The Protective Effect of a Functional Food Consisting of Astragalus membranaceus, Trichosanthes kirilowii, and Angelica gigas or Its Active Component Formononetin against Inflammatory Skin Disorders through Suppression of TSLP via MDM2/HIF1α Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Cell Culture and Treatment
2.3. Cell Viability
2.4. ELISA
2.5. qPCR
2.6. Western Blotting Analysis
2.7. Analysis Immunofluorescence Staining
2.8. Animal Studies
2.9. HPLC Analysis
2.10. Statistical Analyses
3. Results
3.1. JRP-SNF102 or FMN Inhibited TSLP Secretion in Activated HMC-1 Cells
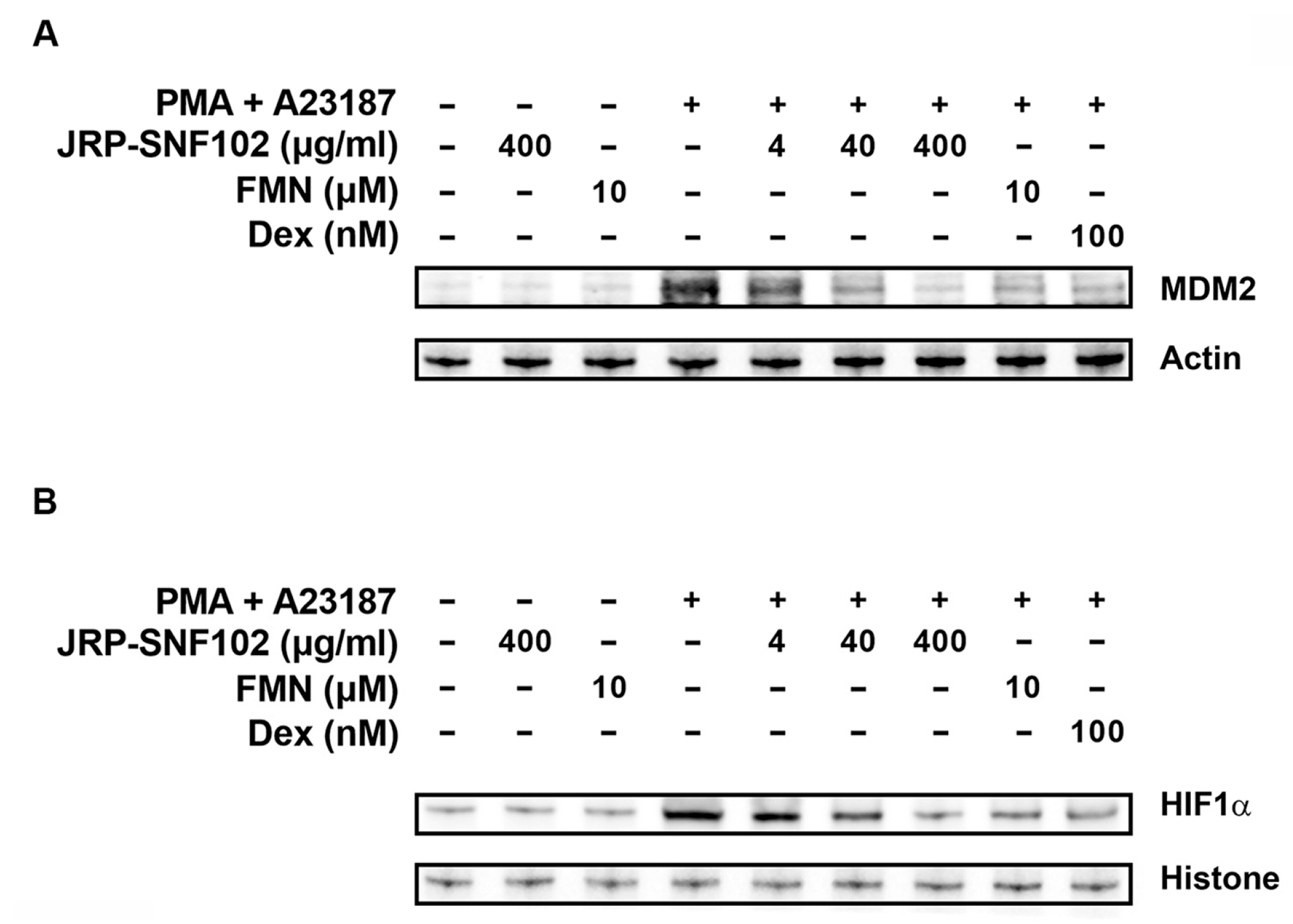
3.2. JRP-SNF102 or FMN Suppressed MDM2 Expression in Activated HMC-1 Cells
3.3. JRP-SNF102 or FMN Suppressed Nuclear NF-κB Expression in Activated HMC-1 Cells
3.4. JRP-SNF102 or FMN Suppressed PMA-Induced Ear Edema
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, K.; Song, X.; Jia, R.; Yin, Z.; Zou, Y.; Li, L.; Yin, L.; He, C.; Liang, X.; Yue, G.; et al. Evaluation of Analgesic and Anti-Inflammatory Activities of Water Extract of Galla Chinensis In Vivo Models. Evid.-Based Complement. Altern. Med. 2018, 2018, 6784032. [Google Scholar] [CrossRef] [Green Version]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudeck, A.; Dudeck, J.; Scholten, J.; Petzold, A.; Surianarayanan, S.; Köhler, A.; Peschke, K.; Vöhringer, D.; Waskow, C.; Krieg, T.; et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 2011, 34, 973–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collington, S.J.; Williams, T.J.; Weller, C.L. Mechanisms underlying the localisation of mast cells in tissues. Trends Immunol. 2011, 32, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Katsoulis-Dimitriou, K.; Edler, H.J.; Dudeck, J.; Drube, S.; Dudeck, A. Mast cells initiate the vascular response to contact allergens by sensing cell stress. J. Allergy Clin. Immunol. 2020, 145, 1476–1479.e3. [Google Scholar] [CrossRef] [Green Version]
- Okayama, Y.; Okumura, S.; Sagara, H.; Yuki, K.; Sasaki, T.; Watanabe, N.; Fueki, M.; Sugiyama, K.; Takeda, K.; Fukuda, T.; et al. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur. Respir. J. 2009, 34, 425–435. [Google Scholar] [CrossRef] [Green Version]
- Boehme, S.A.; Franz-Bacon, K.; Chen, E.P.; Sásik, R.; Sprague, L.J.; Ly, T.W.; Hardiman, G.; Bacon, K.B. A small molecule CRTH2 antagonist inhibits FITC-induced allergic cutaneous inflammation. Int. Immunol. 2009, 21, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Scuron, M.D.; Fay, B.L.; Connell, A.J.; Peel, M.T.; Smith, P.A. Ruxolitinib Cream Has Dual Efficacy on Pruritus and Inflammation in Experimental Dermatitis. Front. Immunol. 2021, 11, 620098. [Google Scholar] [CrossRef]
- Kabata, H.; Flamar, A.L.; Mahlakõiv, T.; Moriyama, S.; Rodewald, H.R.; Ziegler, S.F.; Artis, D. Targeted deletion of the TSLP receptor reveals cellular mechanisms that promote type 2 airway inflammation. Mucosal Immunol. 2020, 13, 626–636. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Z.; Liu, J.; Yang, B. Structure Differences of Water Soluble Polysaccharides in Astragalus membranaceus Induced by Origin and Their Bioactivity. Foods 2021, 10, 1755. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Liu, P.; Duan, J.A.; Dong, L.; Shang, E.X.; Qian, D.W.; Zhu, Z.H.; Li, H.W.; Li, W.W. Comparative Analysis of Carbohydrates, Nucleosides and Amino Acids in Different Parts of Trichosanthes kirilowii Maxim. by (Ultra) High-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry and Evaporative Light Scattering Detector Methods. Molecules 2019, 24, 1440. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.M.; Park, K.I.; Yang, J.H.; Cho, W.K.; Lee, B.; Ma, J.Y. Anti-allergic actions of F-PASA, a novel herbal cocktail, in IgE/antigen-mediated allergic responses in RBL-2H3 cells and passive cutaneous anaphylaxis in mice. Phytomedicine 2019, 55, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, K.; Yoon, J.H.; Cho, S.G.; Kim, Y.G.; Jeong, M.; Hwang, H.H.; Lee, S.Y.; Jung, S.E.; Ko, S.G. SH003 and Docetaxel Show Synergistic Anticancer Effects by Inhibiting EGFR Activation in Triple-Negative Breast Cancer. BioMed Res. Int. 2022, 2022, 3647900. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.S.; Lee, K.W.; Choi, Y.J.; Kim, Y.G.; Hwang, H.H.; Lee, S.Y.; Jung, S.E.; Park, S.A.; Lee, J.H.; Joo, Y.J.; et al. Synergistic Antitumor Activity of SH003 and Docetaxel via EGFR Signaling Inhibition in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 8405. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, K.; Choi, Y.K.; Choi, Y.J.; Seo, H.S.; Ko, S.G. SH003-induced G1 phase cell cycle arrest induces apoptosis in HeLa cervical cancer cells. Mol. Med. Rep. 2017, 16, 8237–8244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.W.; Cheon, C.; Ko, S.G. SH003 activates autophagic cell death by activating ATF4 and inhibiting G9a under hypoxia in gastric cancer cells. Cell Death Dis. 2020, 11, 717. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.; Ko, S.G.; Kim, W. Analgesic Effect of SH003 and Trichosanthes kirilowii Maximowicz in Paclitaxel-Induced Neuropathic Pain in Mice. Curr. Issues Mol. Biol. 2022, 44, 718–730. [Google Scholar] [CrossRef]
- Lee, K.; Ku, J.M.; Choi, Y.J.; Hwang, H.H.; Jeong, M.; Kim, Y.G.; Kim, M.J.; Ko, S.G. Herbal Prescription SH003 Alleviates Docetaxel-Induced Neuropathic Pain in C57BL/6 Mice. Evid.-Based Complement. Altern. Med. 2021, 2021, 4120334. [Google Scholar] [CrossRef]
- Han, N.R.; Kim, K.C.; Kim, J.S.; Ko, S.G.; Park, H.J.; Moon, P.D. The immune-enhancing effects of a mixture of Astragalus membranaceus (Fisch.) Bunge, Angelica gigas Nakai, and Trichosanthes Kirilowii (Maxim.) or its active constituent nodakenin. J. Ethnopharmacol. 2022, 285, 114893. [Google Scholar] [CrossRef]
- Li, S.; Sun, Y.; Huang, J.; Wang, B.; Gong, Y.; Fang, Y.; Liu, Y.; Wang, S.; Guo, Y.; Wang, H.; et al. Anti-tumor effects and mechanisms of Astragalus membranaceus (AM) and its specific immunopotentiation: Status and prospect. J. Ethnopharmacol. 2020, 258, 112797. [Google Scholar] [CrossRef]
- Shin, J.W.; Son, J.Y.; Kang, J.K.; Han, S.H.; Cho, C.K.; Son, C.G. Trichosanthes kirilowii tuber extract induces G2/M phase arrest via inhibition of tubulin polymerization in HepG2 cells. J. Ethnopharmacol. 2008, 115, 209–216. [Google Scholar] [CrossRef]
- Shaw, P.C.; Chan, W.L.; Yeung, H.W.; Ng, T.B. Minireview: Trichosanthin--a protein with multiple pharmacological properties. Life Sci. 1994, 55, 253–262. [Google Scholar] [CrossRef]
- Park, S.J.; Jung, H.J.; Son, M.S.; Jung, J.M.; Kim, D.H.; Jung, I.H.; Cho, Y.B.; Lee, E.H.; Ryu, J.H. Neuroprotective effects of INM-176 against lipopolysaccharide-induced neuronal injury. Pharmacol. Biochem. Behav. 2012, 101, 427–433. [Google Scholar] [CrossRef]
- Yan, J.J.; Kim, D.H.; Moon, Y.S.; Jung, J.S.; Ahn, E.M.; Baek, N.I.; Song, D.K. Protection against beta-amyloid peptide-induced memory impairment with long-term administration of extract of Angelica gigas or decursinol in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ke, X.; Ma, N.; Wang, W.; Fu, W.; Zhang, H.; Zhao, M.; Gao, X.; Hao, X.; Zhang, Z. Formononetin, an active compound of Astragalus membranaceus (Fisch) Bunge, inhibits hypoxia-induced retinal neovascularization via the HIF-1α/VEGF signaling pathway. Drug Des. Dev. Ther. 2016, 10, 3071–3081. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Chen, H.; Yin, K.; Shen, Y.; Lin, K.; Guo, X.; Zhang, X.; Wang, N.; Xin, W.; Xu, Y.; et al. Formononetin Attenuates Renal Tubular Injury and Mitochondrial Damage in Diabetic Nephropathy Partly via Regulating Sirt1/PGC-1α Pathway. Front. Pharmacol. 2022, 13, 901234. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, J. Screening of Immune-Related Genes and Predicting the Immunotherapeutic Effects of Formononetin in Breast Cancer: A Bioinformatics Analysis. Evid.-Based Complement. Altern. Med. 2022, 2022, 9942373. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; An, J. Formononetin ameliorates mast cell-mediated allergic inflammation via inhibition of histamine release and production of pro-inflammatory cytokines. Exp. Ther. Med. 2017, 14, 6201–6206. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.H.; Kim, H.Y.; Lee, J.S.; Kim, H.M.; Jeong, H.J. Dp44mT regulates the levels of inflammatory mediators through blocking NF-κB nuclear translocation in LPS-stimulated RAW 264.7 macrophages. In Vitro Cell. Dev. Biol. Anim. 2021, 57, 332–341. [Google Scholar] [CrossRef]
- Kim, H.Y.; Han, N.R.; Kim, H.M.; Jeong, H.J. The Iron Chelator and Anticancer Agent Dp44mT Relieves Allergic Inflammation in Mice With Allergic Rhinitis. Inflammation 2018, 41, 1744–1754. [Google Scholar] [CrossRef]
- Jeong, H.J.; Ryu, K.J.; Kim, H.M. Anticancer agent ABT-737 possesses anti-atopic dermatitis activity via blockade of caspase-1 in atopic dermatitis in vitro and in vivo models. Immunopharmacol. Immunotoxicol. 2018, 40, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Jeong, H.J.; Kim, H.M. Anti-allergic and anti-inflammatory effects of the Bcl-2 inhibitor ABT-737 on experimental allergic rhinitis models. Eur. J. Pharmacol. 2018, 833, 34–43. [Google Scholar] [CrossRef]
- Nam, S.Y.; Han, N.R.; Yoon, K.W.; Kim, H.M.; Jeong, H.J. Di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT), an anticancer agent, exerts an anti-inflammatory effect in activated human mast cells. Inflamm. Res. 2017, 66, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.Y.; Cheon, C.; Ko, S.G. Development and Validation of a New Analytical HPLC-PDA Method for Simultaneous Determination of Cucurbitacins B and D from the Roots of Trichosanthes kirilowii. J. Chem. 2022, 2022, 2109502. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Wang, X.; Tao, Y.; Bao, K.; Hua, Y.; Jiang, G.; Hong, M. Formononetin attenuated allergic diseases through inhibition of epithelial-derived cytokines by regulating E-cadherin. Clin. Immunol. 2018, 195, 67–76. [Google Scholar] [CrossRef]
- Ogasawara, N.; Poposki, J.A.; Klingler, A.I.; Tan, B.K.; Weibman, A.R.; Hulse, K.E.; Stevens, W.W.; Peters, A.T.; Grammer, L.C.; Schleimer, R.P.; et al. IL-10, TGF-β, and glucocorticoid prevent the production of type 2 cytokines in human group 2 innate lymphoid cells. J. Allergy Clin. Immunol. 2018, 141, 1147–1151.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, N.R.; Ko, S.G.; Moon, P.D.; Park, H.J. Ginsenoside Rg3 attenuates skin disorders via down-regulation of MDM2/HIF1α signaling pathway. J. Ginseng Res. 2021, 45, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Ko, S.G.; Moon, P.D.; Park, H.J. Chloroquine attenuates thymic stromal lymphopoietin production via suppressing caspase-1 signaling in mast cells. Biomed. Pharmacother. 2021, 141, 111835. [Google Scholar] [CrossRef]
- Joung, E.J.; Lee, M.S.; Choi, J.W.; Kim, J.S.; Shin, T.; Jung, B.M.; Yoon, N.Y.; Lim, C.W.; Kim, J.I.; Kim, H.R. Anti-inflammatory effect of ethanolic extract from Myagropsis myagroides on murine macrophages and mouse ear edema. BMC Complement. Altern. Med. 2012, 12, 171. [Google Scholar] [CrossRef]
- Mulay, S.R.; Thomasova, D.; Ryu, M.; Anders, H.J. MDM2 (murine double minute-2) links inflammation and tubular cell healing during acute kidney injury in mice. Kidney Int. 2012, 81, 1199–1211. [Google Scholar] [CrossRef] [Green Version]
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. HIF transcription factors, inflammation, and immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.C.; Skovbakke, S.L.; Masoudi, H.; Hancock, R.; Franzyk, H. In vivo Anti-inflammatory Activity of Lipidated Peptidomimetics Pam-(Lys-βNspe)6-NH2 and Lau-(Lys-βNspe)6-NH2 Against PMA-Induced Acute Inflammation. Front. Immunol. 2020, 11, 2102. [Google Scholar] [CrossRef]
- Biedermann, T.; Kneilling, M.; Mailhammer, R.; Maier, K.; Sander, C.A.; Kollias, G.; Kunkel, S.L.; Hültner, L.; Röcken, M. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J. Exp. Med. 2000, 192, 1441–1452. [Google Scholar] [CrossRef] [Green Version]
- Shaik-Dasthagirisaheb, Y.B.; Varvara, G.; Murmura, G.; Saggini, A.; Potalivo, G.; Caraffa, A.; Antinolfi, P.; Tete, S.; Tripodi, D.; Conti, F.; et al. Vascular endothelial growth factor (VEGF), mast cells and inflammation. Int. J. Immunopathol. Pharmacol. 2013, 26, 327–335. [Google Scholar] [CrossRef]
- He, R.; Oyoshi, M.K.; Garibyan, L.; Kumar, L.; Ziegler, S.F.; Geha, R.S. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 11875–11880. [Google Scholar] [CrossRef] [Green Version]
- Al-Shami, A.; Spolski, R.; Kelly, J.; Keane-Myers, A.; Leonard, W.J. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005, 202, 829–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Comeau, M.R.; De Smedt, T.; Liggitt, H.D.; Dahl, M.E.; Lewis, D.B.; Gyarmati, D.; Aye, T.; Campbell, D.J.; Ziegler, S.F. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005, 6, 1047–1053. [Google Scholar] [CrossRef]
- Imazeki, M.; Noma, H.; Yasuda, K.; Motohashi, R.; Goto, H.; Shimura, M. Anti-VEGF Therapy Reduces Inflammation in Diabetic Macular Edema. Ophthalmic Res. 2021, 64, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.F.; Yu, X.; Wei, X.; Gui, L.L.; Liu, H.L.; Wang, X.Y.; Tao, Y.; Jiang, G.R.; Hong, M. Astragaloside IV ameliorates allergic inflammation by inhibiting key initiating factors in the initial stage of sensitization. Sci. Rep. 2016, 6, 38241. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Tao, X.; Ma, J. Yi-Qi-Ping-Chuan-Fang Reduces TSLP Elevation Caused by LPS + Poly(I:C) via Inhibiting TLR4/MYD88/NF-κB Signaling Pathway. Evid.-Based Complement. Altern. Med. 2017, 2017, 3209407. [Google Scholar] [CrossRef] [Green Version]
- Ku, J.M.; Hong, S.H.; Kim, S.R.; Choi, H.S.; Kim, H.I.; Kim, D.U.; Oh, S.M.; Seo, H.S.; Kim, T.Y.; Shin, Y.C.; et al. The prevention of 2,4-dinitrochlorobenzene-induced inflammation in atopic dermatitis-like skin lesions in BALB/c mice by Jawoongo. BMC Complement. Altern. Med. 2018, 18, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, W.; Chen, Y.; Zhou, Y.; Bao, K.; Yu, X.; Xu, Y.; Zhang, Y.; Zheng, J.; Jiang, G.; Hong, M. Formononetin attenuates atopic dermatitis by upregulating A20 expression via activation of G protein-coupled estrogen receptor. J. Ethnopharmacol. 2021, 266, 113397. [Google Scholar] [CrossRef] [PubMed]
- Thomasova, D.; Mulay, S.R.; Bruns, H.; Anders, H.J. p53-independent roles of MDM2 in NF-κB signaling: Implications for cancer therapy, wound healing, and autoimmune diseases. Neoplasia 2012, 14, 1097–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; Findley, H.W.; Zhou, M. MDM2 induces NF-kappaB/p65 expression transcriptionally through Sp1-binding sites: A novel, p53-independent role of MDM2 in doxorubicin resistance in acute lymphoblastic leukemia. Blood 2002, 99, 3367–3375. [Google Scholar] [CrossRef] [Green Version]
- Han, N.R.; Oh, H.A.; Nam, S.Y.; Moon, P.D.; Kim, D.W.; Kim, H.M.; Jeong, H.J. TSLP induces mast cell development and aggravates allergic reactions through the activation of MDM2 and STAT6. J. Investig. Dermatol. 2014, 134, 2521–2530. [Google Scholar] [CrossRef] [Green Version]
- Han, N.R.; Moon, P.D.; Kim, H.M.; Jeong, H.J. TSLP Exacerbates Septic Inflammation via Murine Double Minute 2 (MDM2) Signaling Pathway. J. Clin. Med. 2019, 8, 1350. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Qin, J.J.; Rajaei, M.; Li, X.; Yu, X.; Hunt, C.; Zhang, R. Targeting MDM2 for novel molecular therapy: Beyond oncology. Med. Res. Rev. 2020, 40, 856–880. [Google Scholar] [CrossRef]
- Imtiyaz, H.Z.; Simon, M.C. Hypoxia-inducible factors as essential regulators of inflammation. Curr. Top. Microbiol. Immunol. 2010, 345, 105–120. [Google Scholar] [CrossRef] [Green Version]
- Miyake, T.; Miyake, T.; Sakaguchi, M.; Nankai, H.; Nakazawa, T.; Morishita, R. Prevention of Asthma Exacerbation in a Mouse Model by Simultaneous Inhibition of NF-κB and STAT6 Activation Using a Chimeric Decoy Strategy. Mol. Ther. Nucleic Acids 2018, 10, 159–169. [Google Scholar] [CrossRef]
- Ye, M.; Chen, H.; Deng, Y. The mechanism of Astragalus membranaceus’ effects on the proliferation of human basal-like breast cancer cell line MDA-MB-468 from p53/MDM2 pathway. Cancer Res. 2009, 69 (Suppl. 2), 2116. [Google Scholar] [CrossRef]
- Dat, N.T.; Jin, X.; Hong, Y.S.; Lee, J.J. An isoaurone and other constituents from Trichosanthes kirilowii seeds inhibit hypoxia-inducible factor-1 and nuclear factor-kappaB. J. Nat. Prod. 2010, 73, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Jeon, S.K.; Kim, O.H.; Ahn, J.Y.; Kim, C.H.; Park, S.D.; Lee, J.H. Anti-tumor effects of the ethanolic extract of Trichosanthes kirilowii seeds in colorectal cancer. Chin. Med. 2019, 14, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.; Yoon, S.H.; Jang, H.; Jeong, J.H.; Lee, Y.M. Decursin promotes HIF-1α proteasomal degradation and immune responses in hypoxic tumour microenvironment. Phytomedicine 2020, 78, 153318. [Google Scholar] [CrossRef]





| Target Gene | Forward | Reverse |
|---|---|---|
| TSLP | 5′-CGC CAC AAT CCT TGT AAT TGT G-3′ | 5′-CCC AGG CTA TTC GGA AAC TCA G-3′ |
| VEGF | 5′-AGG CCC ACA GGG ATT TTC TT-3′ | 5′-ATC AAA CCT CAC CAA GGC CA-3′ |
| GAPDH | 5′-TCG ACA GTC AGC CGC ATC TTC TTT-3′ | 5′-ACC AAA TCC GTT GAC TCC GAC CTT-3′ |
| Group | Pre-Thickness (mm) | Post-Thickness (mm) | Increase (mm) |
|---|---|---|---|
| Normal | 0.3380 ± 0.0017 | 0.3447 ± 0.0022 | 0.0067 ± 0.0012 |
| PMA | 0.3367 ± 0.0020 | 0.4361 ± 0.0091 | 0.0995 ± 0.0075 # |
| PMA + JRP-SNF102 | 0.3378 ± 0.0021 | 0.3818 ± 0.0048 | 0.0440 ± 0.0037 * |
| PMA + FMN | 0.3385 ± 0.0019 | 0.4028 ± 0.0043 | 0.0643 ± 0.0026 * |
| PMA + Dex | 0.3381 ± 0.0017 | 0.3948 ± 0.0052 | 0.0567 ± 0.0064 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, N.-R.; Park, H.-J.; Ko, S.-G.; Moon, P.-D. The Protective Effect of a Functional Food Consisting of Astragalus membranaceus, Trichosanthes kirilowii, and Angelica gigas or Its Active Component Formononetin against Inflammatory Skin Disorders through Suppression of TSLP via MDM2/HIF1α Signaling Pathways. Foods 2023, 12, 276. https://doi.org/10.3390/foods12020276
Han N-R, Park H-J, Ko S-G, Moon P-D. The Protective Effect of a Functional Food Consisting of Astragalus membranaceus, Trichosanthes kirilowii, and Angelica gigas or Its Active Component Formononetin against Inflammatory Skin Disorders through Suppression of TSLP via MDM2/HIF1α Signaling Pathways. Foods. 2023; 12(2):276. https://doi.org/10.3390/foods12020276
Chicago/Turabian StyleHan, Na-Ra, Hi-Joon Park, Seong-Gyu Ko, and Phil-Dong Moon. 2023. "The Protective Effect of a Functional Food Consisting of Astragalus membranaceus, Trichosanthes kirilowii, and Angelica gigas or Its Active Component Formononetin against Inflammatory Skin Disorders through Suppression of TSLP via MDM2/HIF1α Signaling Pathways" Foods 12, no. 2: 276. https://doi.org/10.3390/foods12020276
APA StyleHan, N.-R., Park, H.-J., Ko, S.-G., & Moon, P.-D. (2023). The Protective Effect of a Functional Food Consisting of Astragalus membranaceus, Trichosanthes kirilowii, and Angelica gigas or Its Active Component Formononetin against Inflammatory Skin Disorders through Suppression of TSLP via MDM2/HIF1α Signaling Pathways. Foods, 12(2), 276. https://doi.org/10.3390/foods12020276








