Possibility of Using Different Calcium Compounds for the Manufacture of Fresh Acid Rennet Cheese from Goat’s Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Goat’s Milk Analysis
2.3. Concentrations of Macro- and Micro-Elements in Milk and Cheese
2.4. The Effect of the Type and Dose of Calcium Compound on the pH Value of Goat’s Milk after Pasteurization
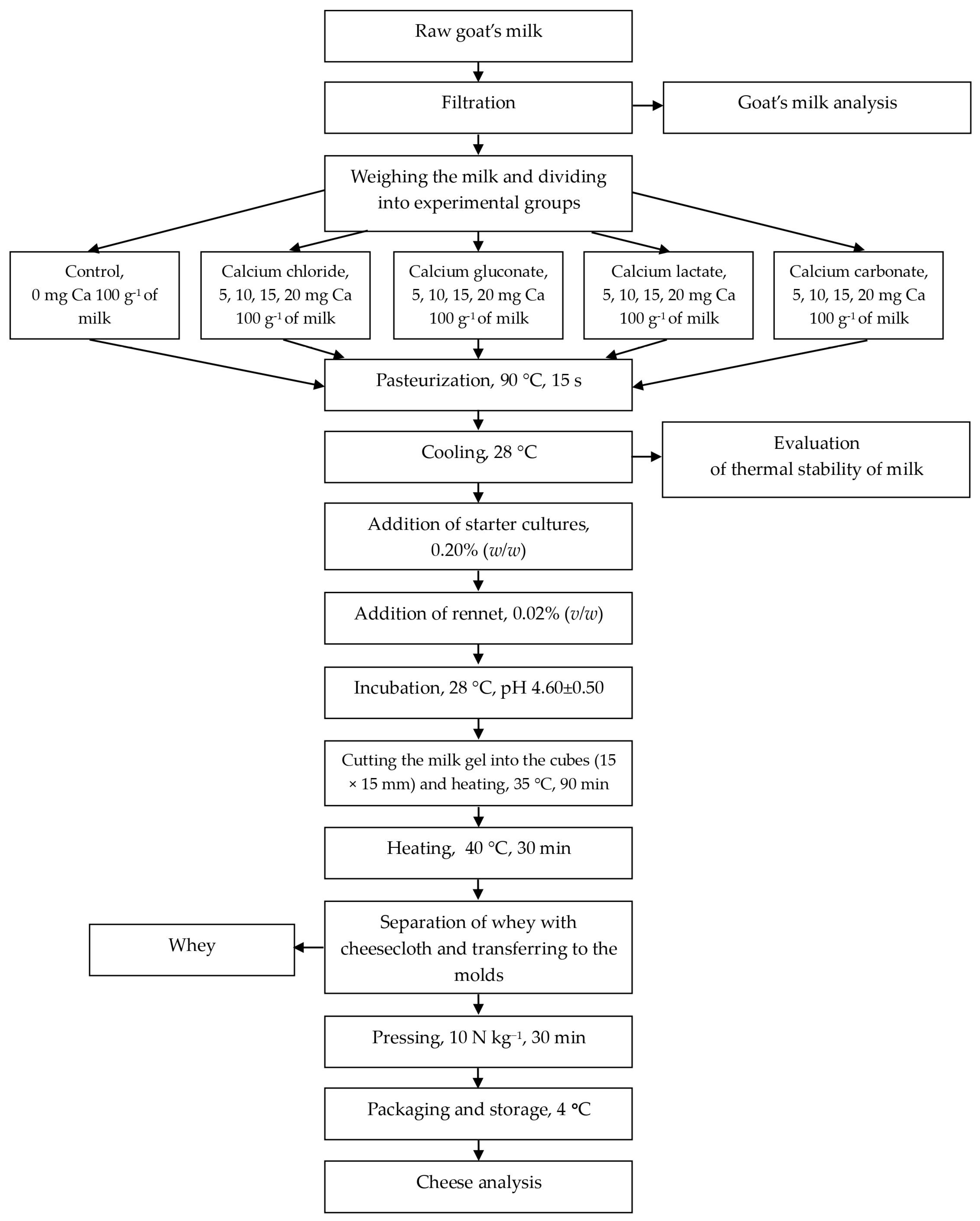
2.5. Cheese Manufacture
2.6. pH of Cheese
2.7. Fat and Moisture Content in Cheese
2.8. Protein Retention in Cheese
2.9. Cheese Yield
2.10. Texture Analysis of Cheese
2.11. Organoleptic Evaluation of Cheese
2.12. Statistical Analysis
3. Results and Discussion
3.1. Caprine Milk Properties
3.2. Effect of Calcium Compound Type and Dose on Goat Milk pH Value after Pasteurization
3.3. Quality of Fresh Acid Rennet Goat Cheese
3.3.1. Physicochemical Properties and Yield of Cheese
3.3.2. Texture of Cheese
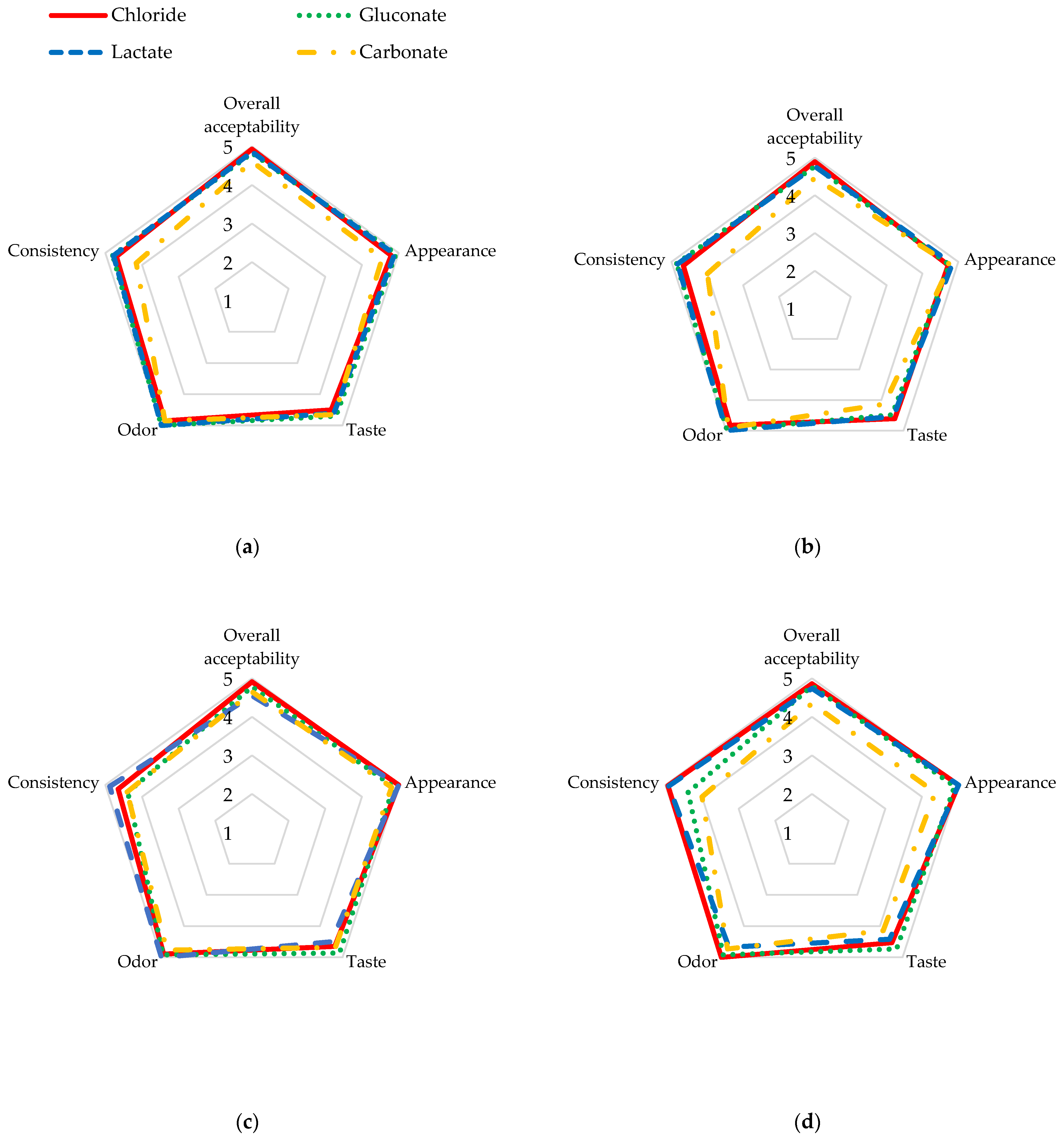
3.3.3. Organoleptic Evaluation of Cheese
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Liu, X.T.; Zhang, H.; Wang, F.; Luo, J.; Guo, H.Y.; Ren, F.Z. Rheological and structural properties of differently acidified and renneted milk gels. J. Dairy Sci. 2014, 97, 3292–3299. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, X.; Wang, B. A Review on the General Cheese Processing Technology, Flavor Biochemical Pathways and the Influence of Yeasts in Cheese. Front. Microbiol. 2021, 12, 703284. [Google Scholar] [CrossRef]
- Bouroutzika, E.; Proikakis, S.; Anagnostopoulos, A.K.; Katsafadou, A.I.; Fthenakis, G.C.; Tsangaris, G.T. Proteomics Analysis in Dairy Products: Cheese, a Review. Appl. Sci. 2021, 11, 7622. [Google Scholar] [CrossRef]
- Tarapata, J.; Smoczyński, M.; Maciejczyk, M.; Zulewska, J. Effect of calcium chloride addition on properties of acid-rennet gels. Int. Dairy J. 2020, 106, 104707. [Google Scholar] [CrossRef]
- Beltrán, M.C.; Sanna, A.; Quintanilla, P.; Montes, R.; Molina, M.P. Quinolones in goats’ milk: Effect on the cheese-making process, chemical and microbial characteristics of acid-coagulated cheeses. Int. Dairy J. 2023, 138, 105538. [Google Scholar] [CrossRef]
- Juan, B.; Trujillo, A.-J. Acid and Rennet Coagulation Properties of A2 Milk. Foods 2022, 11, 3648. [Google Scholar] [CrossRef]
- Pazzola, M. Coagulation Traits of Sheep and Goat Milk. Animals 2019, 9, 540. [Google Scholar] [CrossRef]
- Clark, S.; García, M.B.M. A 100-Year Review: Advances in goat milk research. J. Dairy Sci. 2017, 100, 10026–10044. [Google Scholar] [CrossRef]
- Miller, B.A.; Lu, C.D. Current status of global dairy goat production: An overview. Asian-Australas J. Anim. Sci. 2019, 32, 1219–1232. [Google Scholar] [CrossRef]
- Rahimi, J.; Fillol, E.; Mutua, J.Y.; Cinardi, G.; Robinson, T.P.; Notenbaert, A.M.O.; Ericksen, P.J.; Graham, M.W.; Butterbach-Bahl, K. A shift from cattle to camel and goat farming can sustain milk production with lower inputs and emissions in north sub-Saharan Africa’s drylands. Nat. Food 2022, 3, 523–531. [Google Scholar] [CrossRef]
- Verduci, E.; D’Elios, S.; Cerrato, L.; Comberiati, P.; Calvani, M.; Palazzo, S.; Martelli, A.; Landi, M.; Trikamjee, T.; Peroni, D.G. Cow’s Milk Substitutes for Children: Nutritional Aspects of Milk from Different Mammalian Species, Special Formula and Plant-Based Beverages. Nutrients 2019, 11, 1739. [Google Scholar] [CrossRef]
- Ceballos, L.S.; Morales, E.R.; de la Torre Adarve, G.; Castro, J.D.; Martínez, L.P.; Sampelayo, M.R.S. Composition of Goat and Cow Milk Produced under Similar Conditions and Analyzed by Identical Methodology. J. Food Compos. Anal. 2009, 22, 322–329. [Google Scholar] [CrossRef]
- Muehlhoff, E.; Bennet, A.; McMahon, D. Food and Agriculture Organisation of the United Nations (FAO). Milk and Dairy Products in Human Nutrition (2013). Dairy Technol. 2014, 67, 303–304. [Google Scholar] [CrossRef]
- Pastuszka, R.; Barłowska, J.; Litwińczuk, Z. Walory odzywcze i prozdrowotne mleka koziego. Nutritional value and health—Promoting properties of goat milk. Med. Weter. 2015, 71, 480–485. (In Polish) [Google Scholar]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rum. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Huppertz, T.; Chia, L.W. Milk protein coagulation under gastric conditions: A review. Int. Dairy J. 2021, 113, 104882. [Google Scholar] [CrossRef]
- Nian, Y.; Chen, B.Y.; Aikman, P.; Grandison, A.; Lewis, M. Naturally Occurring Variations in Milk pH and Ionic Calcium and Their Effects on Some Properties and Processing Characteristics of Milk. Int. J. Dairy Technol. 2012, 65, 490–497. [Google Scholar] [CrossRef]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef]
- Kowalska, M.; Ambroziak, A.; Aljewicz, M.; Cichosz, G. Wzbogacone w wapń i magnez produkty mleczarskie. Fortification of dairy products by calcium and magnesium. Postępy Technol. Przetwórstwa Spożywczego 2012, 1, 93–98. (In Polish) [Google Scholar]
- Siemianowski, K.; Szpendowski, J. Możliwość zwiększania zawartości wapnia w serach twarogowych w świetle dotychczasowych badań. Possibilities of tvarog cheeses enrichment with calcium in the light of hitherto existing research. Nauk. Inżynierskie I Technol. 2012, 4, 83–98. [Google Scholar]
- Ziarno, M.; Zaręba, D.; Piskorz, J. Wzbogacanie maślanki w wapń, magnez oraz białka serwatkowe. Fortifying buttermilk with calcium, magnesium, and whey proteins. Żywność Nauka Technol. Jakość 2009, 2, 14–27. (In Polish) [Google Scholar]
- Augustin, M.A.; Clarke, P.T. Effects of added salts on the heat stability of recombined concentrated milk. J. Dairy Res. 1990, 57, 213–226. [Google Scholar] [CrossRef]
- Szpendowski, J.; Kłobukowski, J.; Prokop, E. Wpływ dodatku chlorku wapnia i ogrzewania mleka na skład chemiczny serów twarogowych. The effect of calcium chloride added to milk and milk heating on the chemical composition of cottage cheeses. Żywność Nauka Technol. Jakość 2005, 3, 36–45. (In Polish) [Google Scholar]
- Ziarno, M.; Semeniuk, E.; Kycia, K. Wpływ dodatku soli wapnia na stabilność mleka przeznaczonego do produkcji sera typu cottage cheese. The impact of the calcium salts addition on the stability of milk used in the cottage cheese production. Żywność Nauka Technol. Jakość 2004, 2, 81–91. (In Polish) [Google Scholar]
- Dumpler, J.; Huppertz, T.; Kulozik, U. Invited review: Heat stability of milk and concentrated milk: Past, present, and future research objectives. J. Dairy Sci. 2020, 103, 10986–11007. [Google Scholar] [CrossRef]
- Szpendowski, J.; Śmietana, Z.; Płodzień, T.; Lewandowski, K.; Owczarzak, A.; Buczma, E. Technologia serów twarogowych o podwyższonej wartości odżywczej. Technology of acid curd cheese with increased nutritional value. Przegląd. Mleczarski. 2007, 1, 4–9. (In Polish) [Google Scholar]
- Znamirowska, A.; Szajnar, K.; Pawlos, M. Organic magnesium salts fortification in fermented goat’s milk. Int. J. Food Prop. 2019, 22, 1615–1625. [Google Scholar] [CrossRef]
- Pawlos, M.; Znamirowska-Piotrowska, A.; Kowalczyk, M.; Zaguła, G. Application of Calcium Citrate in the Manufacture of Acid Rennet Cheese Produced from High-Heat-Treated Goat’s Milk from Spring and Autumn Season. Molecules 2022, 27, 5523. [Google Scholar] [CrossRef]
- PN-A-86232:1973; Mleko i Przetwory Mleczarskie Sery Metody Badań. Milk and Dairy Products Cheeses Methods of Analysis. Polski Komitet Normalizacyjny: Warszawa, Poland, 1973. (In Polish)
- Kowalska, M.; Janas, S.; Woźniak, M. Innovative application of the moisture analyzer for determination of dry mass content of processed cheese. Heat. Mass. Transf. 2018, 54, 3071–3080. [Google Scholar] [CrossRef]
- Siemianowski, K.; Szpendowski, J.; Bohdziewicz, K.; Kołakowski, P.; Pawlikowska, K.; Żylińska, J.; Bardowski, J.K. Wpływ Zawartości Suchej Masy w Mleku na Skład Oraz Cechy Sensoryczne Twarogu Kwasowego. Effect of the Dry Matter Content in Milk on the Composition and Sensory Properties of Acid Tvarog Cheese. Folia Pomer. Univ. Technol. Stetin. Agric. Aliment. Pisc. Zootech. 2013, 302, 113–124. (In Polish) [Google Scholar]
- Jarzynkowska, A.; Kłopotek, E. Charakterystyka składu chemicznego i frakcji lipidowej półtwardego sera dojrzewającego produkowanego z mleka owczego i owczo-krowiego w sezonie letnim. Characterization of the chemical composition and lipid fraction of semi-hard ripened cheese produced from sheep’s and sheep-cow’s milk during the summer season. Rocz. Nauk. Pol. Tow. Zootech. 2013, 9, 39–52. (In Polish) [Google Scholar]
- ISO 4121:2003; Sensory Analysis—Guidelines for the Use of Quantitative Response Scales. International Organization for Standardization (ISO): Geneva, Switzerland, 2003.
- Barłowska, J.; Wolanciuk, A.; Kędzierska-Matysek, M.; Litwińczuk, Z. Wpływ sezonu produkcji na podstawowy skład chemiczny oraz zawartość makro- i mikroelementów w mleku krowim i kozim. Effect of production season on basic chemical composition and content of macro- and microelements in cow’s and goat’s milk. Zywnosc-Nauka Technol. Jakosc 2013, 91, 69–78. (In Polish) [Google Scholar]
- Mohsin, A.Z.; Sukor, R.; Selamat, J.; Hussin, A.S.M.; Ismail, I.H. Chemical and mineral composition of raw goat milk as affected by breed varieties available in Malaysia. Int. J. Food Prop. 2019, 22, 815–824. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Barłowska, J.; Litwińczuk, Z.; Koperska, N. Content of macro- and microelements in goat milk in relation to the lactation stage and region of production. J. Elem. 2015, 20, 107–114. [Google Scholar] [CrossRef]
- Zucali, M.; Bava, L.; Tamburini, A.; Brasca, M.; Vanoni, L.; Sandrucci, A. Effects of season, milking routine and cow cleanliness on bacterial and somatic cell counts of bulk tank milk. J. Dairy Res. 2011, 78, 436–441. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, X.; Kong, F.; Guo, W. Quantitatively determining the total bacterial count of raw goat milk using dielectric spectra. J. Dairy Sci. 2019, 102, 7895–7903. [Google Scholar] [CrossRef]
- Commission Regulation (EC). No 1662/2006 of 6 November 2006 Amending Regulation (EC) No 853/2004 of the European Parliament and of the Council Laying down Specific Hygiene Rules for Food of Animal Origin (Text with EEA Relevance). Off. J. Eur. Union 2006, L320, 1–10. [Google Scholar]
- Boyazoglu, J.; Morand-Fehr, P. Mediterranean dairy sheep and goat products and their quality. Small Rumin. Res. 2001, 40, 1–11. [Google Scholar] [CrossRef]
- Jimenez-Granado, R.; Sanchez-Rodriguez, M.; Arce, C.; Rodriguez-Estevez, V. Factors affecting somatic cell count in dairy goats: A review. Span. J. Agric. Res. 2014, 12, 133–150. [Google Scholar] [CrossRef]
- Paape, M.J.; Poutrel, B.; Contreras, A.; Marco, J.C.; Capuco, A.V. Milk somatic cells and lactation in small ruminants. J. Dairy Sci. 2001, 84 (Suppl. E), E237–E244. [Google Scholar] [CrossRef]
- Raynal-Ljutovac, K.; Gaborit, P.; Lauret, A. The relationship between quality criteria of goat milk, its technological properties and the quality of the final products. Small Rum. Res. 2005, 60, 167–177. [Google Scholar] [CrossRef]
- Kuchtık, J.; Sustova, K.; Sykora, V.; Kalhotka, L.; Pavlata, L.; Konecna, L. Changes in the somatic cells counts and total bacterial counts in raw goatmilk during lactation and their relationships to selected milk traits. Ital. J. Anim. Scin. 2021, 20, 911–917. [Google Scholar] [CrossRef]
- Otwinowska-Mindur, A.; Ptak, E.; Grzesiak, A. Factors affecting the freezing point of milk from Polish Holstein-Friesian cows. Ann. Anim. Sci. 2017, 17, 873–885. [Google Scholar] [CrossRef]
- Janštová, B.; Dračková, M.; Navrátilová, P.; Hadra, L.; Vorlová, L. Freezing point of raw and heat-treated goat milk. Czech J. Anim. Sci. 2007, 52, 394–398. [Google Scholar] [CrossRef]
- Mayer, H.K.; Fiechter, G. Physicochemical characteristics of goat’s milk in Austria-seasonal variations and differences between six breeds. Dairy Sci. Technol. 2012, 92, 167–177. [Google Scholar] [CrossRef]
- Kljajevic, N.V.; Tomasevic, I.B.; Miloradovic, Z.N.; Nedeljkovic, A.; Miocinovic, J.B.; Jovanovic, S.T. Seasonal variations of Saanen goat milk composition and the impact of climatic conditions. J. Food Sci. Technol. 2018, 55, 299–303. [Google Scholar] [CrossRef]
- Paszczyk, B.; Czarnowska-Kujawska, M.; Klepacka, J.; Tońska, E. Health-Promoting Ingredients in Goat’s Milk and Fermented Goat’s Milk Drinks. Animals 2023, 13, 907. [Google Scholar] [CrossRef]
- Pandya, A.J.; Ghodke, K.M. Goat and sheep products rather than cheeses and yoghurts. Small Rumin. Res. 2007, 68, 193–206. [Google Scholar] [CrossRef]
- Alyaqoubi, S.; Abdullah, A.; Samudi, M.; Abdullah, N.; Addai, Z.R.; Al-Ghazali, M. Physicochemical Properties and Antioxidant Activity of Milk Samples Collected from Five Goat Breeds in Malaysia. Adv. J. Food. Sci. Technol. 2015, 7, 235–241. [Google Scholar] [CrossRef]
- Zamberlin, Š.; Antunac, N.; Havranek, J.; Samaržija, D. Mineral elements in milk and dairy products. Mljekarstvo 2012, 62, 111–125. [Google Scholar]
- Panta, R.; Kumar Paswan, V.; Kumar Gupta, P.; Narayan Kohar, D. Goat’s Milk (GM), a Booster to Human Immune System against Diseases; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Bilandžić, N.; Sedaka, M.; Đokić, M.; Božić, Đ. Determination of Macro- and Microelements in Cow, Goat, and Human Milk Using Inductively Coupled Plasma Optical Emission Spectrometry. Spectrosc. Lett. 2015, 48, 677–684. [Google Scholar] [CrossRef]
- Li, X.Y.; Cheng, M.; Li, J.; Zhao, X.; Qin, Y.S.; Chen, D.; Wang, J.M.; Wang, C.F. Change in the structural and functional properties of goat milk protein due to pH and heat. J. Dairy Sci. 2020, 103, 1337–1351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, C.; Cheng, M.; Zhang, X.; Jiang, H. Influence of calcium on the properties of micellar casein in goat milk. LWT 2021, 150, 111935. [Google Scholar] [CrossRef]
- Nair, P.K.; Douglas, G.; Dalgleish, D.G.; Corredig, M. Colloidal properties of concentrated heated milk. Soft Matter. 2013, 14, 3815–3824. [Google Scholar] [CrossRef]
- Xu, Z.; Li, S.; Gong, G.; Liu, Z.; Wu, Z.; Ma, C. Influence of Different Acidifying Strains of Lactobacillus delbrueckii subsp. bulgaricus on the Quality of Yogurt. Food Sci. Technol. Res. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Lajnaf, R.; Picart-Palmade, L.; Attia, H.; Marchesseau, S.; Ayadi, M.A. The effect of pH and heat treatments on the foaming properties of purified α-lactalbumin from camel milk. Colloids Surf. B. 2017, 156, 55–61. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, H.; Liu, Q.; Pang, X.; Zhao, X.; Yang, H. Sanitising efficacy of lactic acid combined with low-concentration sodium hypochlorite on Listeria innocua in organic broccoli sprouts. Int. J. Food Microbiol. 2019, 295, 41–48. [Google Scholar] [CrossRef]
- Huang, M.; Wang, C.; Cheng, M.; Zhang, X.; Jiang, H.; Wang, J. Effects of Quantity and Source of Calcium on the Behavior of Goat Milk after Heating and Acidification. LWT 2022, 153, 112535. [Google Scholar] [CrossRef]
- Omoarukhe, E.D.; On-Nom, N.; Grandison, A.S.; Lewis, M.J. Effects of different calcium salts on properties of milk related to heat stability. Int. J. Dairy Technol. 2010, 63, 504–511. [Google Scholar] [CrossRef]
- Ong, L.; Dagastine, R.R.; Kentish, S.E.; Gras, S.L. The effect of calcium chloride addition on the microstructure and composition of Cheddar cheese. Int. Dairy J. 2013, 33, 135–141. [Google Scholar] [CrossRef]
- Soodam, K.; Ong, L.; Powell, I.B.; Kentish, S.E.; Gras, S.L. Effect of calcium chloride addition and draining pH on the microstructure and texture of full fat Cheddar cheese during ripening. Food Chem. 2015, 181, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.N.C.; Silva, C.C.C.V.; Domingues, J.R.; Cortez, M.A.S.; Freitas, D.D.G.C.; Chiappini, C.C.J.; Araujo, K.G.L. Effect of calcium addition and pH on yield and texture of Minas cured cheese. Arq. Bras. Med. Vet. Zootec. 2013, 65, 601–609. [Google Scholar] [CrossRef]
- Hickey, C.D.; Auty, M.A.E.; Wilkinson, M.G.; Sheehan, J.J. The influence of cheese manufacture parameters on cheese microstructure, microbial localisation and their interactions during ripening: A review. Trends Food Sci. Technol. 2015, 41, 135–148. [Google Scholar] [CrossRef]
- Lamichhane, P.; Kelly, A.L.; Sheehan, J.J. Symposium review: Structure-function relationships in cheese. J. Dairy Sci. 2018, 101, 2692–2709. [Google Scholar] [CrossRef] [PubMed]
- Feeney, E.L.; Lamichhane, P.; Sheehan, J.J. The cheese matrix: Understanding the impact of cheese structure on aspects of cardiovascular health—A food science and a human nutrition perspective. Int. J. Dairy Technol. 2021, 74, 656–670. [Google Scholar] [CrossRef]
- Wątróbska-Świetlikowska, D. Compatibility of Maximum Inorganic and Organic Calcium and Phosphate Content in Neonatal Parenteral Solutions. Sci. Rep. 2019, 19, 10525. [Google Scholar] [CrossRef]
- Goss, L.S.; Lemons, K.A.; Kerstetter, K.E.; Bogner, R.H. Determination of calcium salt solubility with changes in pH and PCO2, simulating varying gastrointestinal environments. J. Pharm. Pharmacol. 2007, 59, 1485–1492. [Google Scholar] [CrossRef]
- Tan, P.Y.; Tey, B.T.; Chan, E.S.; Lai, O.M.; Chang, H.W.; Tan, T.B.; Liu, Y.; Wang, Y.; Tan, C.P. Stabilization and Release of Palm Tocotrienol Emulsion Fabricated Using pH-Sensitive Calcium Carbonate. Foods 2021, 10, 358. [Google Scholar] [CrossRef]
- Jia, R.; Zhang, F.; Song, Y.; Lou, Y.; Zhao, A.; Liu, Y.; Peng, H.; Hui, Y.; Ren, R.; Wang, B. Physicochemical and textural characteristics and volatile compounds of semihard goat cheese as affected by starter cultures. J. Dairy Sci. 2021, 104, 270–280. [Google Scholar] [CrossRef]
- Kajak-Siemaszko, K.; Zielińska, D.; Łepecka, A.; Jaworska, D.; Okoń, A.; Neffe-Skocińska, K.; Trząskowska, M.; Sionek, B.; Szymański, P.; Dolatowski, Z.J.; et al. Effect of Lactic Acid Bacteria on Nutritional and Sensory Quality of Goat Organic Acid-Rennet Cheeses. Appl. Sci. 2022, 12, 8855. [Google Scholar] [CrossRef]
- Hovjecki, M.; Miloradovic, Z.; Barukčić, I.; Blažić, M.; Miocinovic, J. Rheological Properties of Goat Milk Coagulation as Affected by Rennet Concentration, pH and Temperature. Fermentation 2022, 8, 291. [Google Scholar] [CrossRef]
- Miloradovic, Z.; Kljajevic, N.; Miocinovic, J.; Tomic, N.; Smiljanic, J.; Macej, O. High heat treatment of goat cheese milk. The effect on yield, composition, proteolysis, texture and sensory quality of cheese during ripening. Int. Dairy J. 2017, 68, 1–8. [Google Scholar] [CrossRef]
- Salaün, F.; Mietton, B.; Gaucheron, F. Buffering capacity of dairy products. Int. Dairy J. 2005, 15, 95–109. [Google Scholar] [CrossRef]
- Gaber, S.M.; Grethe Johansen, A.; Devold, T.G.; Rukke, E.O.; Borghild Skeie, S. Manufacture and characterization of acid-coagulated fresh cheese made from casein concentrates obtained by acid diafiltration. J. Dairy Sci. 2021, 104, 6598–6608. [Google Scholar] [CrossRef] [PubMed]
- Sakr, H.S.A.; El-Nawasany, L.I.; Mehanna, N.M. Fotification of Karish Cheese with Calcium by Means of Using Calcium Chloride or Casein Co-Precipitates Containing High Calcium. J. Food Dairy Sci. 2019, 10, 309–313. [Google Scholar] [CrossRef]
- Dmytrów, I.; Mituniewicz-Małek, A.; Dmytrów, K. Fizykochemiczne i sensoryczne cechy sera twarogowego kwasowego wyprodukowanego z mleka koziego oraz mieszaniny mleka koziego i krowiego. Physicochemical and sensory features of acid curd cheese (tvarog) produced from goat’s milk and mixture of cow’s and goat’s milk. Żywn. Nauka Technol. Jakość. 2010, 2, 46–61. [Google Scholar]
- Vilela, P.T.; Gomes, A.M.; Ferreira, J.P. Probing the structure-holding interactions in cheeses by dissociating agents—A review and an experimental evaluation with emmental cheese. Curr. Res. Food Sci. 2020, 3, 201–206. [Google Scholar] [CrossRef]
- Ong, L.; Pax, A.P.; Ong, A.; Vongsvivut, J.; Tobin, M.J.; Kentish, S.E.; Gras, S.L. The effect of pH on the fat and protein within cream cheese and their influence on textural and rheological properties. Food Chem. 2020, 332, 127327. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, P.; Tian, Y.; Liang, Q.; Ren, F. Rennet-induced coagulation properties of yak casein micelles: A comparison with cow casein micelles. Food Res. Int. 2017, 102, 25–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, P.; Liang, Q.; Zhang, Y.; Ren, F. The factors influencing rennet-induced coagulation properties of yak milk: The importance of micellar calcium during gelation. LWT 2019, 111, 500–505. [Google Scholar] [CrossRef]
- Britten, M.; Giroux, H.J. Acid-induced gelation of whey protein polymers: Effects of pH and calcium concentration during polymerization. Food Hydrocoll. 2001, 15, 609–617. [Google Scholar] [CrossRef]
- Ong, L.; Dagastine, R.R.; Sandra, E.; Kentish, S.E.; Gras, S.L. The effect of pH at renneting on the microstructure, composition and texture of Cheddar cheese. Food Res. Int. 2012, 48, 119–130. [Google Scholar] [CrossRef]
- Verdier-Metz, I.; Baptiste Coulon, J.; Pradel, P. Relationship between milk fat and protein contents and cheese yield. Anim. Res. 2001, 50, 365–371. [Google Scholar] [CrossRef]
- Herman-Lara, E.; Bolívar-Moreno, D.; Toledo-Lopez, V.M.; Cuevas-Glory, L.F.; Lope-Navarrete, M.C.; Barron-Zambrano, J.A.; Díaz-Rivera, P.; de Jesús Ramírez-Rivera, E. Minerals multi-element analysis and its relationship with geographical origin of artisanal Mexican goat cheeses. Food Sci.Technol. Camp. 2019, 39 (Suppl. S2), 517–525. [Google Scholar] [CrossRef]
- Lima, M.J.R.; Teixeira-Lemos, E.; Oliveira, J.; Teixeira-Lemos, L.P.; Monteiro, A.M.C.; Costa, J.M. Nutritional and Health Profile of Goat Products: Focus on Health Benefits of Goat Milk. InTechOpen 2018, 10, 189–232. [Google Scholar] [CrossRef]
- da Paixão Teixeira, J.L.; Lima Pallone, J.A.; Andrade, C.D.; Mesías, M.; Seiquer, I. Bioavailability evaluation of calcium, magnesium and zinc in Brazilian cheese through a combined model of in vitro digestion and Caco-2 cells. J. Food Compos. Anal. 2022, 107, 104365. [Google Scholar] [CrossRef]
- Pawlos, M.; Znamirowska, A.; Zaguła, G.; Buniowska, M. Use of Calcium Amino Acid Chelate in the Production of Acid-Curd Goat Cheese. Foods 2020, 9, 994. [Google Scholar] [CrossRef]
- Baran, J.; Pieczonka, W.; Pompa-Roborzyński, M. Składniki mineralne w serach i w serwatce otrzymanych z mleka owczego i koziego. Mineral components in cheeses and whey made from ewe’s and goat’s milk. Żywność Nauka Technol. Jakość 2011, 78, 132–140. [Google Scholar]
- Park, Y.W. Nutrient Profiles of Commercial Goat Milk Cheeses Manufactured in the United States. J. Dairy Sci. 1990, 73, 3059–3067. [Google Scholar] [CrossRef]
- Stocco, G.; Summer, A.; Cipolat-Gotet, C.; Malacarne, M.; Cecchinato, A.; Amalfitano, N.; Bittante, G. The Mineral Profile Affects the Coagulation Pattern and Cheese-Making Efficiency of Bovine Milk. J. Dairy Sci. 2021, 104, 8439–8453. [Google Scholar] [CrossRef]
- Chumak, S. Mineral substances in bryndza from goat milk of the steppe zone of Ukraine. Sci. Eur. 2020, 57, 54–58. [Google Scholar]
- Rojo-Gutiérrez, E.; Sánchez-Vega, R.; Olivas, G.I.; Gutiérrez-Méndez, N.; Baeza-Jiménez, R.; Rios-Velasco, C.; Sepúlveda, D.R. Manufacture of magnesium-fortified Chihuahua cheese. J. Dairy Sci. 2022, 105, 4915–4924. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hernandez, M.C.; Juarez, M. Retention of Main and Trace Elements in Four Types of Goat Cheese. J. Dairy Sci. 1989, 72, 1092–1097. [Google Scholar] [CrossRef]
- Herrera García, M.I.; Puerto, P.P.; Baquero, M.F.; Rodríguez Rodríguez, E.; Martín, J.D.; Díaz Romero, C. Mineral and trace element concentrations of dairy products from goats’ milk produced in Tenerife (Canary Islands). Int. Dairy J. 2006, 16, 182–185. [Google Scholar] [CrossRef]
- da Silva, V.B.; da Bahia, U.F.; Barreto Bezerra, L.S.M.; de Oliveira Alves, B.P.; dos Santos, B.C. Rheology and texture applied to goat and sheep milk cheeses: Principles, applications and future trends. In Avanços em Ciência e Tecnologia de Alimentos; Editora Científica Digital: São Paulo, Brazil, 2020; Volume 2, pp. 409–436. [Google Scholar]
- Lucey, J.A.; Johnson, M.E.; Horne, D.S. Invited Review: Perspectives on the Basis of the Rheology and Texture Properties of Cheese. J. Dairy Sci. 2003, 86, 2725–2743. [Google Scholar] [CrossRef]
- Kumar, S.; Kanawjia, S.K.; Kumar, S.; Khatkar, S. Effect of rate of addition of starter culture on textural characteristics of buffalo milk Feta type cheese during ripening. J. Food Sci. Technol. 2014, 51, 800–804. [Google Scholar] [CrossRef]
- Chevanan, N.; Muthukumarappan, K.; Upreti, P.; Metzger, L.E. Effect of calcium and phosphorus, residual lactose and salt-to-moisture ratio on textural properties of cheddar cheese during ripening. J. Texture Stud. 2006, 37, 711–730. [Google Scholar] [CrossRef]
- Sant’Ana, A.M.; Bezerril, S.F.F.; Madruga, M.S.; Batista, A.S.M.; Magnani, M.; Souza, E.L.; Queiroga, R.C.R.E. Nutritional and sensory characteristics of Minas fresh cheese made with goat milk, cow milk, or a mixture of both. J. Dairy Sci. 2013, 96, 7442–7453. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, Y.; Luo, J.; Wang, P.; Liu, B.; Du, Y.; Jiao, Y.; Hu, Y.; Chen, C.; Ren, F.; et al. Effect of anthocyanin-absorbed whey protein microgels on physicochemical and textural properties of reduced-fat Cheddar cheese. J. Dairy Sci. 2021, 104, 228–242. [Google Scholar] [CrossRef]
- Rogers, N.R.; Drake, M.A.; Daubert, C.R.; McMahon, D.J.; Bletsch, T.K.; Foegeding, E.A. The effect of aging on low-fat, reduced-fat, and full-fat Cheddar cheese texture. J. Dairy Sci. 2009, 92, 4756–4772. [Google Scholar] [CrossRef]
- Liu, F.; Yin, J.; Wang, J.; Xu, X. Food for the elderly based on sensory perception: A review. Curr. Res. Food Sci. 2022, 13, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, Z.; Mo, B. Texture Profile Analysis of Sliced Cheese in Relation to Chemical Composition and Storage Temperature. J. Chem. 2016, 2016, 8690380. [Google Scholar] [CrossRef]
- McMahon, D.J.; Paulson, B.; Oberg, C.J. Influence of calcium, pH, and moisture on protein matrix structure and functionality in direct-acidified nonfat Mozzarella cheese. J. Dairy Sci. 2005, 88, 3754–3763. [Google Scholar] [CrossRef]
- Lepesioti, S.; Zoidou, E.; Lioliou, D.; Moschopoulou, E.; Moatsou, G. Quark-Type Cheese: Effect of Fat Content, Homogenization, and Heat Treatment of Cheese Milk. Foods 2021, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Huma, N.; Javed, A. Compositional and textural properties of goat’s milk cheese prepared using dahi (yogurt) as the starter culture. Braz. J. Food Technol. 2019, 22, e2018289. [Google Scholar] [CrossRef]
- Smith, J.L.; Niro, S.; Genovese, D.B. Comparative proteomic analysis of goat cheese made with indigenous lactic acid bacteria or commercial starter cultures. J. Dairy Sci. 2018, 101, 1434–1445. [Google Scholar]
- Foegeding, E.A.; Drake, M.A. Invited Review: Sensory and Mechanical Properties of Cheese Texture. J. Dairy Sci. 2007, 90, 1611–1624. [Google Scholar] [CrossRef]
- Carpino, S.; Mallia, S.; Licitra, G. Influence of Coagulant Type on Proteolysis in Cheeses Made from Goat Milk. J. Agric. Food Chem. 2017, 65, 7224–7230. [Google Scholar]
- Gonçalves, M.C.; Cardarelli, H.R. Mozzarella Cheese Stretching: A Minireview. Food Technol. Biotechnol. 2021, 59, 82–91. [Google Scholar] [CrossRef]
- Lefebvre-Cases, E.; Gastaldi, E.; Vidal, V.; Marchessau, S.; Lagaude, A.; Cuq, J.-L.; Tarodo De La Fuente, B. Identification of Interactions Among Casein Gels Using Dissociating Chemical Agents. J. Dairy Sci. 1998, 81, 932–938. [Google Scholar] [CrossRef]
- Kubantseva, N.; Hartel, R.W.; Swearingen, P.A. Factors Affecting Solubility of Calcium Lactate in Aqueous Solutions. J. Dairy Sci. 2004, 87, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Pawlos, M.; Znamirowska, A.; Szajnar, K. Effect of Calcium Compound Type and Dosage on the Properties of Acid Rennet Goat’s Milk Gels. Molecules 2021, 26, 5563. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, J.A.; McSweeney, P.L.H.; Lucey, J.A. A Model System for Studying the Effects of Colloidal Calcium Phosphate Concentration on the Rheological Properties of Cheddar Cheese. J. Dairy Sci. 2006, 89, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Priyashantha, H.; Lundh, Å.; Höjer, A.; Hetta, M.; Johansson, M.; Langton, M. Interactive effects of casein micelle size and calcium and citrate content on rennet-induced coagulation in bovine milk. J. Texture Stud. 2019, 50, 508–519. [Google Scholar] [CrossRef]
- Sandra, S.; Ho, M.; Alexander, M.; Corredig, M. Effect of soluble calcium on the renneting properties of casein micelles as measured by rheology and diffusing wave spectroscopy. J. Dairy Sci. 2012, 95, 75–82. [Google Scholar] [CrossRef]
- Salles, C.; Sommerer, N.; Septier, C.; Issanchou, S.; Chabanet, C.; Garem, A.; Le Quere, J.L. Goat Cheese Flavor: Sensory Evaluation of Branched-Chain Fatty Acids and Small Peptides. J. Food Sci. 2002, 67, 835–841. [Google Scholar] [CrossRef]
- Talavera, M.; Chambers, D.H. Flavor lexicon and characteristics of artisan goat cheese from the United States. J. Sens. Stud. 2016, 31, 492–506. [Google Scholar] [CrossRef]
- Szwocer, J.; Wituszyńska, B.; Obrusiewicz, T.; Najdeker, M.; Januszewska, H. Próby zastosowania ultrafiltracji w produkcji serków twarogowych z mleka koziego. Attempts to use ultrafiltration in the production of acid-curd cheese from goat’s milk. Postępy Tech. PrzetwÓRstwa Spożywczego 2001, 1, 10–16. [Google Scholar]
- Palacios, C.; Cormick, G.; Hofmeyr, G.J.; Garcia-Casal, M.N.; Peña-Rosas, J.P.; Betrán, A.P. Calcium-fortified foods in public health programs: Considerations for implementation. Ann. N. Y. Acad. Sci. 2021, 1485, 3–21. [Google Scholar] [CrossRef]
- Trailokya, A.; Srivastava, A.; Bhole, M.; Zalte, N. Calcium and Calcium Salts. J. Assoc. Physic. India 2017, 65, 100–103. [Google Scholar]
| Properties | Mean ± SD |
|---|---|
| TBC, cfu 1 mL−1 | 207,780 ± 7600 |
| SCC, in 1 mL−1 | 646,300 ± 6200 |
| Freezing point, °C | −0.562 ± 0.010 |
| pH | 6.69 ± 0.05 |
| Protein, g 100 g−1 | 2.79 ± 0.39 |
| Fat, g 100 g−1 | 2.93 ± 0.26 |
| Lactose, g 100 g−1 | 4.54 ± 0.15 |
| Ca, mg 100 g−1 | 158.17 ± 3.00 |
| K, mg 100 g−1 | 174.20 ± 2.03 |
| Mg, mg 100 g−1 | 21.20 ± 0.56 |
| P, mg 100 g−1 | 123.12 ± 4.00 |
| Mn, µg 100 g−1 | 4.01 ± 0.62 |
| Mo, µg 100 g−1 | 6.00 ± 1.30 |
| Se, µg 100 g−1 | 3.47 ± 0.49 |
| Calcium Dose, mg Ca 100 g−1 of Milk | Calcium Compound | |||
|---|---|---|---|---|
| Chloride | Gluconate | Lactate | Carbonate | |
| 0 | 6.70 aB ± 0.08 | 6.70 aB ± 0.08 | 6.70 aB ± 0.08 | 6.70 aA ± 0.08 |
| 5 | 6.53 aA ± 0.06 | 6.68 abAB ± 0.11 | 6.70 bB ± 0.07 | 6.72 bA ± 0.08 |
| 10 | 6.53 aA ± 0.06 | 6.66 abAB ± 0.11 | 6.63 bAB ± 0.03 | 6.76 bAB ± 0.11 |
| 15 | 6.51 aA ± 0.01 | 6.53 aA ± 0.05 | 6.56 aA ± 0.04 | 6.80 bAB ± 0.13 |
| 20 | 6.47 aA ± 0.06 | 6.52 aA ± 0.03 | 6.58 aA ± 0.02 | 6.81 bB ± 0.14 |
| Properties | Calcium Compound | Calcium Dose, mg Ca 100 g−1 of Milk | r | ||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |||
| pH | Chloride | 4.56 abA ± 0.07 | 4.59 bAB ± 0.10 | 4.59 bA ± 0.02 | 4.53 aA ± 0.01 | 4.52 bA ± 0.04 | −0.3129 |
| Gluconate | 4.65 abA ± 0.06 | 4.68 bB ± 0.04 | 4.65 abB ± 0.03 | 4.64 abC ± 0.01 | 4.61 aB ± 0.01 | −0.4116 | |
| Lactate | 4.61 bA ± 0.06 | 4.60 abA ± 0.01 | 4.59 abA ± 0.02 | 4.59 abB ± 0.02 | 4.54 aA ± 0.02 | −0.9521 * | |
| Carbonate | 4.63 aA ± 0.07 | 4.69 abB ± 0.05 | 4.74 bC ± 0.02 | 4.78 bcD ± 0.06 | 4.82 cC ± 0.07 | 0.9973 * | |
| Fat, mg 100 g−1 | Chloride | 13.49 aA ± 2.08 | 15.67 abA ± 3.84 | 16.17 abA ± 2.21 | 16.67 bA ± 0.61 | 15.00 abA ± 0.55 | 0.1010 |
| Gluconate | 12.72 aA ± 3.16 | 16.38 aA ± 2.75 | 15.13 aA ± 2.56 | 15.74 aA ± 0.49 | 15.15 aA ± 4.54 | 0.4602 | |
| Lactate | 12.41 aA ± 1.70 | 15.38 bA ± 3.52 | 15.50 bA ± 1.29 | 15.79 bA ± 1.00 | 15.21 abA ± 2.38 | 0.9642 * | |
| Carbonate | 14.04 aA ± 2.34 | 15.46 aA ± 4.84 | 14.62 aA ± 1.31 | 17.55 aA ± 2.25 | 17.60 aA ± 3.69 | 0.5430 * | |
| Moisture, % | Chloride | 71.49 aA ± 3.74 | 69.08 aA ± 2.83 | 69.73 aA ± 1.46 | 70.12 aB ± 0.30 | 71.05 aA ± 3.44 | −0.1535 |
| Gluconate | 68.24 aA ± 1.63 | 68.75 aA ± 2.01 | 68.99 aA ± 1.29 | 68.19 aA ± 0.63 | 68.18 aA ± 0.72 | 0.2605 | |
| Lactate | 67.72 aA ± 1.63 | 68.59 aA ± 1.74 | 68.32 aA ± 1.88 | 68.90 aA ± 0.90 | 69.95 aA ± 1.37 | 0.5915 * | |
| Carbonate | 68.65 aA ± 1.07 | 69.65 aA ± 0.09 | 69.13 aA ± 2.44 | 68.96 aA ± 0.95 | 68.67 aA ± 5.61 | 0.4559 | |
| Protein retention in cheese, % | Chloride | 75.28 aA ± 1.33 | 75.41 aB ± 0.15 | 74.73 aA ± 1.07 | 75.33 aB ± 0.15 | 75.91 aC ± 0.46 | 0.2294 |
| Gluconate | 73.57 bA ± 2.71 | 72.45 bAB ± 3.09 | 72.10 abA ± 4.08 | 73.73 bAB ± 1.80 | 65.91 aA ± 0.89 | −0.7689 * | |
| Lactate | 75.44 bA ± 1.25 | 74.24 abAB ± 2.33 | 72.94 aA ± 1.58 | 73.88 abA ± 0.41 | 73.62 abB ± 0.37 | −0.7561 * | |
| Carbonate | 74.25 bA ± 3.05 | 73.78 bA ± 0.22 | 70.00 aA ± 3.01 | 73.03 abA ± 0.76 | 71.83 abB ± 1.51 | −0.9966 * | |
| Yield, % | Chloride | 16.35 aA ± 3.48 | 18.37 aA ± 2.56 | 16.55 aA ± 1.70 | 16.15 aB ± 1.37 | 18.90 aB ± 1.10 | 0.4462 |
| Gluconate | 13.66 aA ± 3.34 | 14.51 aA ± 2.41 | 14.54 aA ± 5.07 | 16.80 aB ± 0.21 | 17.70 aAB ± 3.94 | 0.5987 * | |
| Lactate | 15.53 bA ± 1.18 | 13.50 abA ± 3.03 | 14.24 abA ± 1.42 | 13.00 aA ± 0.71 | 13.23 abA ± 1.26 | −0.5311 * | |
| Carbonate | 16.69 aA ± 2.53 | 17.85 aA ± 1.81 | 15.77 aA ± 3.37 | 16.80 aB ± 1.01 | 16.70 aB ± 3.40 | 0.0436 | |
| Properties | Calcium Compound | Calcium Dose, mg Ca 100 g−1 of Milk | r | ||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |||
| Ca, mg 100 g−1 | Chloride | 140.82 aB ± 3.20 | 152.26 bC ± 2.90 | 152.82 bC ± 1.09 | 156.05 cC ± 1.80 | 160.81 dC ± 1.73 | 0.9567 * |
| Gluconate | 151.20 aC ± 1.70 | 156.62 bC ± 1.31 | 160.27 cD ± 1.33 | 165.72 dD ± 0.52 | 170.52 eD ± 0.54 | 0.8404 * | |
| Lactate | 129.61 aA ± 2.18 | 134.19 bB ± 2.41 | 136.35 bB ± 1.54 | 145.94 cB ± 0.66 | 150.42 dB ± 2.04 | 0.9739 * | |
| Carbonate | 125.09 aA ± 2.59 | 127.48 aA ± 0.62 | 130.61 bA ± 0.90 | 134.11 bA ± 2.75 | 140.15 cA ± 1.89 | 0.9275 * | |
| K, mg 100 g−1 | Chloride | 69.96 dC ± 1.00 | 44.68 bA ± 2.11 | 57.85 cB ± 2.46 | 54.60 cA ± 3.01 | 32.00 aA ± 4.04 | −0.7755 * |
| Gluconate | 58.30 aB ± 2.02 | 62.54 bC ± 0.98 | 61.05 bC ± 1.02 | 69.16 cB ± 2.33 | 72.96 cC ± 4.58 | 0.7427 * | |
| Lactate | 60.18 cB ± 0.77 | 57.87 bB ± 1.11 | 57.09 bB ± 1.12 | 55.73 bA ± 2.45 | 52.94 aB ± 0.75 | −0.5664 * | |
| Carbonate | 50.52 bA ± 1.99 | 43.69 abA ± 1.66 | 42.90 aA ± 1.59 | 67.19 cB ± 2.34 | 66.93 cC ± 2.00 | 0.5554 * | |
| Mg, mg 100 g−1 | Chloride | 18.35 cB ± 0.15 | 18.25 cB ± 0.29 | 13.90 aB ± 0.52 | 14.81 bAB ± 1.15 | 16.86 bB ± 1.00 | −0.7755 * |
| Gluconate | 13.60 aA ± 1.00 | 25.11 bC ± 0.15 | 25.40 bC ± 0.10 | 26.21 bC ± 0.12 | 25.81 bC ± 0.14 | 0.7470 * | |
| Lactate | 18.35 dB ± 0.68 | 17.29 cB ± 0.40 | 12.51 aA ± 0.24 | 14.37 bA ± 0.15 | 14.52 bA ± 0.17 | −0.7037 * | |
| Carbonate | 12.29 aA ± 0.19 | 13.76 abA ± 1.50 | 13.83 abAB ± 1.51 | 15.70 bB ± 0.87 | 14.93 bA ± 0.50 | 0.7385 * | |
| P, mg 100 g−1 | Chloride | 136.95 aA ± 2.15 | 133.86 aA ± 2.41 | 144.39 bB ± 1.47 | 143.56 bB ± 1.80 | 143.80 bB ± 1.77 | 0.4908 |
| Gluconate | 148.07 aB ± 4.12 | 152.66 aC ± 4.88 | 167.97 bC ± 3.50 | 175.58 cC ± 4.00 | 179.60 cC ± 4.44 | 0.8973 * | |
| Lactate | 134.95 aA ± 1.44 | 137.65 bB ± 0.90 | 137.57 bA ± 0.78 | 137.62 bA ± 1.02 | 139.45 cA ± 0.82 | 0.8321 * | |
| Carbonate | 135.97 aA ± 1.00 | 134.84 aA ± 1.11 | 142.44 bB ± 2.33 | 140.67 bAB ± 2.41 | 144.01 bB ± 2.90 | 0.5265 * | |
| Mn, µg 100 g−1 | Chloride | 6.03 aA ± 0.33 | 6.03 aA ± 0.40 | 6.11 aA ± 0.36 | 6.04 aA ± 0.30 | 6.07 aA ± 0.25 | 0.0952 |
| Gluconate | 6.09 aA ± 0.25 | 6.09 aA ± 0.35 | 6.10 aA ± 0.40 | 6.09 aA ± 0.40 | 6.10 aA ± 0.30 | 0.1112 | |
| Lactate | 6.09 aA ± 0.12 | 6.07 aA ± 0.36 | 6.09 aA ± 0.39 | 6.07 aA ± 0.45 | 6.09 aA ± 0.27 | 0.0087 | |
| Carbonate | 6.20 aA ± 0.30 | 6.20 aA ± 0.49 | 6.20 aA ± 0.30 | 6.19 aA ± 0.47 | 6.19 aA ± 0.29 | −0.0045 | |
| Mo, µg 100 g−1 | Chloride | 2.61 aA ± 0.47 | 2.70 aA ± 0.64 | 2.72 aA ± 0.36 | 2.73 aA ± 0.50 | 2.63 aA ± 0.70 | 0.1871 |
| Gluconate | 2.57 aA ± 0.54 | 2.75 aA ± 0.43 | 2.79 aA ± 0.54 | 2.70 aA ± 0.56 | 2.57 aA ± 0.51 | 0.0078 | |
| Lactate | 2.59 aA ± 0.33 | 2.79 aA ± 0.29 | 2.83 aA ± 0.40 | 2.59 aA ± 0.60 | 2.61 aA ± 0.29 | 0.2543 | |
| Carbonate | 2.94 aA ± 0.51 | 2.95 aA ± 0.22 | 2.94 aA ± 0.45 | 2.92 aA ± 0.30 | 2.93 aA ± 0.41 | 0.0033 | |
| Se, µg 100 g−1 | Chloride | 6.77 aA ± 0.90 | 7.22 aA ± 2.22 | 6.94 aA ± 1.50 | 6.29 aA ± 1.00 | 6.98 aA ± 0.55 | 0.2013 |
| Gluconate | 6.73 aA ± 1.01 | 6.56 aA ± 1.41 | 6.55 aA ± 1.53 | 6.20 aA ± 1.41 | 6.78 aA ± 1.01 | 0.0621 | |
| Lactate | 7.02 aA ± 1.51 | 6.98 aA ± 1.89 | 7.00 aA ± 0.97 | 6.52 aA ± 1.10 | 7.01 aA ± 0.59 | 0.0112 | |
| Carbonate | 6.53 aA ± 0.64 | 6.67 aA ± 1.90 | 6.55 aA ± 1.22 | 6.91 aA ± 1.60 | 6.67 aA ± 1.03 | 0.1127 | |
| Properties | Calcium Compound | Calcium Dose, mg Ca 100 g−1 of Milk | r | ||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |||
| Hardness, N | Chloride | 2.42 aA ± 0.48 | 2.55 abAB ± 0.85 | 2.39 aAB ± 0.57 | 2.57 abA ± 0.19 | 2.68 bA ± 0.24 | 0.2325 |
| Gluconate | 2.75 aA ± 0.67 | 2.66 aAB ± 0.38 | 3.19 abB ± 0.29 | 3.09 abB ± 0.15 | 3.53 bB ± 0.36 | 0.5003 * | |
| Lactate | 2.33 aA ± 0.22 | 3.04 bB ± 0.52 | 3.34 bB ± 0.49 | 3.37 bB ± 0.21 | 3.45 bB ± 0.23 | 0.7444 * | |
| Carbonate | 1.87 aA ± 0.56 | 1.75 aA ± 0.49 | 1.78 aA ± 0.44 | 1.94 aA ± 0.42 | 2.29 bA ± 0.06 | 0.2253 | |
| Cohesiveness | Chloride | 0.26 aA ± 0.07 | 0.19 aA ± 0.11 | 0.16 aA ± 0.07 | 0.27 aAB ± 0.10 | 0.21 aA ± 0.07 | −0.1293 |
| Gluconate | 0.23 aA ± 0.06 | 0.24 aA ± 0.08 | 0.23 aA ± 0.04 | 0.30 aB ± 0.01 | 0.22 aA ± 0.07 | 0.1321 | |
| Lactate | 0.23 aA ± 0.08 | 0.25 aA ± 0.05 | 0.24 aA ± 0.10 | 0.24 aA ± 0.01 | 0.24 aA ± 0.02 | 0.2400 | |
| Carbonate | 0.30 aA ± 0.04 | 0.30 aA ± 0.10 | 0.31 aA ± 0.09 | 0.31 aB ± 0.05 | 0.30 aA ± 0.04 | 0.1607 | |
| Springiness, mm | Chloride | 2.17 bA ± 0.63 | 1.27 aA ± 0.11 | 1.93 abA ± 0.87 | 2.94 bAB ± 0.22 | 2.43 bAB ± 0.38 | 0.5529 * |
| Gluconate | 2.82 aA ± 0.41 | 3.17 abB ± 0.46 | 2.86 abA ± 0.76 | 3.94 bB ± 0.23 | 3.23 abAB ± 0.96 | 0.5439 * | |
| Lactate | 2.15 aA ± 0.37 | 2.46 aB ± 0.17 | 2.50 aA ± 0.33 | 2.87 abA ± 0.32 | 3.27 bB ± 0.43 | 0.7303 * | |
| Carbonate | 2.33 aA ± 0.89 | 2.57 aB ± 0.43 | 2.71 aA ± 0.30 | 2.82 aAB ± 0.92 | 2.18 aA ± 0.36 | 0.0360 | |
| Adhesiveness, mJ | Chloride | 1.41 aAB ± 0.72 | 1.67 aB ± 0.26 | 1.87 aB ± 0.43 | 1.77 aB ± 0.91 | 1.82 aA ± 0.86 | 0.7896 * |
| Gluconate | 0.81 aA ± 0.06 | 0.99 abAB ± 0.61 | 1.27 abAB ± 0.48 | 1.57 bB ± 0.35 | 1.28 abA ± 0.60 | 0.5465 * | |
| Lactate | 1.01 abAB ± 0.67 | 0.55 aA ± 0.20 | 0.54 aA ± 0.52 | 0.53 aA ± 0.12 | 1.32 bA ± 0.36 | 0.0563 | |
| Carbonate | 1.84 aB ± 0.53 | 1.53 aB ± 0.50 | 1.63 aAB ± 0.98 | 1.56 aB ± 0.67 | 1.85 aA ± 0.94 | 0.0345 | |
| Properties | Calcium Compound | Calcium Dose, mg Ca 100 g−1 of Milk | r | ||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |||
| Overall acceptability | Chloride | 4.93 aA ± 0.16 | 4.93 aA ± 0.12 | 4.89 aA ± 0.18 | 4.91 aA ± 0.19 | 4.86 aA ± 0.11 | −0.2356 |
| Gluconate | 4.82 aA ± 0.28 | 4.83 aA ± 0.21 | 4.77 aA ± 0.32 | 4.78 aA ± 0.32 | 4.80 aA ± 0.25 | −0.0032 | |
| Lactate | 4.84 aA ± 0.27 | 4.86 aA ± 0.24 | 4.77 aA ± 0.17 | 4.55 aA ± 0.05 | 4.74 aA ± 0.26 | −0.3312 | |
| Carbonate | 4.80 bA ± 0.32 | 4.61 abA ± 0.38 | 4.46 abA ± 0.36 | 4.65 abA ± 0.48 | 4.35 aA ± 0.58 | −0.5043 * | |
| Appearance | Chloride | 4.83 aA ± 0.39 | 4.80 aA ± 0.35 | 4.72 aA ± 0.44 | 5.00 aA ± 0.02 | 5.00 aA ± 0.00 | 0.1570 |
| Gluconate | 5.00 aA ± 0.00 | 4.93 aA ± 0.26 | 4.75 aA ± 0.48 | 4.86 aA ± 0.38 | 4.89 aB ± 0.30 | −0.0127 | |
| Lactate | 5.00 aA ± 0.00 | 4.89 aA ± 0.33 | 4.83 aA ± 0.35 | 5.00 aA ± 0.00 | 5.00 aA ± 0.00 | 0.1239 | |
| Carbonate | 4.92 aA ± 0.29 | 4.55 aA ± 0.55 | 4.77 aA ± 0.52 | 4.81 aA ± 0.46 | 4.36 aA ± 0.78 | −0.2302 | |
| Taste | Chloride | 4.40 aA ± 0.62 | 4.50 aA ± 0.53 | 4.61 aA ± 0.55 | 4.65 aA ± 0.47 | 4.53 aA ± 0.66 | −0.0159 |
| Gluconate | 4.00 aA ± 0.00 | 4.70 bA ± 0.41 | 4.48 abA ± 0.60 | 4.86 bA ± 0.38 | 4.73 bA ± 0.44 | 0.6777 * | |
| Lactate | 4.57 aA ± 0.45 | 4.61 aA ± 0.49 | 4.54 aA ± 0.45 | 4.50 aA ± 0.41 | 4.42 aA ± 0.92 | −0.0637 | |
| Carbonate | 4.75 aA ± 0.58 | 4.65 aA ± 0.53 | 4.13 aA ± 0.72 | 4.68 aA ± 0.64 | 4.14 aA ± 0.84 | −0.2794 | |
| Odor | Chloride | 4.54 aA ± 1.44 | 4.85 aA ± 0.34 | 4.83 aA ± 0.35 | 4.90 aA ± 0.32 | 5.00 aA ± 0.00 | −0.1058 |
| Gluconate | 4.00 aA ± 0.00 | 5.00 bA ± 0.00 | 4.97 bA ± 0.13 | 4.92 bA ± 0.19 | 4.92 bA ± 0.28 | 0.7685 * | |
| Lactate | 4.86 aA ± 0.38 | 5.00 aA ± 0.00 | 5.00 aA ± 0.00 | 5.00 aA ± 0.00 | 4.67 aA ± 0.52 | −0.2052 | |
| Carbonate | 4.88 aA ± 0.31 | 4.85 aA ± 0.34 | 4.91 aA ± 0.30 | 4.77 aA ± 0.41 | 4.73 aA ± 0.41 | −0.1763 | |
| Consistency | Chloride | 4.75 aA ± 0.40 | 4.70 aA ± 0.42 | 4.67 aA ± 0.56 | 4.65 aA ± 0.41 | 4.93 aB ± 0.16 | −0.0435 |
| Gluconate | 4.90 bA ± 0.00 | 4.80 abA ± 0.37 | 4.86 abA ± 0.57 | 4.39 aA ± 0.48 | 4.38 aAB ± 0.47 | −0.5646 * | |
| Lactate | 4.93 aA ± 0.19 | 4.78 aA ± 0.44 | 4.83 aA ± 0.35 | 4.88 aA ± 0.25 | 4.91 aB ± 0.20 | 0.1622 | |
| Carbonate | 4.79 aA ± 0.58 | 4.16 aA ± 0.75 | 4.00 aA ± 0.71 | 4.41 aA ± 0.63 | 4.00 aA ± 0.70 | −0.5109 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlos, M.; Znamirowska-Piotrowska, A.; Kowalczyk, M.; Zaguła, G.; Szajnar, K. Possibility of Using Different Calcium Compounds for the Manufacture of Fresh Acid Rennet Cheese from Goat’s Milk. Foods 2023, 12, 3703. https://doi.org/10.3390/foods12193703
Pawlos M, Znamirowska-Piotrowska A, Kowalczyk M, Zaguła G, Szajnar K. Possibility of Using Different Calcium Compounds for the Manufacture of Fresh Acid Rennet Cheese from Goat’s Milk. Foods. 2023; 12(19):3703. https://doi.org/10.3390/foods12193703
Chicago/Turabian StylePawlos, Małgorzata, Agata Znamirowska-Piotrowska, Magdalena Kowalczyk, Grzegorz Zaguła, and Katarzyna Szajnar. 2023. "Possibility of Using Different Calcium Compounds for the Manufacture of Fresh Acid Rennet Cheese from Goat’s Milk" Foods 12, no. 19: 3703. https://doi.org/10.3390/foods12193703
APA StylePawlos, M., Znamirowska-Piotrowska, A., Kowalczyk, M., Zaguła, G., & Szajnar, K. (2023). Possibility of Using Different Calcium Compounds for the Manufacture of Fresh Acid Rennet Cheese from Goat’s Milk. Foods, 12(19), 3703. https://doi.org/10.3390/foods12193703









