Biochemical Properties of a Promising Milk-Clotting Enzyme, Moose (Alces alces) Recombinant Chymosin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of an Integrative Plasmid Vector
2.2. Preparation of Kluyveromyces Lactis Strain Alc-D Transgenic for Moose ProChn
2.3. Producer Cultivation
2.4. Zymogen Activation
2.5. Partial Purification of Moose rChn
2.6. Biochemical Characterization of rChn
2.6.1. Enzymatic Kinetics
2.6.2. Dependence of MA on the Temperature of the Substrate (Temperature Optimum)
2.6.3. Influence of Divalent Metal Cations on Milk-Clotting Activity
2.6.4. Determination of Proteolytic Specificity by Electrophoresis
2.6.5. Preparations of Cow and Camel rChns
2.6.6. Protein Concentration
2.7. Statistical Processing
2.8. Crystallization of Moose rChn and Acquisition of X-ray Diffraction Data
3. Results and Discussion
3.1. Construction of the Producer Strain
3.2. Cultivation of the Producer Strain
3.3. Activation of the Zymogen and Yield of the Target Enzyme
3.4. Partial Purification of Moose rChn
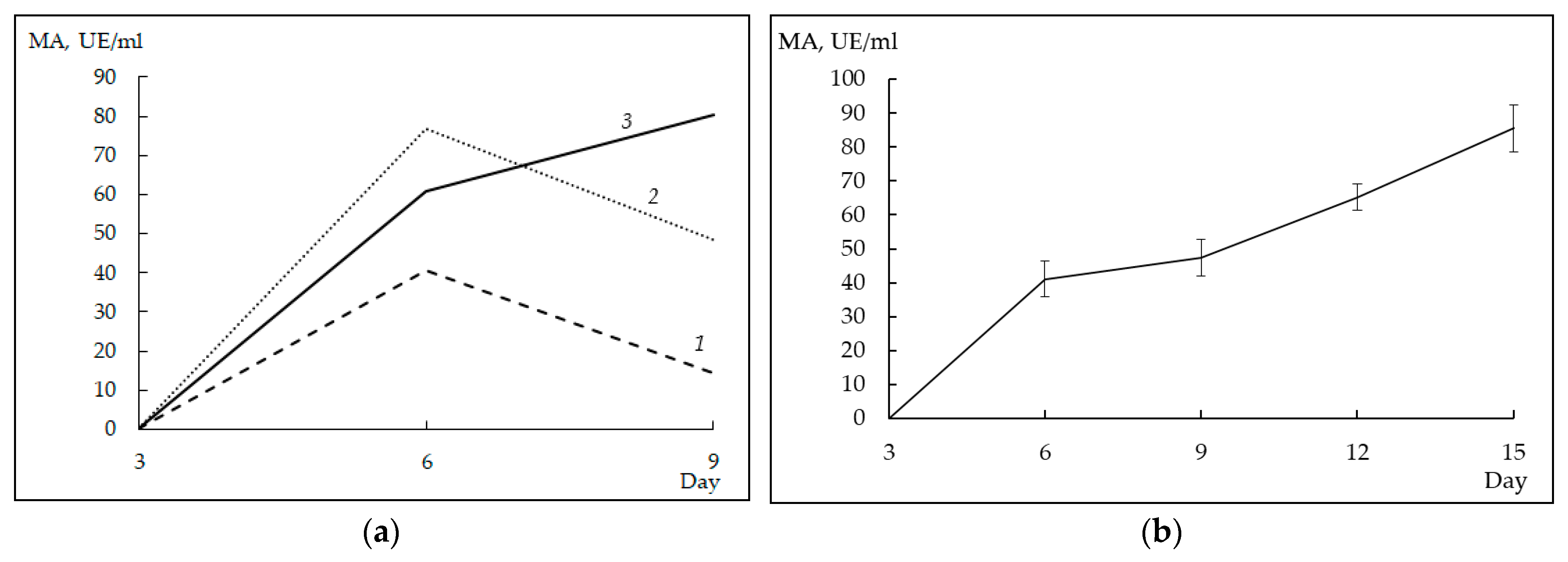
3.5. Milk-Clotting Activity
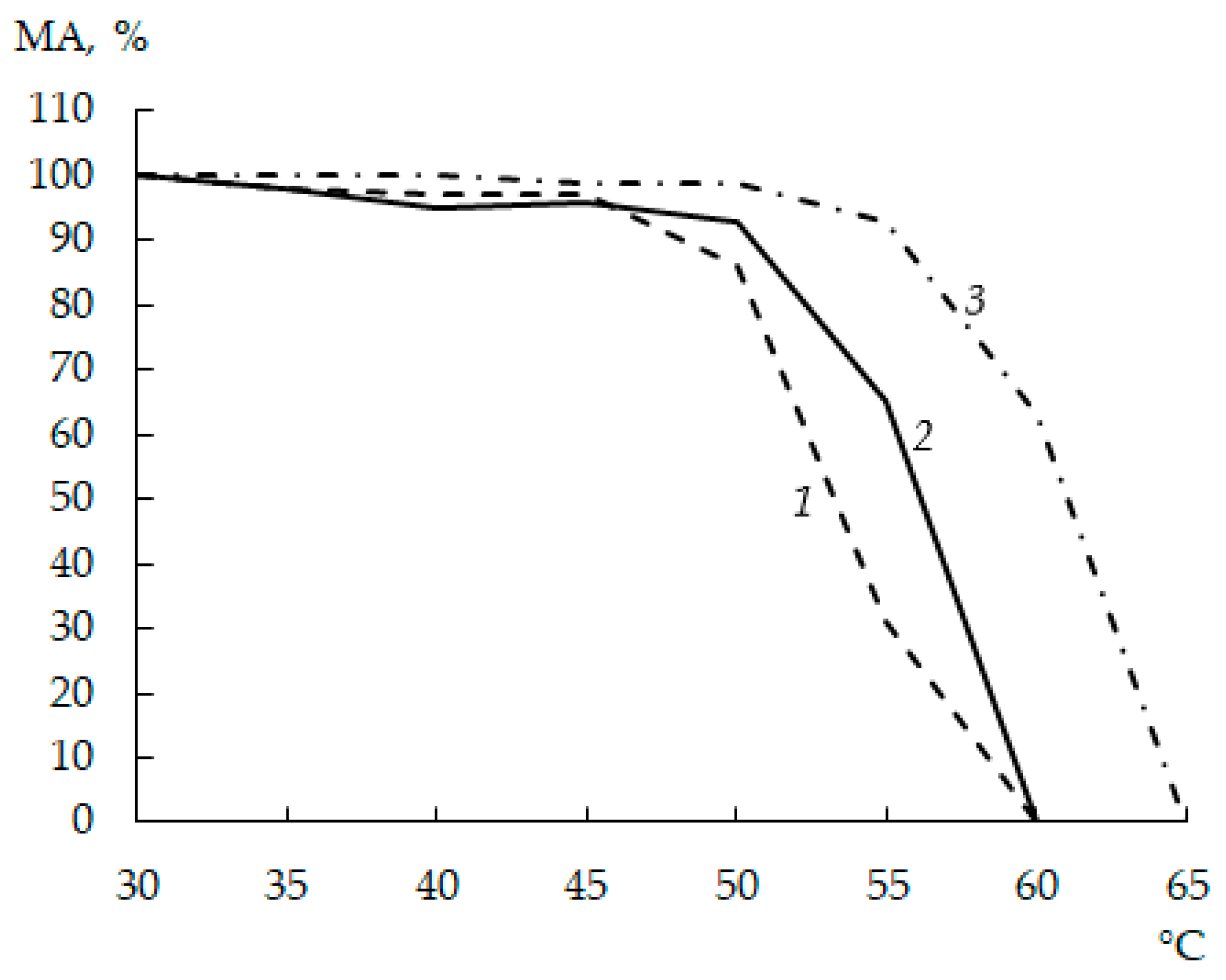
3.6. Thermal Stability
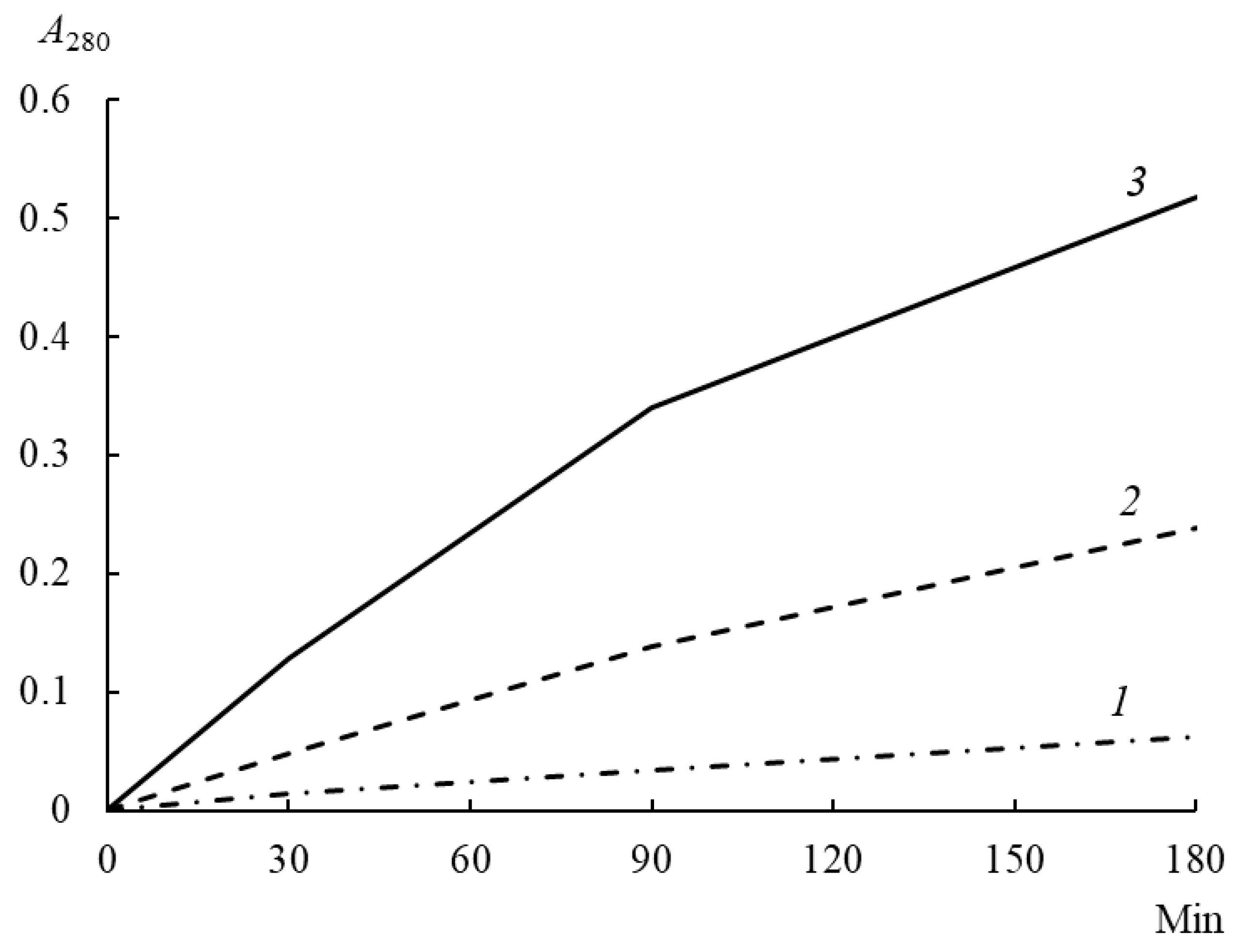
3.7. Proteolytic Activity
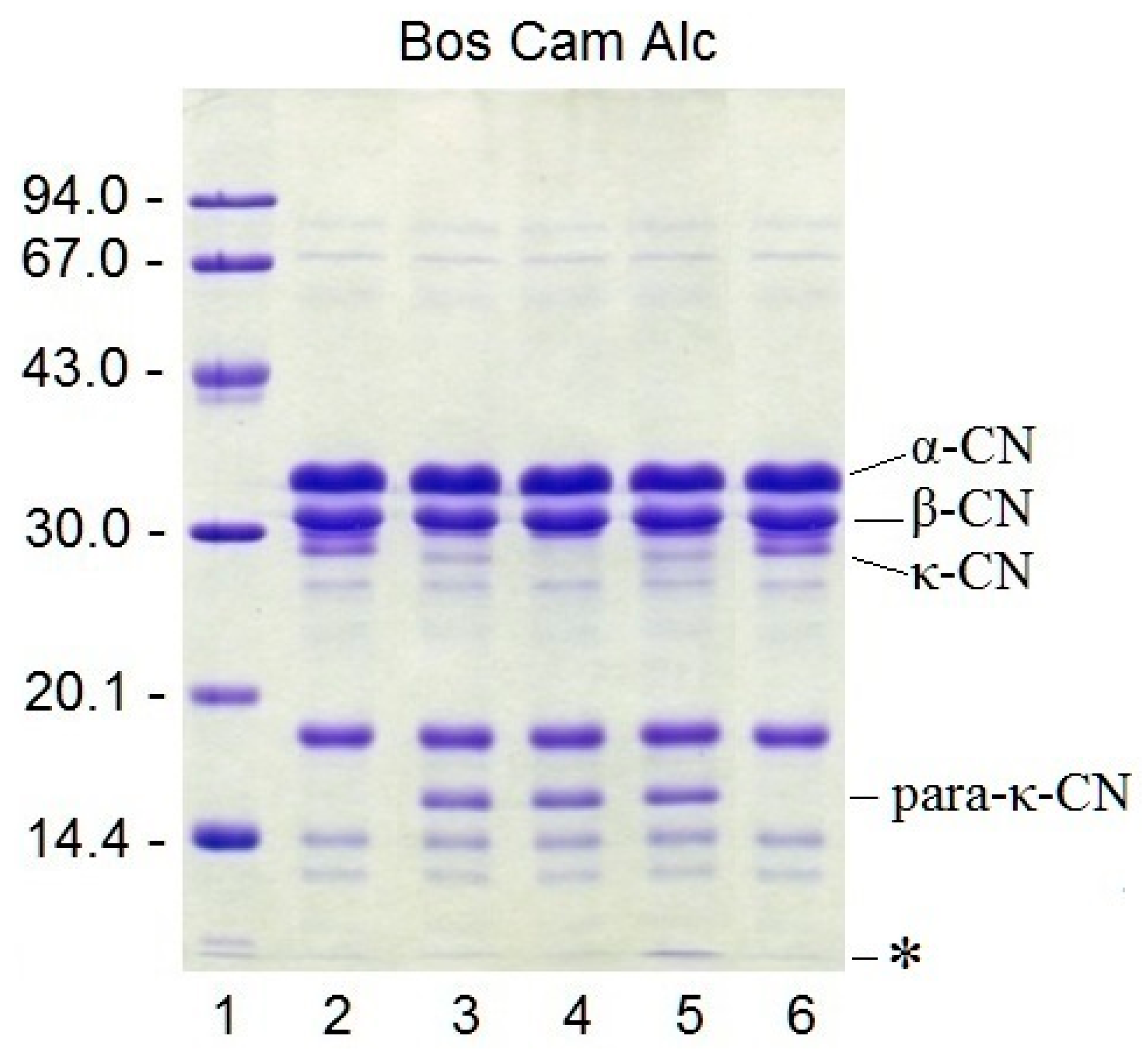
3.8. Proteolytic Specificity
3.9. Parameters of Enzyme Kinetics
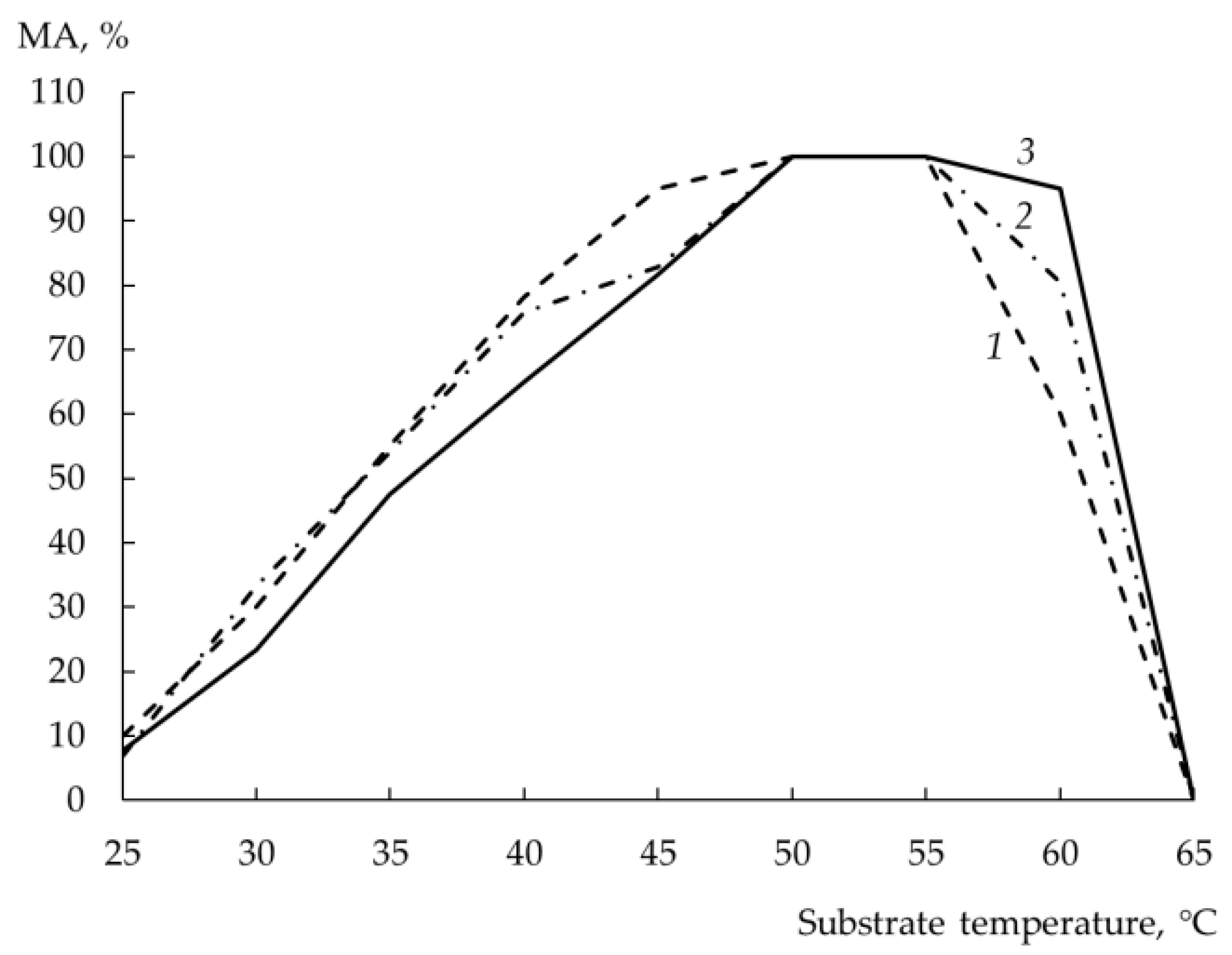
3.10. Temperature Optimum
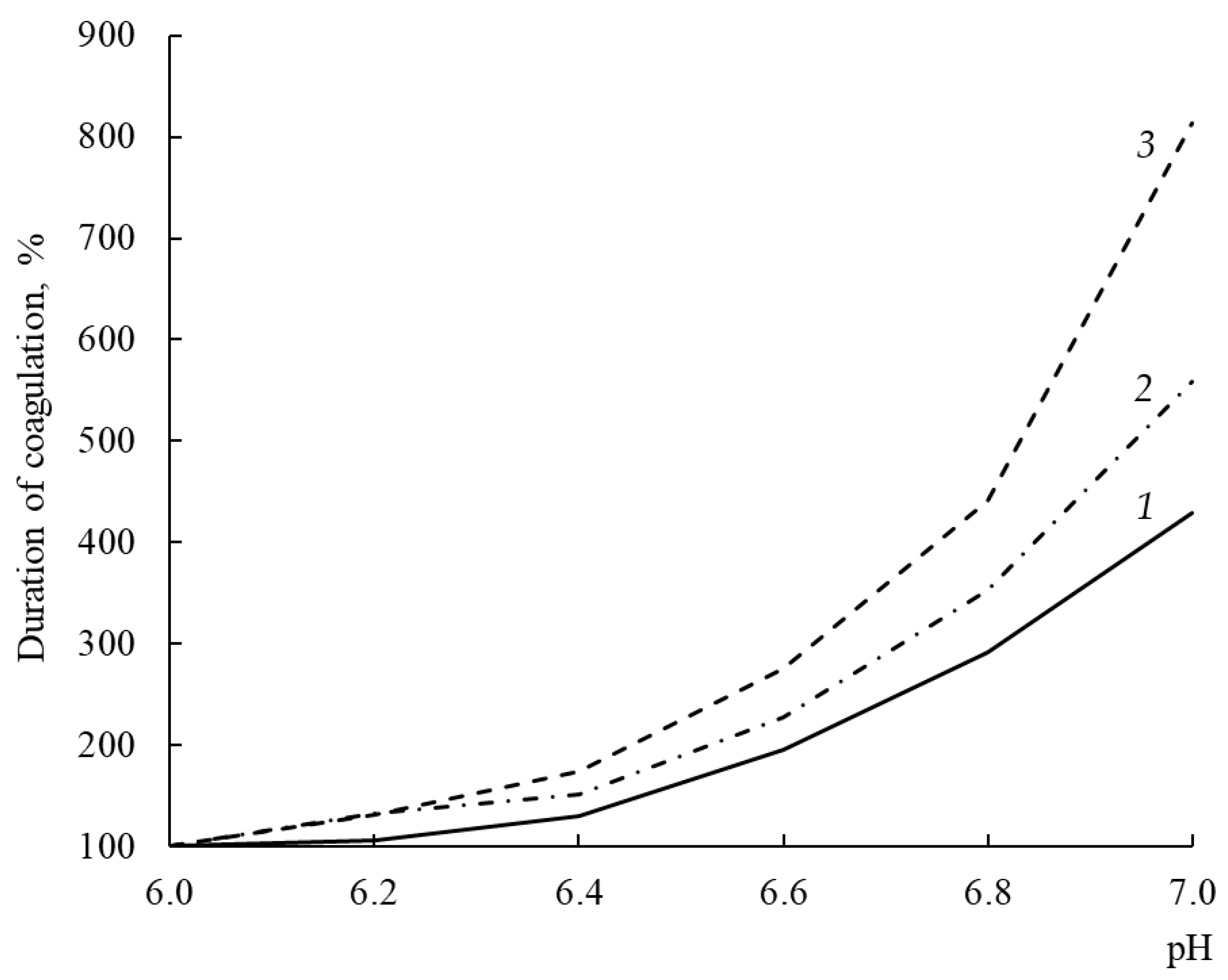
3.11. Dependence of Milk-Clotting Activity on pH of Milk
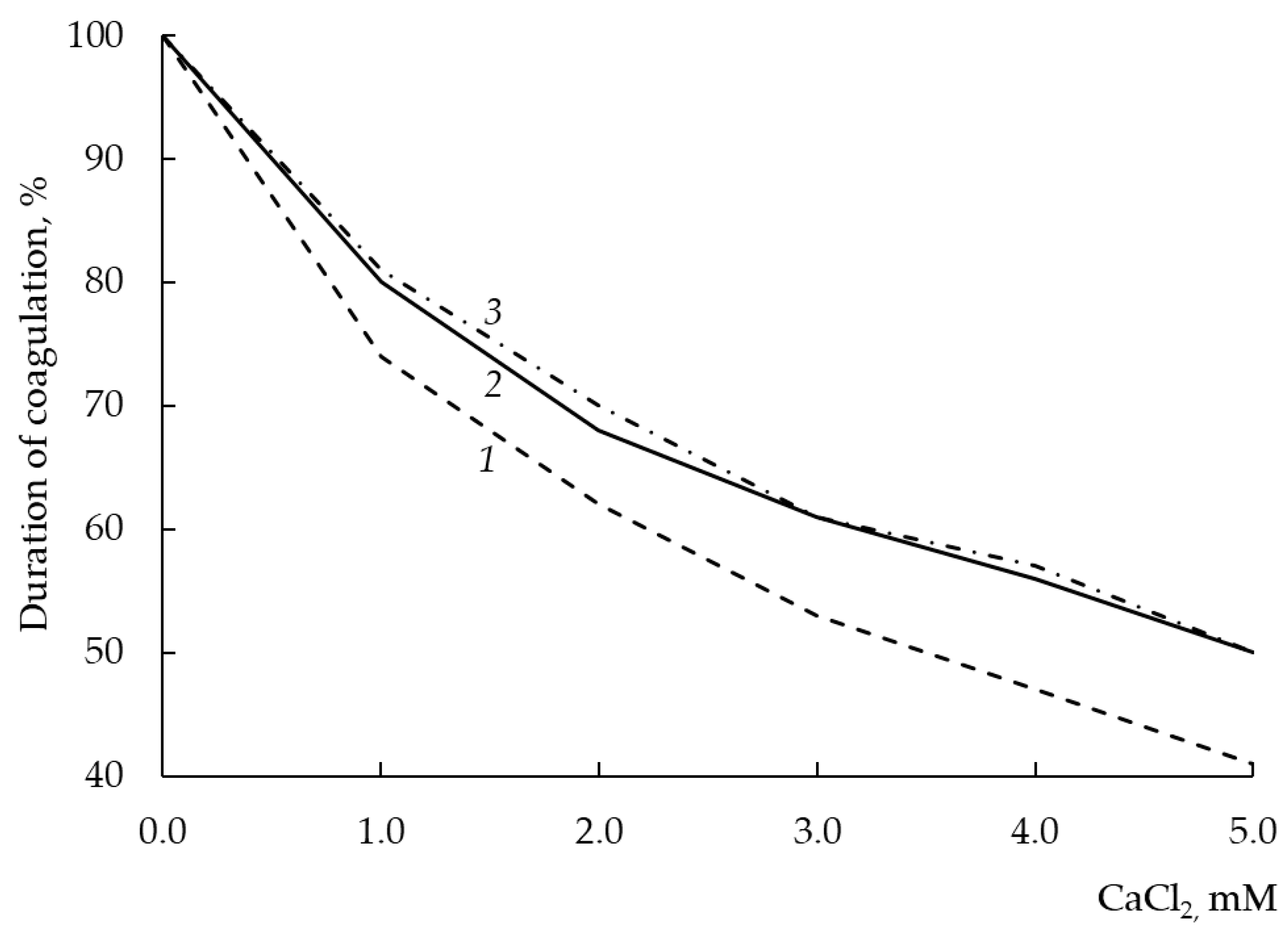
3.12. Dependence of Milk-Clotting Activity on the Concentration of Calcium Chloride
3.13. Influence of Divalent Metal Cations on Milk-Clotting Activity
3.14. X-ray Diffraction Data
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uniacke-Lowe, T.; Fox, P.F. Chymosin, pepsins and other aspartyl proteinases: Structures, functions, catalytic mechanism and milk-clotting properties. In Cheese, 4th ed.; McSweeney, P.L.H., Cotter, P.D., Fox, P.F., Everett, D.W., Eds.; Elsevier Academic Press: Oxford, UK, 2017; pp. 69–113. [Google Scholar] [CrossRef]
- Foltmann, B. Chymosin: A short review on foetal and neonatal gastric proteases. Scand. J. Clin. Lab. Investig. 1992, 210, 65–79. [Google Scholar] [CrossRef]
- Holt, C.; Carver, J.A.; Ecroyd, H.; Thorn, D.C. Invited review: Caseins and the casein micelle: Their biological functions, structures, and behavior in foods. J. Dairy Sci. 2013, 96, 6127–6146. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Marques, M.; Ruivo, R.; Fonseca, E.; Teixeira, A.; Castro, L.F.C. Unusual loss of chymosin in mammalian lineages parallels neo-natal immune transfer strategies. Mol. Phylogenet. Evol. 2017, 116, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Harboe, M.; Broe, M.L.; Qvist, K.B. The Production, Action and Application of Rennet and Coagulants. In Technology of Cheesemaking; Law, B.A., Tamime, A.Y., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; Chapter 3; pp. 98–129. [Google Scholar] [CrossRef]
- Uchiyama, H.; Uozumi, T.; Beppu, T.; Arima, K. Purification of prorennin mRNA and its translation in vitro. Agric. Biol. Chem. 1980, 44, 1373–1381. [Google Scholar] [CrossRef]
- Flamm, E.L. How FDA approved chymosin: A case history. Nat. Biotechnol. 1991, 9, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.L. Production of chymosin for the dairy industry by recombinant DNA technology. Australas. Biotechnol. 1994, 4, 19–23. [Google Scholar]
- Kappeler, S.R.; van den Brink, H.J.M.; Rahbek-Nielsen, H.; Farah, Z.; Puhan, Z.; Hansen, E.B.; Johansen, E. Characterization of recombinant camel chymosin reveals superior properties for the coagulation of bovine and camel milk. Biochem. Biophys. Res. Commun. 2006, 2, 647–654. [Google Scholar] [CrossRef]
- Jensen, J.L.; Mølgaard, A.; Poulsen, J.-C.N.; Harboe, M.K.; Simonsen, J.B.; Lorentzen, A.M.; Hjernø, K.; van den Brink, J.M.; Qvist, K.B.; Larsen, S. Camel and bovine chymosin: The relationship between their structures and cheese-making properties. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 901–913. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Enzymatic Coagulation of Milk. In Fundamentals of Cheese Science; Fox, P.F., Guinee, T.P., Cogan, T.M., McSweeney, P.L.H., Eds.; Springer: New York, NY, USA, 2017; Chapter 7; pp. 185–229. [Google Scholar] [CrossRef]
- Yelchaninov, V.V. The Problem of Searching for New Milk-Enzymes for Cheese-Making: Criteria for Selecting Gene Sources for Recombinant Chymosins: A Monograph; Altai State University: Barnaul, Russia, 2021; 170p. [Google Scholar]
- Rogelj, I.; Perko, B.; Francky, A.; Penca, V.; Purgenčar, J. Recombinant Lamb Chymosin as an Alternative Coagulating Enzyme in Cheese Production. J. Dairy Sci. 2001, 84, 1020–1026. [Google Scholar] [CrossRef]
- Vega-Hernandes, M.C.; Gomes-Coello, A.; Villar, J.; Claverie-Martin, F. Molecular cloning and expression in yeast of caprine prochymosin. J. Biotechnol. 2004, 114, 69–79. [Google Scholar] [CrossRef]
- Vallejo, J.A.; Ageitos, J.M.; Poza, M.; Villa, T.G. Short communication: A comparative analysis of recombinant chymosins. J. Dairy Sci. 2012, 95, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Kumar, A.; Yadav, A.K.; Saklani, A.C.; Grover, S.; Batish, V.K. Functional expression of recombinant goat chymosin in Pichia pastoris bioreactor cultures: A commercially viable alternate. LWT Food Sci. Technol. 2016, 69, 217–224. [Google Scholar] [CrossRef]
- Vallejo, J.A.; Ageitos, J.M.; Poza, M.; Villa, T.G. Cloning and Expression of Buffalo Active Chymosin in Pichia pastoris. J. Agric. Food Chem. 2008, 56, 10606–10610. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Kumar, A.; Mohanty, A.K.; Kaushik, J.K.; Grover, S.; Batish, V.K. Expression of buffalo chymosin in Pichia pastoris for application in mozzarella cheese. LWT Food Sci. Technol. 2017, 84, 733–739. [Google Scholar] [CrossRef]
- Luo, F.; Jiang, W.H.; Yang, Y.X.; Li, J.; Jiang, M.F. Cloning and Expression of Yak Active Chymosin in Pichia pastoris. Asian-Australas. J. Anim. Sci. 2016, 29, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Ersöz, F.; İnan, M. Large-scale production of yak (Bos grunniens) chymosin A in Pichia pastoris. Prot. Expr. Purif. 2019, 154, 126–133. [Google Scholar] [CrossRef]
- Belenkaya, S.V.; Rudometov, A.P.; Shcherbakov, D.N.; Balabova, D.V.; Kriger, A.V.; Belov, A.N.; Koval, A.D.; Elchaninov, V.V. Biochemical Properties of Recombinant Chymosin in Alpaca (Vicugna pacos L.). Appl. Biochem. Microbiol. 2018, 54, 569–576. [Google Scholar] [CrossRef]
- Belenkaya, S.V.; Shcherbakov, D.N.; Balabova, D.V.; Belov, A.N.; Koval, A.D.; Elchaninov, V.V. Production of Maral (Cervus elaphus sibiricus Severtzov) Recombinant Chymosin in the Prokaryotic Expression System and the Study of the Aggregate of Its Biochemical Properties Relevant for the Cheese-Making Industry. Appl. Biochem. Microbiol. 2020, 56, 647–656. [Google Scholar] [CrossRef]
- Belenkaya, S.V.; Bondar, A.A.; Kurgina, T.A.; Elchaninov, V.V.; Bakulina, A.Y.; Rukhlova, E.A.; Lavrik, O.I.; Ilyichev, A.A.; Shcherbakov, D.N. Characterization of the Altai Maral Chymosin Gene, Production of a Chymosin Recombinant Analog in the Prokaryotic Expression System, and Analysis of Its Several Biochemical Properties. Biochemistry 2020, 85, 781–791. [Google Scholar] [CrossRef]
- Belenkaya, S.V.; Elchaninov, V.V.; Shcherbakov, D.N. Development of a producer of recombinant maral chymosin based on the yeast Kluyveromyces lactis. Biotechnology 2021, 37, 20–27. [Google Scholar] [CrossRef]
- Belenkaya, S.V.; Chirkova, V.Y.; Sharlaeva, E.A.; Elchaninov, V.V.; Shcherbakov, D.N. Parameters of enzymatic kinetics of recombinant chymosin of the Altai red deer (Cervus elaphus sibiricus) obtained in pro- and eukaryotic expression systems. Biotechnology 2022, 38, 11–16. [Google Scholar] [CrossRef]
- Alihanoğlu, S.; Ektiren, D.; Karaaslan, M. Recombinant expression and characterization of Oryctolagus cuniculus chymosin in Komagataella phaffii (Pichia pastoris). Protein Expr. Purif. 2021, 183, 105874. [Google Scholar] [CrossRef]
- Filkin, S.Y.; Chertova, N.V.; Zatsepin, S.S.; Sadykhov, E.G.; Fedorov, A.N.; Lipkin, A.V. Production of Beluga Whale (Delphinapterus leucas) Chymosin in the Methylotrophic Yeast Komagataella phaffii and Characteristics of the Recombinant Enzyme. Appl. Biochem. Microbiol. 2021, 57, 297–302. [Google Scholar] [CrossRef]
- Akishev, Z.; Kiribayeva, A.; Mussakhmetov, A.; Baltin, K.; Ramankulov, Y.; Khassenov, B. Constitutive expression of Camelus bactrianus prochymosin B in Pichia pastoris. Heliyon 2021, 7, e07137. [Google Scholar] [CrossRef] [PubMed]
- Akishev, Z.; Aktayeva, S.; Kiribayeva, A.; Abdullayeva, A.; Baltin, K.; Mussakhmetov, A.; Tursunbekova, M.; Ramankulov, Y.; Khassenov, B. Obtaining of Recombinant Camel Chymosin and Testing Its Milk-Clotting Activity on Cow’s, Goat’s, Ewes’, Camel’s and Mare’s Milk. Biology 2022, 11, 1545. [Google Scholar] [CrossRef]
- Balabova, D.V.; Belenkaya, S.V.; Volosnikova, E.A.; Hermes, M.; Chirkova, V.Y.; Sharlaeva, E.A.; Shcherbakov, D.N.; Belov, A.N.; Koval, A.D.; Elchaninov, V.V. Can Recombinant Tree Shrew (Tupaia belangeri chinensis) Chymosin Coagulate Cow (Bos taurus) Milk? Appl. Biochem. Microbiol. 2022, 58, 763–772. [Google Scholar] [CrossRef]
- Balabova, D.V.; Rudometov, A.P.; Belenkaya, S.V.; Belov, A.N.; Koval, A.D.; Bondar, A.A.; Bakulina, A.Y.; Rukhlova, E.A.; Elchaninov, V.V.; Shcherbakov, D.N. Biochemical and technological properties of moose (Alces alces) recombinant chymosin. Vavilovskii Zhurnal Genet. I Sel. (Vavilov. J. Genet. Breed.) 2022, 26, 240–249. [Google Scholar] [CrossRef]
- Liu, W.-G.; Wang, Y.-P.; Zhang, Z.-J.; Wang, M.; Lv, Q.-X.; Liu, H.-W.; Lu, M. Generation and characterization of caprine chymosin in corn seed. Protein Expr. Purif. 2017, 135, 78–82. [Google Scholar] [CrossRef]
- Wei, Z.-Y.; Zhang, Y.-Y.; Wang, Y.-P.; Fan, M.-X.; Zhong, X.-F.; Xu, N.; Xing, S.-C. Production of Bioactive Recombinant Bovine Chymosin in Tobacco Plants. Int. J. Mol. Sci. 2016, 17, 624. [Google Scholar] [CrossRef]
- Kumar, A.; Grover, S.; Sharma, J.; Batish, V.K. Chymosin and other milk coagulants: Sources and biotechnological interventions. Crit. Rev. Biotechnol. 2010, 30, 243–258. [Google Scholar] [CrossRef]
- Kolmer, M.; Õrd, T.; Ulmanen, I. Expression of recombinant calf chymosin in mammalian cell culture J. Biotechnol. 1991, 20, 131–139. [Google Scholar] [CrossRef]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast Expression Systems: Overview and Recent Advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Spohner, S.C.; Schaum, V.; Quitmann, H.; Czermak, P. Kluyveromyces lactis: An emerging tool in biotechnology. J. Biotechnol. 2016, 222, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Patra, P.; Das, M.; Kundu, P.; Ghosh, A. Recent advances in systems and synthetic biology approaches for developing novel cell-factories in non-conventional yeasts. Biotechnol. Adv. 2021, 47, 107695. [Google Scholar] [CrossRef]
- Van den Dungen, M.W.; Boer, R.; Wilms, L.C.; Efimova, Y.; Abbas, H.E. The safety of a Kluyveromyces lactis strain lineage for enzyme production. Regul. Toxicol. Pharmacol. 2021, 126, 105027. [Google Scholar] [CrossRef]
- Sakhtah, H.; Behler, J.; Ali-Reynolds, A.; Causey, T.B.; Vainauskas, S.A.; Taron, C.H. Novel Regulated Hybrid Promoter That Permits Autoinduction of Heterologous Protein Expression in Kluyveromyces lactis. Appl. Environ. Microbiol. 2019, 85, e00542-19. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Warburg, O.; Christian, W. Isolierung und Kristallisation des Garungsterments Enolase. Biochem. Z. 1942, 310, 384–421. [Google Scholar]
- Newman, J.; Egan, D.; Walter, T.S.; Meged, R.; Berry, I.; Jelloul, M.B.; Sussman, J.L.; Stuart, D.; Perrakis, A. Towards rationalization of crystallization screening for small- to mediumsized laboratories: The PACT/JCSG+ strategy. Acta Crystallogr. Sect. D Biol. Crystallogr. 2005, 10, 1426–1431. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Belenkaya, S.V.; Balabova, D.V.; Belov, A.N.; Koval, A.D.; Shcherbakov, D.N.; Elchaninov, V.V. Basic Biochemical Properties of Recombinant Chymosins (Review). Appl. Biochem. Microbiol. 2020, 56, 363–372. [Google Scholar] [CrossRef]
- Myagkonosov, D.S.; Abramov, D.V.; Delitskaya, I.N.; Ovchinnikova, E.G. Proteolytic activity of milk-clotting enzymes of different origin. Food Syst. 2022, 5, 47–54. [Google Scholar] [CrossRef]
- Emmons, D.B.; Beckett, D.C.; Binns, M. Milk-clotting enzymes. 1. Proteolysis during cheese making in relation to estimated losses of yield. J. Dairy Sci. 1990, 73, 2007–2015. [Google Scholar] [CrossRef]
- Singh, T.K.; Drake, M.A.; Cadwallader, K.R. Flavor of Cheddar Cheese: A Chemical and Sensory Perspective. Compr. Rev. Food Sci. Food Safety. 2003, 2, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Costabel, L.M.; Bergamini, C.V.; Pozza, L.; Cuffia, F.; Candioti, M.C.; Hynes, E. Influence of chymosin type and curd scalding temperature on proteolysis of hard cooked cheeses. J. Dairy Res. 2015, 82, 375–384. [Google Scholar] [CrossRef]
- Wang, N.; Wang, K.Y.; Li, G.; Guo, W.; Liu, D. Expression and characterization of camel chymosin in Pichia pastoris. Prot. Expr. Purif. 2015, 111, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Belov, A.N.; Koval, A.D.; Pushkarev, V.A.; Mironova, A.V.; Scherbakov, D.N.; Belenkaya, S.V.; Balabova, D.V.; Yelchaninov, V.V. First experience of application of domestic recombinant chymosin in cheese making with low temperature of the second heating. Cheese-Mak. Butter-Mak. 2022, 3, 28–32. [Google Scholar]
- Belov, A.N.; Koval, A.D.; Pushkarev, V.A.; Mironova, A.V.; Scherbakov, D.N.; Belenkaya, S.V.; Balabova, D.V.; Yelchaninov, V.V. Validation of recombinant maral chymosin in the production of cheese with high second heating temperature. Cheese-Mak. Butter-Mak. 2023, 3, 32–34. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Factors That Affecting Cheese Yield: Rennet Type. In Fundamentals of Cheese Science, 2nd ed.; Fox, P.F., Guinee, T.P., Cogan, T.M., McSweeney, P.L.H., Eds.; Springer: New York, NY, USA, 2017; Chapter 10.5.13; pp. 315–317. [Google Scholar] [CrossRef]
- Hayes, M.G.; Oliveira, J.C.; Mcsweeney, P.L.; Kelly, A.L. Thermal inactivation of chymosin during cheese manufacture. J. Dairy Res. 2002, 69, 269–279. [Google Scholar] [CrossRef]
- Bansal, N.; Fox, P.F.; McSweeney, P.L. Factors affecting the retention of rennet in cheese curd. J. Agric. Food Chem. 2007, 55, 9219–9225. [Google Scholar] [CrossRef]
- D’Incecco, P.; Limbo, S.; Hogenboom, J.; Rosi, V.; Gobbi, S.; Pellegrino, L. Impact of Extending Hard-Cheese Ripening: A Multiparameter Characterization of Parmigiano Reggiano Cheese Ripened up to 50 Months. Foods 2020, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Hynes, E.R.; Aparo, L.; Candioti, M.C. Influence of Residual Milk-Clotting Enzyme on αs1-Casein Hydrolysis During Ripening of Reggianito Argentino Cheese. J. Dairy Sci. 2004, 87, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.J.; Oliveira, J.C.; Kelly, A.L.; McSweeney, P.L.H. Effect of cook temperature on primary proteolysis and predicted residual chymosin activity of a semi-hard cheese manufactured using thermophilic cultures. Int. Dairy J. 2007, 17, 826–834. [Google Scholar] [CrossRef]
- Kelly, A.L.; McSweeney, P.L.H. digenous proteinases in milk. In Advanced Dairy Chemistry—Proteins, 3rd ed.; McSweeney, P.L.H., Fox, P.F., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003; Volume I, pp. 495–521. [Google Scholar]
- Fox, P.F.; Cogan, T.M. Factors that Affect the Quality of Cheese. In Cheese Chemistry, Physics and Microbiology, 3rd ed.; Fox, P.F., Cogan, T.M., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; Elsevier Academic Press: London, UK, 2004; Volume I, pp. 583–608. [Google Scholar]
- Jiang, X.P.; Yin, M.L.; Chen, P.; Yang, Q. Constitutive expression, purification and characterization of bovine prochymosin in Pichia pastoris GS115. World J. Microbiol. Biotechnol. 2012, 28, 2087–2093. [Google Scholar] [CrossRef] [PubMed]

| Preparation | Volume (mL) | MA (AU/mL) | Total MA (AU) | A280 | Relative MA (MA/A280) |
|---|---|---|---|---|---|
| Culture liquid with rChn-Alc-KL | 4800 | 86 | 412,800 | 9.798 | 8.8 |
| Partially purified rChn-Alc-KL | 15 | 14,469 | 217,035 | 0.669 | 21,627.8 |
| Preparation | Total MA (AU/mL) | Protein Concentration (mg/mL) | Specific MA (AU/mg) | Specific MA (%) |
|---|---|---|---|---|
| rChn-Alc-KL | 1293 ± 16 | 0.082 ± 0.001 | 15,768 | 106 |
| rChn-Bos | 1293 ± 16 | 0.087 ± 0.001 | 14,862 | 100 |
| rChn-Cam | 1324 ± 0 | 0.077 ± 0.001 | 17,195 | 116 |
| rChn | Specific MA (%) | PA (A280) | PA (%) | MA/PA |
|---|---|---|---|---|
| rChn-Alc-KL | 106 | 0.332 ± 0.001 | 138 | 0.8 |
| rChn-Bos | 100 | 0.241 ± 0.001 | 100 | 1.0 |
| rChn-Cam | 116 | 0.037 ± 0.000 | 15 | 7.7 |
| rChn | Km, μM | Vmax, nM/s | kcat (s−1) | kcat/Km, (μM−1 s−1) |
|---|---|---|---|---|
| rChn-Alc-KL | 4.69 ± 0.27 | 616.85 ± 28.44 | 98.69 ± 4.55 | 21.11 ± 0.78 |
| rChn-Bos | 2.12 ± 0.09 | 315.31 ± 3.48 | 252.24 ± 2.79 | 119.58 ± 4.74 |
| rChn-Cam | 1.27 ± 0.16 | 190.30 ± 29.21 | 152.24 ± 23.37 | 118.81 ± 6.74 |
| Substrate (±Me2+) | MCA (%) | ||
|---|---|---|---|
| rChn-Alc-KL | rChn-Bos | rChn-Cam | |
| Control | 100.0 | 100.0 | 100.0 |
| Cu2+ | 136.6 ± 2.1 | 67.9 ± 1.3 | 73.5 ± 1.6 |
| Mg2+ | 259.6 ± 3.7 | 204.1 ± 7.2 | 162.5 ± 1.8 |
| Ni2+ | 73.3 ± 0.6 | 71.3 ± 1.4 | 53.0 ± 0.7 |
| Zn2+ | 161.1 ± 5.0 | 109.0 ± 2.3 | 111.5 ± 4.4 |
| Co2+ | 256.2 ± 7.0 | 201.8 ± 9.0 | 145.0 ± 2.5 |
| Ba2+ | 319.3 ± 9.7 | 296.6 ± 10.8 | 246.4 ± 0.0 |
| Fe2+ | 308.1 ± 5.3 | 296.0 ± 5.7 | 224.3 ± 5.9 |
| Mn2+ | 386.8 ± 8.4 | 308.1 ± 10.5 | 321.6 ± 6.8 |
| Ca2+ | 298.4 ± 10.1 | 296.6 ± 10.8 | 217.7 ± 6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balabova, D.V.; Belash, E.A.; Belenkaya, S.V.; Shcherbakov, D.N.; Belov, A.N.; Koval, A.D.; Mironova, A.V.; Bondar, A.A.; Volosnikova, E.A.; Arkhipov, S.G.; et al. Biochemical Properties of a Promising Milk-Clotting Enzyme, Moose (Alces alces) Recombinant Chymosin. Foods 2023, 12, 3772. https://doi.org/10.3390/foods12203772
Balabova DV, Belash EA, Belenkaya SV, Shcherbakov DN, Belov AN, Koval AD, Mironova AV, Bondar AA, Volosnikova EA, Arkhipov SG, et al. Biochemical Properties of a Promising Milk-Clotting Enzyme, Moose (Alces alces) Recombinant Chymosin. Foods. 2023; 12(20):3772. https://doi.org/10.3390/foods12203772
Chicago/Turabian StyleBalabova, Dina V., Ekaterina A. Belash, Svetlana V. Belenkaya, Dmitry N. Shcherbakov, Alexander N. Belov, Anatoly D. Koval, Anna V. Mironova, Alexander A. Bondar, Ekaterina A. Volosnikova, Sergey G. Arkhipov, and et al. 2023. "Biochemical Properties of a Promising Milk-Clotting Enzyme, Moose (Alces alces) Recombinant Chymosin" Foods 12, no. 20: 3772. https://doi.org/10.3390/foods12203772
APA StyleBalabova, D. V., Belash, E. A., Belenkaya, S. V., Shcherbakov, D. N., Belov, A. N., Koval, A. D., Mironova, A. V., Bondar, A. A., Volosnikova, E. A., Arkhipov, S. G., Sokolova, O. O., Chirkova, V. Y., & Elchaninov, V. V. (2023). Biochemical Properties of a Promising Milk-Clotting Enzyme, Moose (Alces alces) Recombinant Chymosin. Foods, 12(20), 3772. https://doi.org/10.3390/foods12203772







