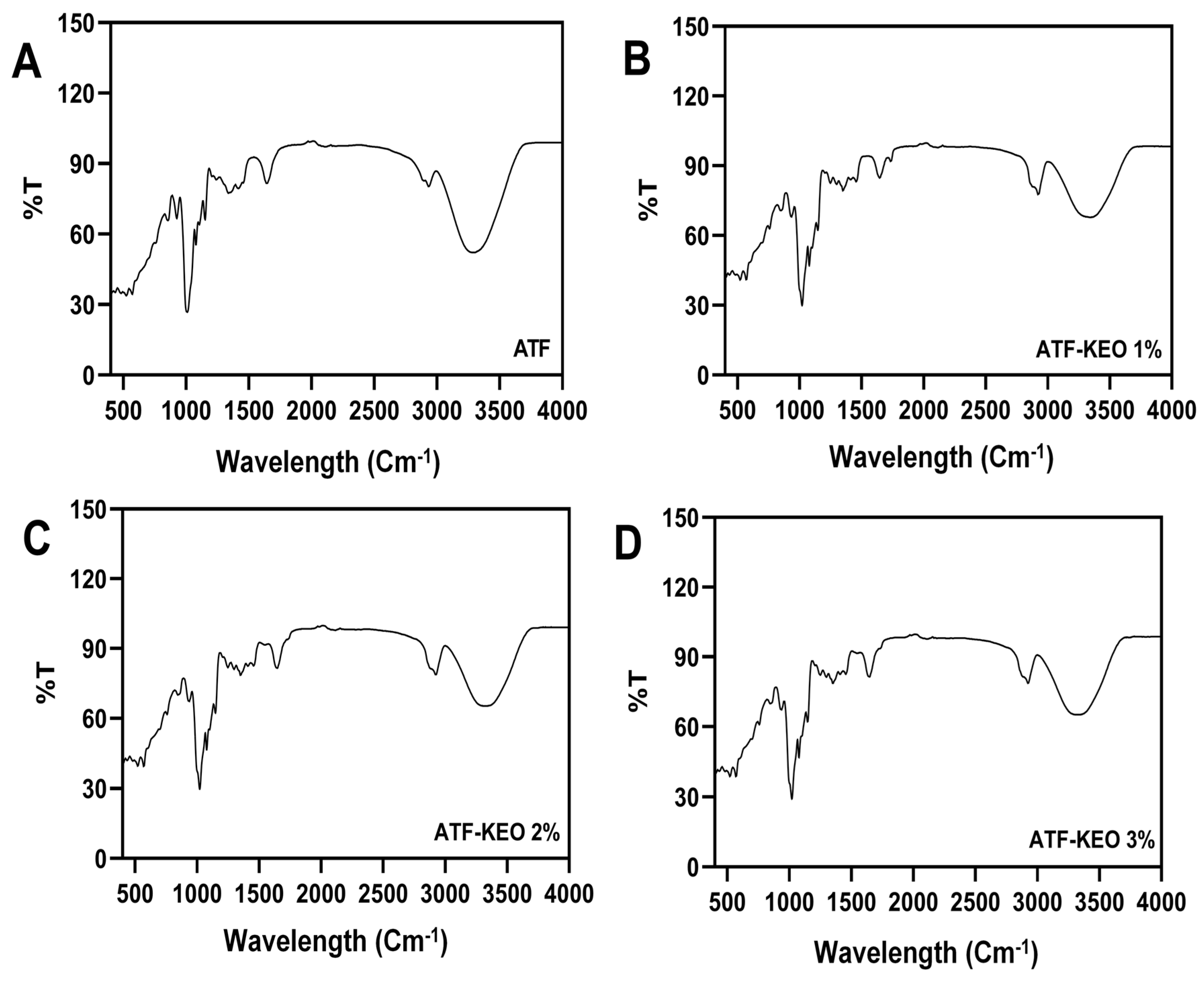
3.2.1. FTIR
3.2.2. Microstructural Observations
3.3. Impact of ATF-KEO Coating on Pork Sausage Qualities
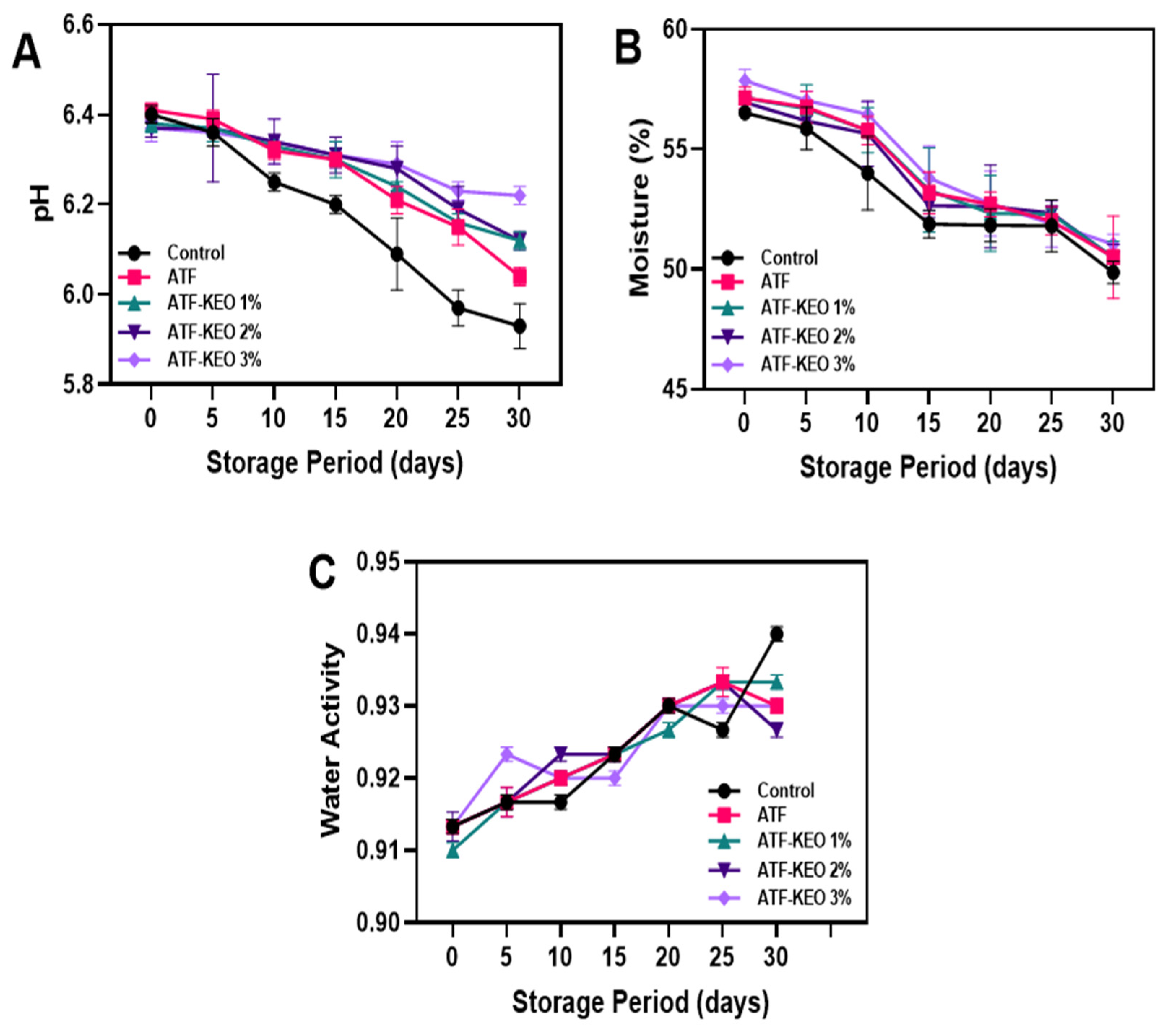
3.3.1. Physiochemical Qualities
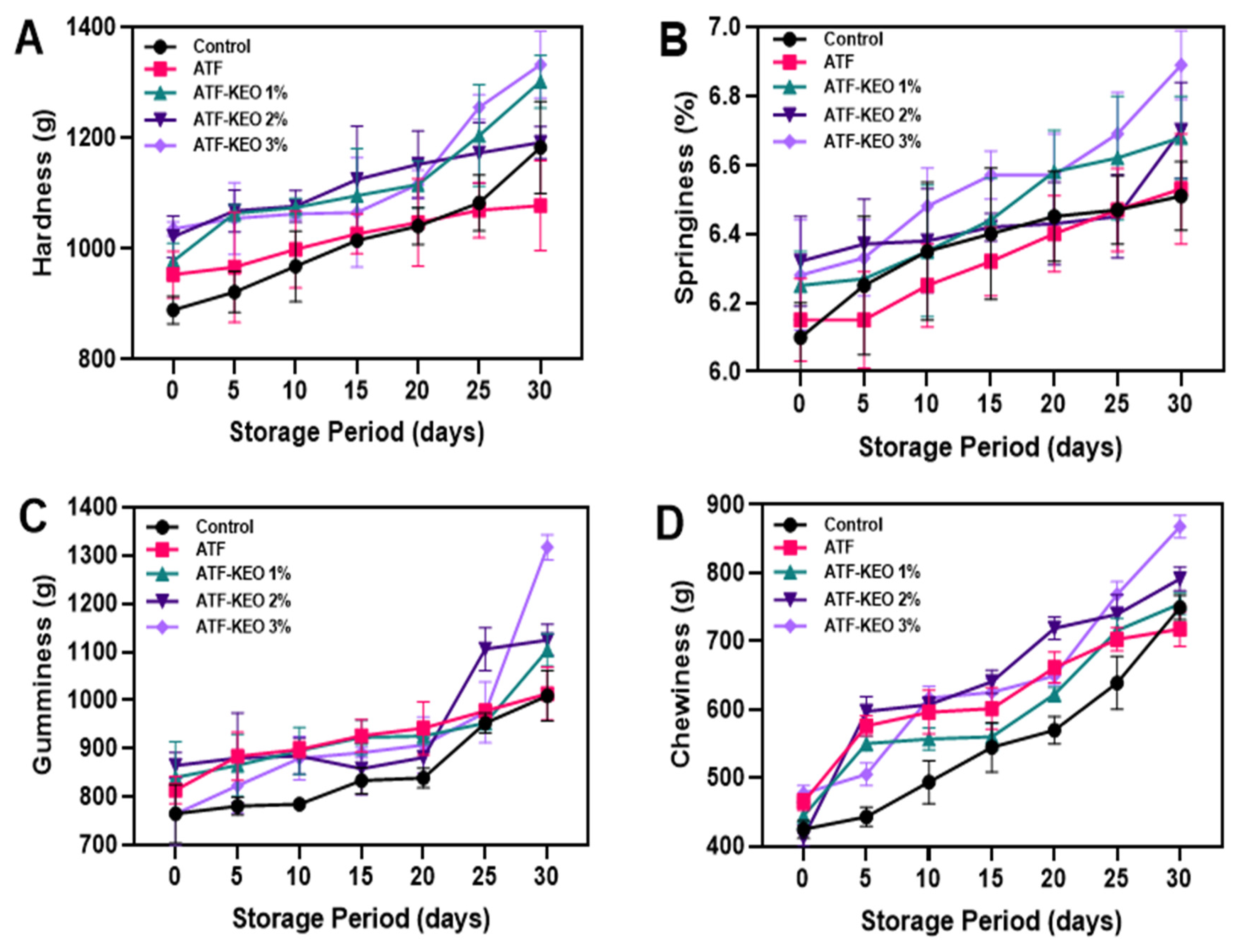
3.3.2. Textural Profile
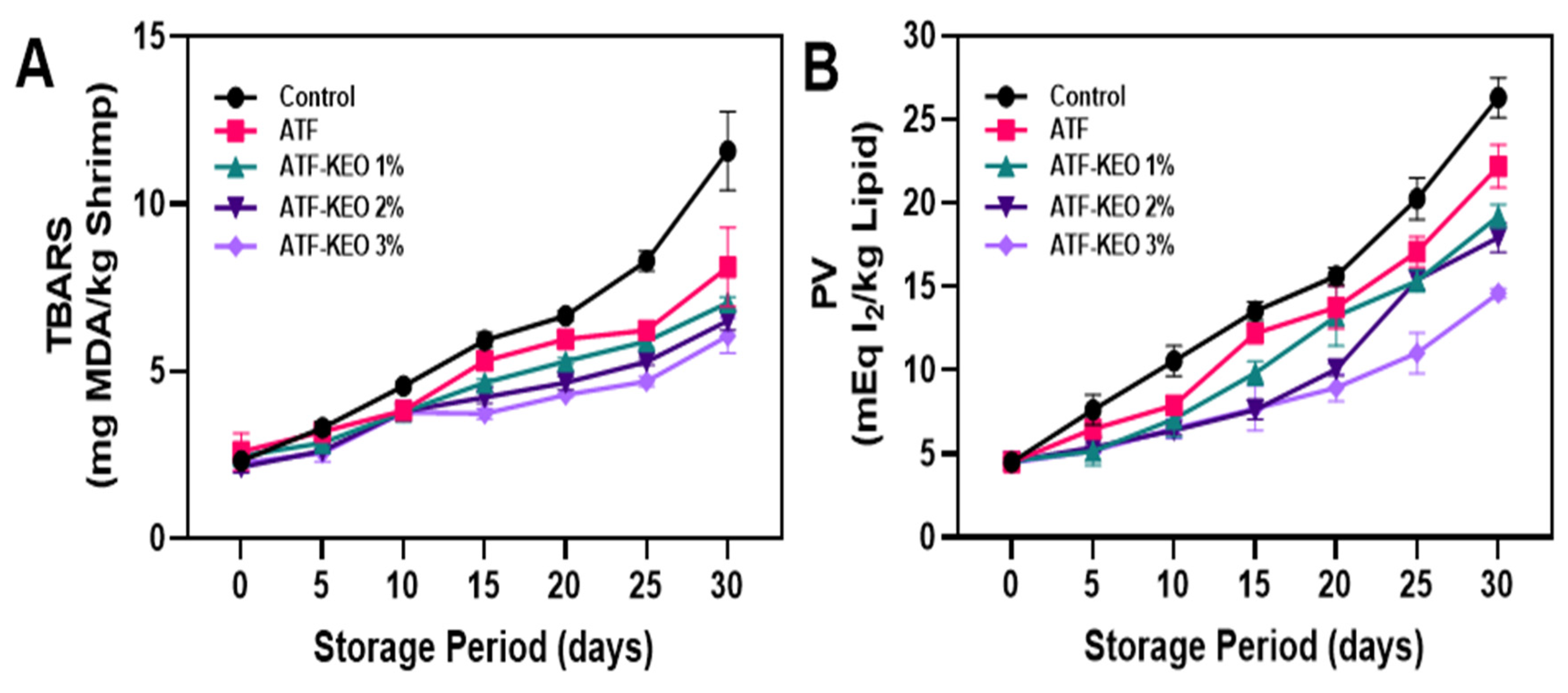
3.3.3. Lipid Oxidation
3.3.4. Microbiological Growth
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Tiwari, R.; Yatoo, M.I.; Karthik, K.; Michalak, I.; Dhama, K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health—A comprehensive review. Vet. Quart. 2021, 41, 1–29. [Google Scholar] [CrossRef]
- Gagaoua, M.; Pinto, V.Z.; Göksen, G.; Alessandroni, L.; Lamri, M.; Dib, A.L.; Boukid, F. Electrospinning as a promising process to preserve the quality and safety of meat and meat products. Coatings 2022, 12, 644. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A. Basics of Sausage Making: Formulation, Processing & Safety. Available online: https://secure.caes.uga.edu/extension/publications/files/pdf/B%201437_1.PDF (accessed on 10 July 2023).
- Mastromatteo, M.; Incoronato, A.L.; Conte, A.; Del Nobile, M.A. Shelf life of reduced pork back-fat content sausages as affected by antimicrobial compounds and modified atmosphere packaging. Int. J. Food Microbiol. 2011, 150, 1–7. [Google Scholar] [CrossRef]
- Gagaoua, M.; Bhattacharya, T.; Lamri, M.; Oz, F.; Dib, A.L.; Oz, E.; Uysal-Unalan, I.; Tomasevic, I. Green coating polymers in meat preservation. Coatings 2021, 11, 1379. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in food packaging, food quality, and safety—Controlled-release antioxidant and/or antimicrobial packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef]
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and bio-based food packaging: A review on past and current design innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Salama, A.; Mohamed, S.A.A. Propolis applications in food industries and packaging. Biomass Convers. Biorefin. 2023, 1–16. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Galactomannans use in the development of edible films/coatings for food applications. Trends Food Sci. Technol. 2011, 22, 662–671. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A sustainable material for food and medical applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Extraction and characterization of Arrowroot (Maranta arundinaceae L.) starch and its application in edible films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef]
- Nordin, N.; Othman, S.H.; Rashid, S.A.; Basha, R.K. Effects of glycerol and thymol on physical, mechanical, and thermal properties of corn starch films. Food Hydrocoll. 2020, 106, 105884. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Leme, B.d.O.; Santos, G.R.S.d.; Silva, J.V.d.; Nascimento, P.B.; Soares, C.T.; Fakhouri, F.M.; de Oliveira, R.A. Edible films and coatings formulated with Arrowroot starch as a non-conventional starch source for plums packaging. Polysaccharides 2021, 2, 373–386. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A.; Sherwani, S.F.K.; Yusuf, J.; Ilyas, R.A. Recent developments in sustainable Arrowroot (Maranta arundinacea Linn) starch biopolymers, fibres, biopolymer composites and their potential industrial applications: A review. J. Mater. Res. Technol. 2021, 13, 1191–1219. [Google Scholar] [CrossRef]
- Abdillah, A.A.; Charles, A.L. Characterization of a natural biodegradable edible film obtained from Arrowroot starch and Iota-carrageenan and application in food packaging. Int. J. Biol. Macromol. 2021, 191, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Utami Hatmi, R.; Apriyati, E.; Cahyaningrum, N. Edible coating quality with three types of starch and sorbitol plasticizer. E3S Web Conf. 2020, 142, 02003. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Xue, F. Effects of conjugation between proteins and polysaccharides on the physical properties of emulsion-based edible films. J. Am. Oil Chem. Soc. 2019, 96, 1249–1263. [Google Scholar] [CrossRef]
- Fadiji, T.; Rashvand, M.; Daramola, M.O.; Iwarere, S.A. A review on antimicrobial packaging for extending the shelf life of food. Processes 2023, 11, 590. [Google Scholar] [CrossRef]
- Budiarto, R.; Poerwanto, R.; Santosa, E.; Efendi, D.; Agusta, A. Production, post-harvest and marketing of Kaffir lime (Citrus hystrix DC) in Tulungagung, Indonesia. J. Trop. Crop Sci. 2019, 6, 138–143. [Google Scholar] [CrossRef]
- Budiarto, R.; Sholikin, M.M. Kaffir lime essential oil variation in the last fifty years: A meta-analysis of plant origins, plant parts and extraction methods. Horticulturae 2022, 8, 1132. [Google Scholar] [CrossRef]
- Cerrón-Mercado, F.; Perez-Alvarez, J.A.; Nolazco-Cama, D.; Salva-Ruíz, B.; Tellez-Monzon, L.; Fernández-López, J.; Viuda-Martos, M. Chemical composition, antioxidant and antibacterial activities of essential oil obtained from Chincho (Tagetes elliptica Sm) leaves grown in the Peruvian Andes. Foods 2023, 12, 894. [Google Scholar] [CrossRef] [PubMed]
- Astuti, I.P.; Palupi, K.D.; Damayanti, F. Essential oils composition of Kaffir lime (Citrus hystrix DC.) collection of Bogor Botanic Gardens from Central Java and East Sumba. J. Trop. Biodivers. Biotechnol. 2022, 7, 66061. [Google Scholar] [CrossRef]
- Waikedre, J.; Dugay, A.; Barrachina, I.; Herrenknecht, C.; Cabalion, P.; Fournet, A. Chemical composition and antimicrobial activity of the essential oils from New Caledonian Citrus macroptera and Citrus hystrix. Chem. Biodivers. 2010, 7, 871–877. [Google Scholar] [CrossRef]
- Soem, B. Effect of Chemical and Organic Fertilizers Application on Growth and Yield of Arrowroot (Maranta arundinacea L.). Master’s Thesis, Rajamangala University of Technology Tawan-ok, Chonburi, Thailand, 2021. [Google Scholar]
- de Oliveira Filho, J.G.; Bezerra, C.C.D.O.N.; Albiero, B.R.; Oldoni, F.C.A.; Miranda, M.; Egea, M.B.; de Azeredo, H.M.C.; Ferreira, M.D. New approach in the development of edible films: The use of carnauba wax micro-or nanoemulsions in Arrowroot starch-based films. Food Packag. Shelf Life 2020, 26, 100589. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Qian, C.; Kan, J.; Jin, C. Effect of grafting method on the physical property and antioxidant potential of chitosan film functionalized with gallic acid. Food Hydrocoll. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Al-Azri, M.S.; Ullah, S.; Bekhit, A.E.-D.A.; Pratap-Singh, A.; Chatli, M.K.; Anwer, M.K.; Aldawsari, M.F. Preparation and physiochemical characterization of bitter orange oil loaded sodium alginate and casein based edible films. Polymers 2022, 14, 3855. [Google Scholar] [CrossRef]
- MacDougall, D.B. Colour of meat. In Quality Attributes and Their Measurement in Meat, Poultry and Fish Products; Pearson, A.M., Dutson, T.R., Eds.; Springer: Boston, MA, USA, 1994; pp. 79–93. [Google Scholar]
- Lekjing, S. A chitosan-based coating with or without clove oil extends the shelf life of cooked pork sausages in refrigerated storage. Meat Sci. 2016, 111, 192–197. [Google Scholar] [CrossRef]
- Horita, C.N.; Messias, V.C.; Morgano, M.A.; Hayakawa, F.M.; Pollonio, M.A.R. Textural, microstructural and sensory properties of reduced sodium frankfurter sausages containing mechanically deboned poultry meat and blends of chloride salts. Food Res. Int. 2014, 66, 29–35. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Low, L.K.; Ng, C.S. Determination of peroxide value. In Laboratory Manual on Analytical Methods and Procedures for Fish and Fish Products; Hasegawa, H., Ed.; Marine Fisheries Research Department, Southeast Asian Fisheries Development Center: Singapore, 1978; pp. C7.1–C7.3. [Google Scholar]
- Rajasekaran, B.; Singh, A.; Nagarajan, M.; Benjakul, S. Effect of chitooligosaccharide and α-tocopherol on physical properties and oxidative stability of shrimp oil-in-water emulsion stabilized by bovine serum albumin-chitosan complex. Food Control 2022, 137, 108899. [Google Scholar] [CrossRef]
- BAM. Bacteriological Analytical Manual Chapter 3: Aerobic Plate Count; US Food and Drug Administration: Silver Spring, MD, USA, 2001.
- Radha Krishnan, K.; Babuskin, S.; Azhagu Saravana Babu, P.; Sasikala, M.; Sabina, K.; Archana, G.; Sivarajan, M.; Sukumar, M. Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. Int. J. Food Microbiol. 2014, 171, 32–40. [Google Scholar] [CrossRef]
- Brito, V.; Nascimento, R.; Narcisa-Oliveira, J.; Joffer, N.; Fattori, A.; Cereda, M.; Oliveira, C.; Costa, R.; Tiburtino-Silva, L.; Maciel, J. Arrowroot (Maranta arundinacea L.): Botany, horticulture, and uses. Hortic. Rev. 2021, 48, 233–274. [Google Scholar]
- Martinescu, D.; Sârbu, N.-R.; Velciov, A.-B.; Stoin, D. Nutritional and sensory evaluation of gluten-free cake obtained from mixtures of rice flour, almond flour and arrowroot flour. J. Agroaliment. Process. Technol. 2020, 26, 368–374. [Google Scholar]
- Grosso, A.L.; Asensio, C.M.; Grosso, N.R.; Nepote, V. Increase of walnuts’ shelf life using a walnut flour protein-based edible coating. LWT-Food Sci. Technol. 2020, 118, 108712. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, S.; Kumar, D. Edible coating and edible film as food packaging material: A review. J. Packag. Technol. Res. 2022, 6, 1–10. [Google Scholar] [CrossRef]
- Mahajan, K.; Kumar, S.; Bhat, Z.F.; Naqvi, Z.; Jayawardena, R. Development of bioactive edible film using phytochemicals from Aloe vera for improved microbial and lipid oxidative stability of frozen dairy products. Food Bioproc. Technol. 2021, 14, 2120–2133. [Google Scholar] [CrossRef]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and science behind modified starch edible films and coatings: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Ragaee, S.; Abdel-Aal, E.S.M. Pasting properties of starch and protein in selected cereals and quality of their food products. Food Chem. 2006, 95, 9–18. [Google Scholar] [CrossRef]
- Sholichah, E.; Deswina, P.; Sarifudin, A.; Andriansyah, C.E.; Rahman, N. Physicochemical, structural and morphological properties of some Arrowroot (Maranta arundinacea) accessions growth in Indonesia. AIP Conf. Proc. 2019, 2175, 020008. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Norulaini, N.A.N.; Omar, A.K.M.; Yamauchi, H.; Noda, T. RVA analysis of mixtures of wheat flour and potato, sweet potato, yam, and cassava starches. Carbohydr. Polym. 2007, 69, 784–791. [Google Scholar] [CrossRef]
- Juhász, R.; Salgó, A. Pasting behavior of amylose, amylopectin and their mixtures as determined by RVA curves and first derivatives. Starch 2008, 60, 70–78. [Google Scholar] [CrossRef]
- Tanavar, H.; Barzegar, H.; Alizadeh Behbahani, B.; Mehrnia, M.A. Investigation of the chemical properties of Mentha pulegium essential oil and its application in Ocimum basilicum seed mucilage edible coating for extending the quality and shelf life of veal stored in refrigerator (4 °C). Food Sci. Nutr. 2021, 9, 5600–5615. [Google Scholar] [CrossRef]
- Alavi, M.; Dehestaniathar, S.; Mohammadi, S.; Maleki, A.; Karimi, N. Antibacterial activities of phytofabricated ZnO and CuO NPs by Mentha pulegium leaf/flower mixture extract against antibiotic resistant bacteria. Adv. Pharm. Bull. 2021, 11, 497–504. [Google Scholar] [CrossRef]
- Lukum, A.; Kadir, A.; Sukamto, K.; Mohamad, E.; Thayban, T.; Rizki Paramata, A. application of shrimp shell waste chitosan as edible coating to extend the shelf-life of tomato (Solanum lycopersicum L.). E3S Web Conf. 2023, 400, 04008. [Google Scholar] [CrossRef]
- Yashaswini, M.; Iyer, P. Chitosan based films incorporated with turmeric/clove/ginger essential oil for food packaging. J. Nanomed. Nanotechnol. 2019, 10, 537. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with Citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Ramadhan, D.S.; Iftitah, E.D. Microwave-assisted synthesis of benzimidazole derivatives from Citronellal in Kaffir Lime (Citrus hystrix DC.) oil. IOP Conf. Ser. Mater. Sci. Eng. 2018, 299, 012076. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Matta Fakhouri, F.; Nogueira, G.F.; de Oliveira, R.A.; Velasco, J.I. Bioactive edible films based on arrowroot starch incorporated with cranberry powder: Microstructure, thermal properties, ascorbic acid content and sensory analysis. Polymers 2019, 11, 1650. [Google Scholar] [CrossRef] [PubMed]
- Gomide, R.A.C.; de Oliveira, A.C.S.; Luvizaro, L.B.; Yoshida, M.I.; de Oliveira, C.R.; Borges, S.V. Biopolymeric films based on whey protein isolate/lignin microparticles for waste recovery. J. Food Process Eng. 2021, 44, e13596. [Google Scholar] [CrossRef]
- Luong, N.D.M.; Jeuge, S.; Coroller, L.; Feurer, C.; Desmonts, M.-H.; Moriceau, N.; Anthoine, V.; Gavignet, S.; Rapin, A.; Frémaux, B.; et al. Spoilage of fresh turkey and pork sausages: Influence of potassium lactate and modified atmosphere packaging. Food Res. Int. 2020, 137, 109501. [Google Scholar] [CrossRef]
- Catarino, M.D.; Alves-Silva, J.M.; Fernandes, R.P.; Gonçalves, M.J.; Salgueiro, L.R.; Henriques, M.F.; Cardoso, S.M. Development and performance of whey protein active coatings with Origanum virens essential oils in the quality and shelf life improvement of processed meat products. Food Control 2017, 80, 273–280. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Triki, M.; Herrero, A.M.; Rodriguez-Salas, L.; Jiménez-Colmenero, F. Konjac gel as pork backfat replacer in dry fermented sausages: Processing and quality characteristics. Meat Sci. 2012, 92, 144–150. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Lekjing, S. A chitosan-based edible film with clove essential oil and nisin for improving the quality and shelf life of pork patties in cold storage. RSC Adv. 2020, 10, 17777–17786. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kerry, J.F.; Kerry, J.P. Application and assessment of extruded edible casings manufactured from pectin and gelatin/sodium alginate blends for use with breakfast pork sausage. Meat Sci. 2007, 75, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Papadima, S.N.; Bloukas, J.G. Effect of fat level and storage conditions on quality characteristics of traditional Greek sausages. Meat Sci. 1999, 51, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Jo, C.; Kwon, J.H.; Kim, J.H.; Chung, H.J.; Byun, M.W. Effect of a pectin-based edible coating containing green tea powder on the quality of irradiated pork patty. Food Control 2007, 18, 430–435. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Zimoch-Korzycka, A.; Jarmoluk, A. Polysaccharide-based edible coatings containing cellulase for improved preservation of meat quality during storage. Molecules 2017, 22, 390. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Behbahani, B.; Noshad, M.; Jooyandeh, H. Improving oxidative and microbial stability of beef using Shahri balangu seed mucilage loaded with Cumin essential oil as a bioactive edible coating. Biocatal. Agric. Biotechnol. 2020, 24, 101563. [Google Scholar] [CrossRef]
- Tosati, J.V.; Messias, V.C.; Carvalho, P.I.N.; Rodrigues Pollonio, M.A.; Meireles, M.A.A.; Monteiro, A.R. Antimicrobial effect of edible coating blend based on turmeric starch residue and gelatin applied onto fresh Frankfurter sausage. Food Bioproc. Technol. 2017, 10, 2165–2175. [Google Scholar] [CrossRef]
- Chorbadzhiev, P.; Zsivanovits, G.; Gradinarska, D.; Danov, K.; Valkova-Jorgova, K.; Zsivanovits, G.; Gradinarska, D.; Danov, K.; Valkova-Jorgova, K. Improvement of texture profile attributes of cooked sausage type “krenvirsh”. Bulg. J. Agric. Sci. 2017, 23, 338–347. [Google Scholar]
- Jokanovic, M.; Hromis, N.; Tomovic, V.; Lazic, V.; Skaljac, S.; Sojic, B.; Ikonic, P.; Peulic, T.; Ivic, M. Effect of biopolymer coating on texture characteristics of dry fermented sausage during storage. IOP Conf. Ser. Earth Environ. Sci. 2019, 333, 012066. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Khalifa, I.; Mesak, M.A.; Lorenzo, J.M.; Farag, M.A. A Comprehensive review of the role of microorganisms on texture change, flavor and biogenic amines formation in fermented meat with their action mechanisms and safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 3538–3555. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Noshad, M.; Falah, F. Cumin essential oil: Phytochemical analysis, antimicrobial activity and investigation of its mechanism of action through scanning electron microscopy. Microb. Pathog. 2019, 136, 103716. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, G.; Barbieri, F.; Soglia, F.; Magnani, R.; Gardini, G.; Petracci, M.; Gardini, F.; Montanari, C. Safety and technological issues of dry fermented sausages produced without nitrate and nitrite. Food Res. Int. 2022, 160, 111685. [Google Scholar] [CrossRef]
- Zhang, H.; He, P.; Kang, H.; Li, X. Antioxidant and antimicrobial effects of edible coating based on chitosan and bamboo vinegar in ready to cook pork chops. LWT Food Sci. Technol. 2018, 93, 470–476. [Google Scholar] [CrossRef]
- Kurek, M.A.; Moczkowska, M.; Pieczykolan, E.; Sobieralska, M. Barley β-D-glucan—Modified starch complex as potential encapsulation agent for fish oil. Int. J. Biol. Macromol. 2018, 120, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.L.; Abdillah, A.A.; Saraswati, Y.R.; Sridhar, K.; Balderamos, C.; Masithah, E.D.; Alamsjah, M.A. Characterization of freeze-dried microencapsulation Tuna fish oil with Arrowroot starch and maltodextrin. Food Hydrocoll. 2021, 112, 106281. [Google Scholar] [CrossRef]
- Klangpetch, W.; Phromsurin, K.; Hannarong, K.; Wichaphon, J.; Rungchang, S. Antibacterial and antioxidant effects of tropical citrus peel extracts to improve the shelf life of raw chicken drumettes. Int. Food. Res. J. 2016, 23, 700–707. [Google Scholar]
- Harrysson, H.; Swolin, B.; Axelsson, M.; Undeland, I. A trout (Oncorhynchus mykiss) perfusion model approach to elucidate the role of blood removal for lipid oxidation and colour changes in ice-stored fish muscle. Int. J. Food Sci. Technol. 2020, 55, 2462–2471. [Google Scholar] [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.S.; Chemat, F. Degradation during application of ultrasound in food processing: A review. Food Control 2013, 31, 593–606. [Google Scholar] [CrossRef]
- Boran, G.; Karaçam, H.; Boran, M. Changes in the quality of fish oils due to storage temperature and time. Food Chem. 2006, 98, 693–698. [Google Scholar] [CrossRef]
- Alparslan, Y.; Metin, C.; Yapıcı, H.H.; Baygar, T.; Günlü, A.; Baygar, T. Combined effect of orange peel essential oil and gelatin coating on the quality and shelf life of shrimps. J. Food Saf. Food Qual. 2017, 68, 69–78. [Google Scholar]
- Abirami, A.; Nagarani, G.; Siddhuraju, P. The medicinal and nutritional role of underutilized citrus fruit- Citrus hystrix (Kaffir lime): A review. Drug Invent. Today 2014, 6, 1–5. [Google Scholar]
- Bhawana; Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef]
- Ji, J.; Shankar, S.; Royon, F.; Salmieri, S.; Lacroix, M. Essential oils as natural antimicrobials applied in meat and meat products—A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 993–1009. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Sreepian, A.; Sreepian, P.M.; Chanthong, C.; Mingkhwancheep, T.; Prathit, P. Antibacterial activity of essential oil extracted from Citrus hystrix (Kaffir lime) peels: An in vitro study. Trop. Biomed. 2019, 36, 531–541. [Google Scholar] [PubMed]
- Utami, R.; Kawiji; Khasanah, L.U.; Solikhah, R. The effect of edible coating enriched with Kaffir lime leaf essential oil (Citrus hystrix DC) on beef sausage quality during frozen storage (−18° ± 2 °C). IOP Conf. Ser. Mater. Sci. Eng. 2018, 333, 012070. [Google Scholar] [CrossRef]
- Gedikoğlu, A. The effect of Thymus vulgaris and Thymbra spicata essential oils and/or extracts in pectin edible coating on the preservation of sliced bolognas. Meat Sci. 2022, 184, 108697. [Google Scholar] [CrossRef]
- Department of Medical Sciences. Microbiological Reference Criteria for Food and Container, 2nd ed.; Deparment of Medical Sciences, Ministry of Public Health: Bangkok, Thailand, 2010. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatachalam, K.; Ieamkheng, S.; Noonim, P.; Lekjing, S. Effect of Edible Coating Made from Arrowroot Flour and Kaffir Lime Leaf Essential Oil on the Quality Changes of Pork Sausage under Prolonged Refrigerated Storage. Foods 2023, 12, 3691. https://doi.org/10.3390/foods12193691
Venkatachalam K, Ieamkheng S, Noonim P, Lekjing S. Effect of Edible Coating Made from Arrowroot Flour and Kaffir Lime Leaf Essential Oil on the Quality Changes of Pork Sausage under Prolonged Refrigerated Storage. Foods. 2023; 12(19):3691. https://doi.org/10.3390/foods12193691
Chicago/Turabian StyleVenkatachalam, Karthikeyan, Supaporn Ieamkheng, Paramee Noonim, and Somwang Lekjing. 2023. "Effect of Edible Coating Made from Arrowroot Flour and Kaffir Lime Leaf Essential Oil on the Quality Changes of Pork Sausage under Prolonged Refrigerated Storage" Foods 12, no. 19: 3691. https://doi.org/10.3390/foods12193691
APA StyleVenkatachalam, K., Ieamkheng, S., Noonim, P., & Lekjing, S. (2023). Effect of Edible Coating Made from Arrowroot Flour and Kaffir Lime Leaf Essential Oil on the Quality Changes of Pork Sausage under Prolonged Refrigerated Storage. Foods, 12(19), 3691. https://doi.org/10.3390/foods12193691








