Amelioration of Chilling Injury by Fucoidan in Cold-Stored Cucumber via Membrane Lipid Metabolism Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Measurement of CI, Weight Loss, and Electrolyte Leakage
2.3. Measurement of MDA and Respiration Rate
2.4. Determination of ω-FDAS, PLD, and LOX Activities
2.5. Determination of Plant Lipids
2.6. Gene Expression Assays
2.7. Statistical Analysis
3. Results
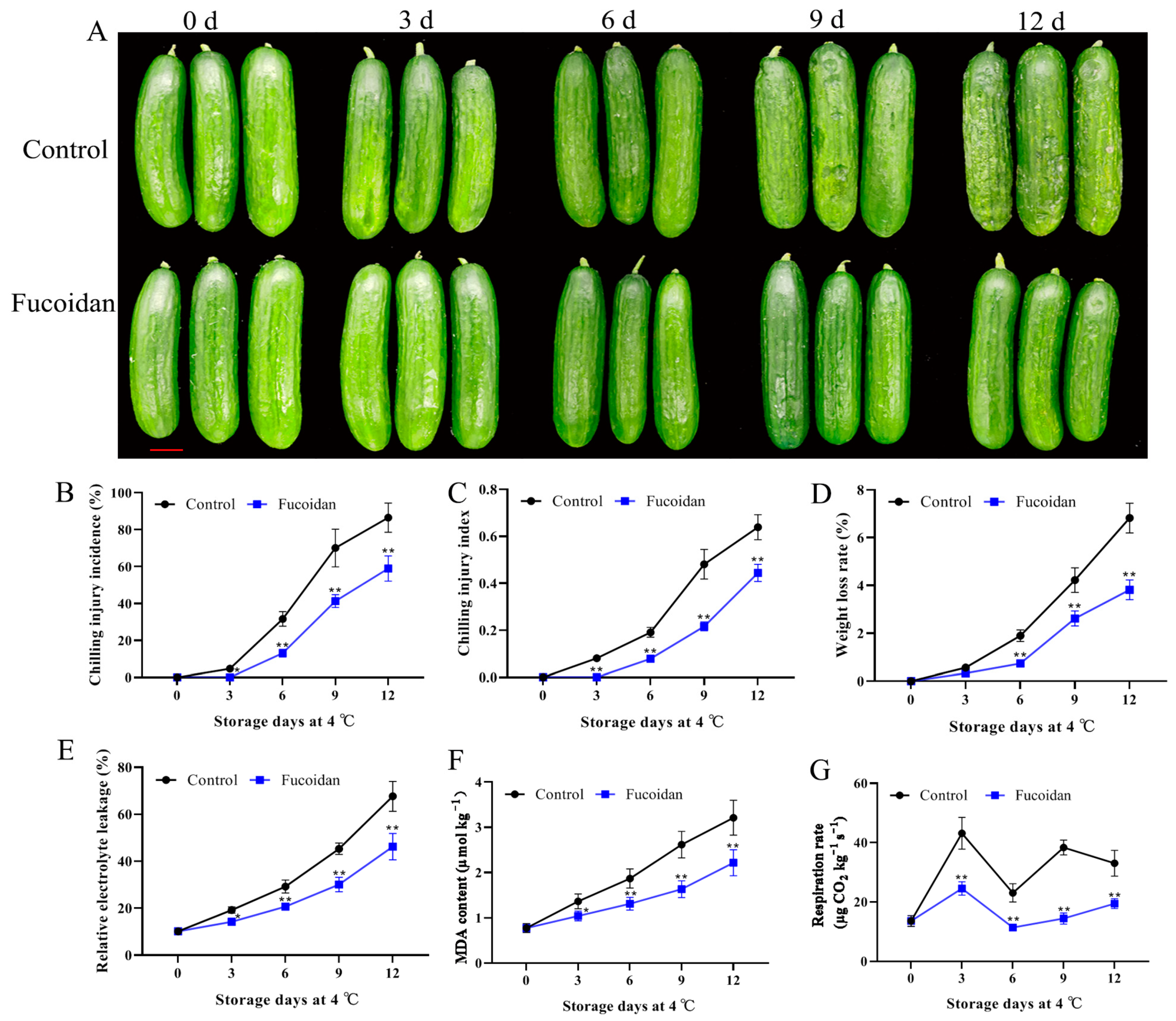
3.1. Fucoidan Treatment Effects on CI of Cucumber Fruit
3.2. Fucoidan Treatment Effects on Weight Loss, Electrolyte Leakage, MDA Content, and Respiration Rate
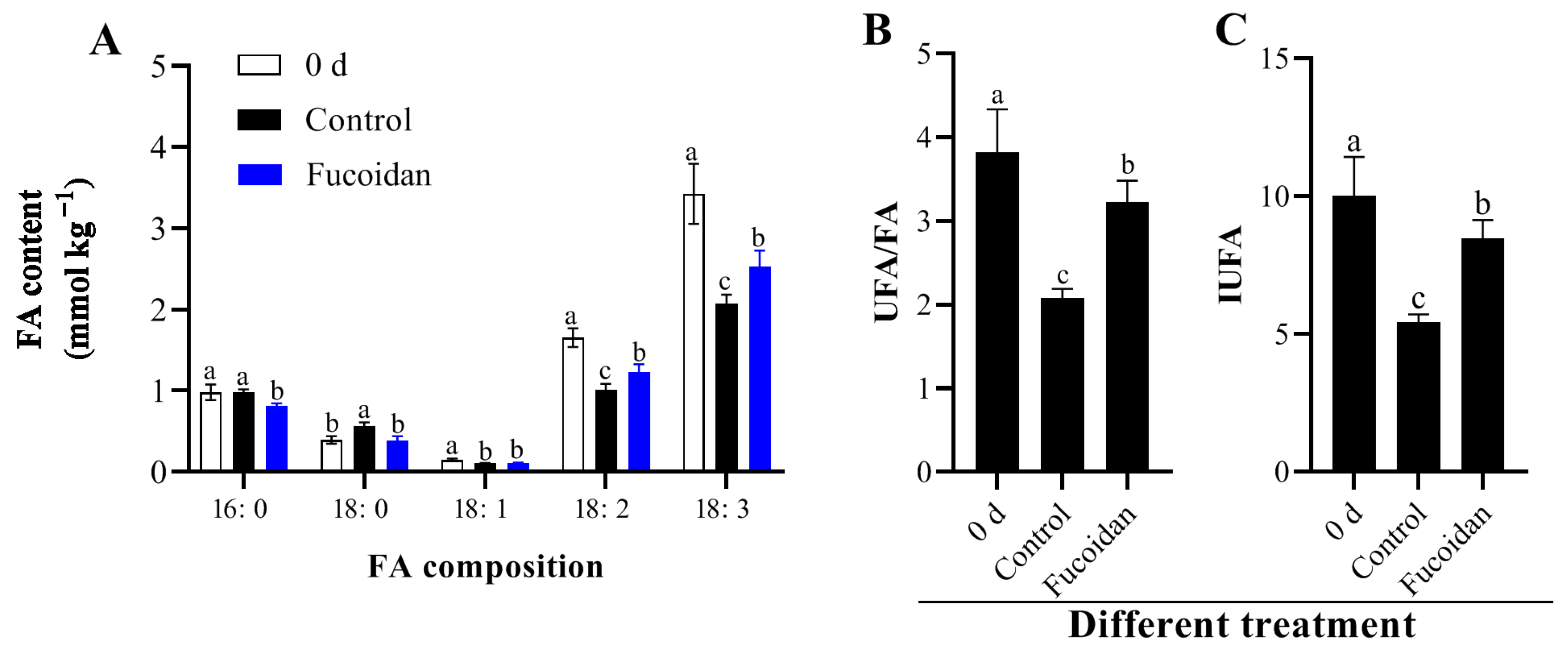
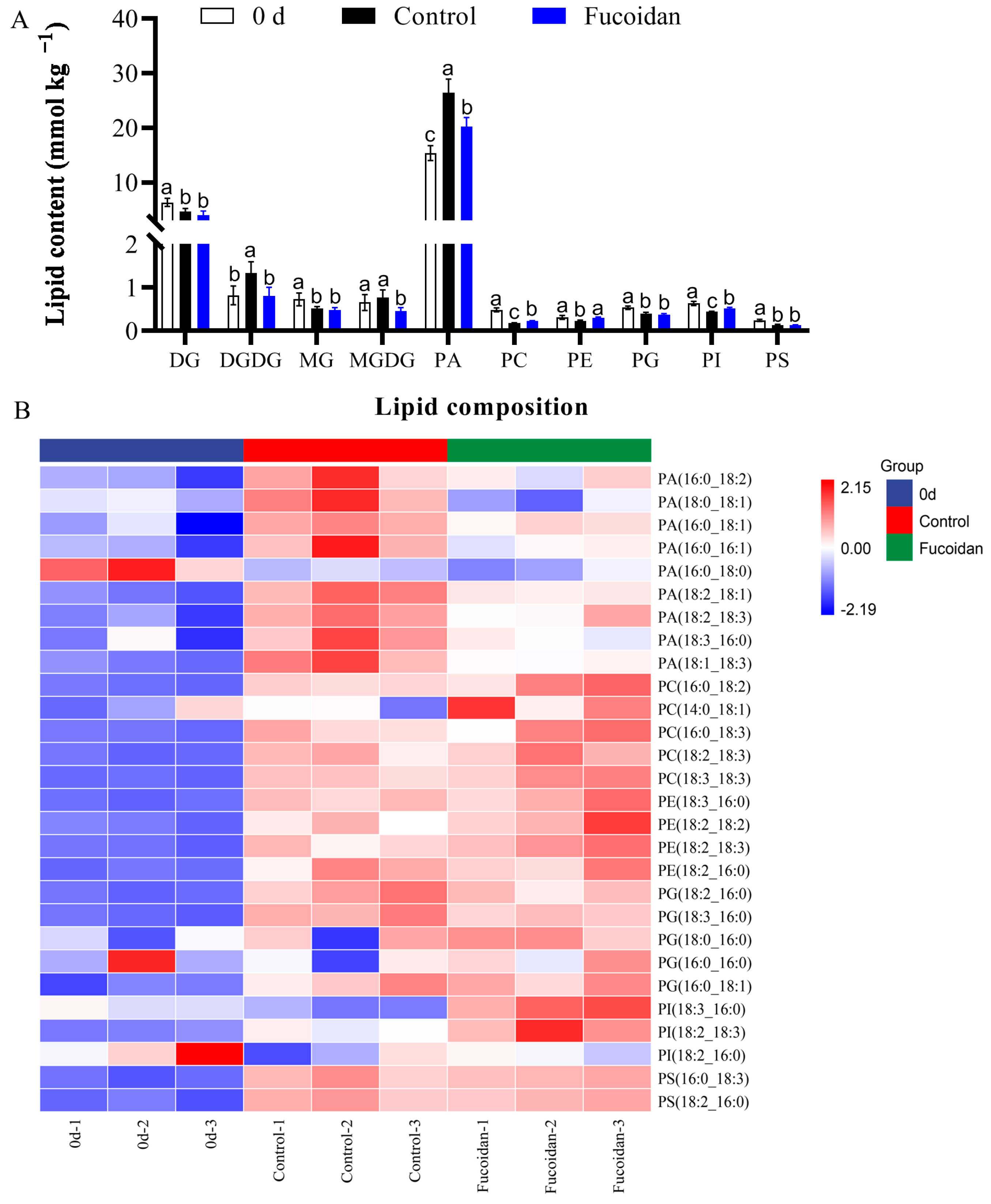
3.3. Fucoidan Treatment Effects on Membrane Lipid Components and Contents
3.4. Fucoidan Treatment Effects on Enzyme Activities and Related Gene Expression Associated with Membrane Lipids
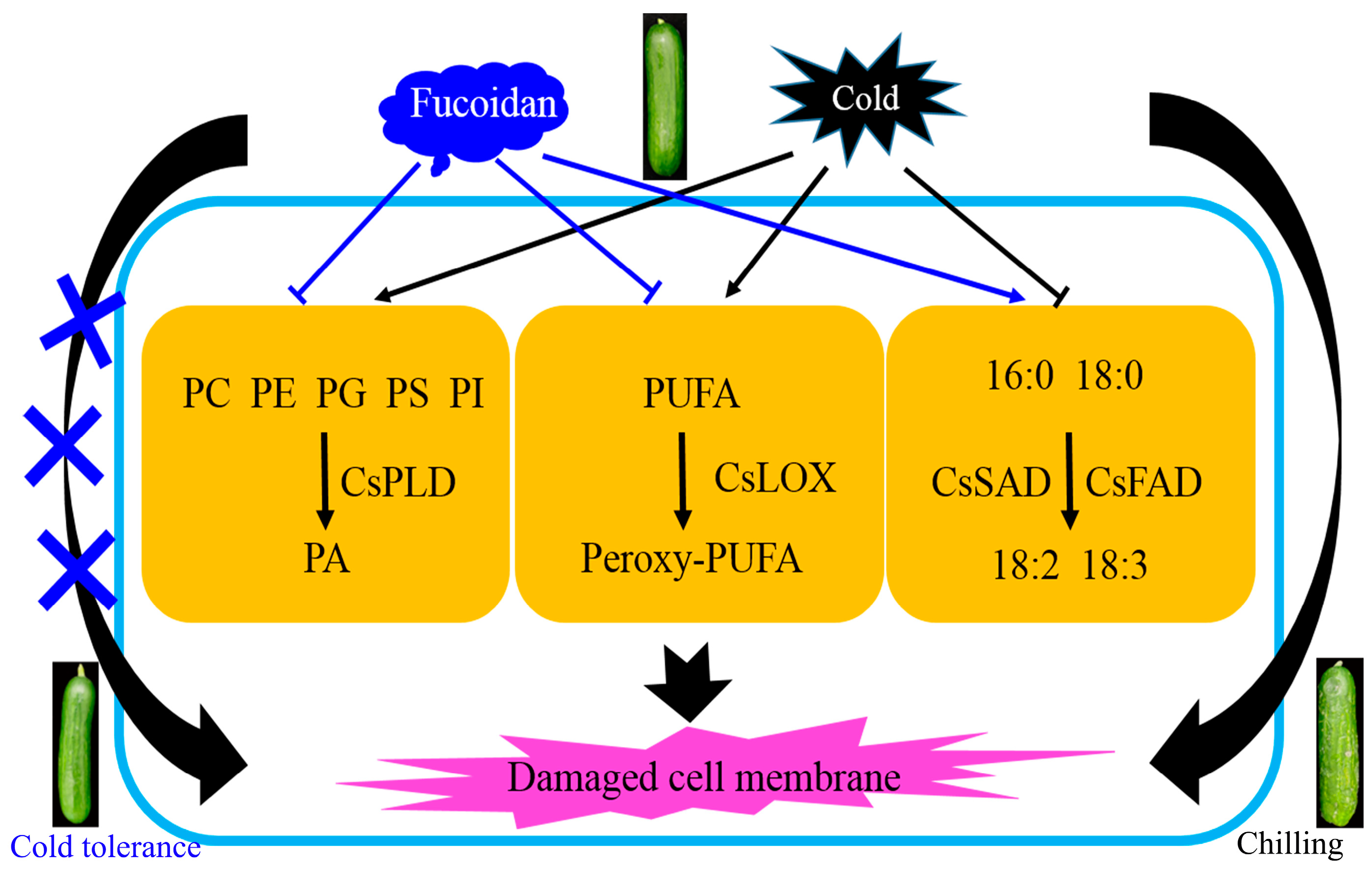
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Si, J.; Fan, Y.; Liu, Z.; Wei, W.; Xiao, X.; Yang, Y.; Shan, W.; Kuang, J.; Lu, W.; Fan, Z.; et al. Comparative transcriptomic analysis reveals the potential mechanism of hot water treatment alleviated-chilling injury in banana fruit. Food Res. Int. 2022, 157, 111296. [Google Scholar] [CrossRef]
- Mao, L.; Pang, H.; Wang, G.; Zhu, C. Phospholipase D and lipoxygenase activity of cucumber fruit in response to chilling stress. Postharvest Biol. Technol. 2007, 44, 42–47. [Google Scholar] [CrossRef]
- Tsuchida, H.; Kozukue, N.; Han, G.-P.; Choi, S.-H.; Levin, C.E.; Friedman, M. Low-temperature storage of cucumbers induces changes in the organic acid content and in citrate synthase activity. Postharvest Biol. Technol. 2010, 58, 129–134. [Google Scholar] [CrossRef]
- Song, C.; Wang, K.; Xiao, X.; Liu, Q.; Yang, M.; Li, X.; Feng, Y.; Li, S.; Shi, L.; Chen, W.; et al. Membrane lipid metabolism influences chilling injury during cold storage of peach fruit. Food Res. Int. 2022, 157, 111249. [Google Scholar] [CrossRef]
- Lyons, J.M. Chilling injury in plants. Annu. Rev. Plant Physiol. 1973, 24, 445–466. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Chen, J.; Jiang, Y. Revealing further insights on chilling injury of postharvest bananas by untargeted lipidomics. Foods 2020, 9, 894. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, T.; Yao, W.; Zhu, H.; Jin, P.; Zheng, Y. Effect of heat treatment on reactive oxygen species metabolism and membrane lipid constituents of cucumber fruit under low temperature stress. J. Nucl. Agric. Sci. 2020, 34, 85–93. [Google Scholar]
- Walley, J.W.; Kliebenstein, D.J.; Bostock, R.M.; Dehesh, K. Fatty acids and early detection of pathogens. Curr. Opin. Plant Biol. 2013, 16, 520–526. [Google Scholar] [CrossRef]
- Ge, W.; Luo, M.; Sun, H.; Wei, B.; Zhou, X.; Zhou, Q.; Ji, S. The CaMYB340 transcription factor induces chilling injury in post-harvest bell pepper by inhibiting fatty acid desaturation. Plant J. 2022, 111, 800–818. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhang, Y.; Li, Q.; Li, R.; Xia, X.; Qin, X.; Guo, H. Identification and expression of a stearoyl-ACP desaturase gene responsible for oleic acid accumulation in Xanthoceras sorbifolia seeds. Plant Physiol. Biochem. 2015, 87, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Ge, W.; Wei, B.; Zhou, Q.; Zhou, X.; Zhao, Y.; Ji, S. Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biol. Technol. 2020, 170, 111315. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Sun, J.; Lin, Y.; Lin, Y.; Lin, M.; Hung, Y.; Ritenour, M.A.; Lin, H. The changes in metabolisms of membrane lipids and phenolics induced by phomopsis longanae chi infection in association with pericarp browning and disease occurrence of postharvest longan fruit. J. Agric. Food Chem. 2018, 66, 12794–12804. [Google Scholar] [CrossRef] [PubMed]
- Nasef, I.N. Short hot water as safe treatment induces chilling tolerance and antioxidant enzymes, prevents decay and maintains quality of cold-stored cucumbers. Postharvest Biol. Technol. 2018, 138, 1–10. [Google Scholar] [CrossRef]
- Wang, B.; Wu, C.; Wang, G.; He, J.; Zhu, S. Transcriptomic analysis reveals a role of phenylpropanoid pathway in the enhancement of chilling tolerance by pre-storage cold acclimation in cucumber fruit. Sci. Hortic. 2021, 288, 110282. [Google Scholar] [CrossRef]
- Wang, J.D.; Zhao, Y.Q.; Ma, Z.Q.; Zheng, Y.H.; Jin, P. Hydrogen sulfide treatment alleviates chilling injury in cucumber fruit by regulating antioxidant capacity, energy metabolism and proline metabolism. Foods 2022, 11, 2749. [Google Scholar] [CrossRef] [PubMed]
- Madebo, M.P.; Luo, S.M.; Wang, L.; Zheng, Y.H.; Jin, P. Melatonin treatment induces chilling tolerance by regulating the contents of polyamine, γ-aminobutyric acid, and proline in cucumber fruit. J. Integr. Agric. 2021, 20, 3060–3074. [Google Scholar] [CrossRef]
- Ru, L.; Jiang, L.; Wills, R.B.H.; Golding, J.B.; Huo, Y.; Yang, H.; Li, Y. Chitosan oligosaccharides induced chilling resistance in cucumber fruit and associated stimulation of antioxidant and HSP gene expression. Sci. Hortic. 2020, 264, 109187. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, C.H.; Li, Z.J.; Cai, Y.P.; Liu, H.Y.; Qi, H.T. Antioxidant and hepatoprotective activities of low molecular weight sulfated polysaccharide from Laminaria Japonica. J. Appl. Phycol. 2004, 16, 111–115. [Google Scholar] [CrossRef]
- Thanh-Sang, V.; Kim, S.-K. Fucoidans as a natural bioactive ingredient for functional foods. J. Funct. Foods 2013, 5, 16–27. [Google Scholar]
- Xu, B.; Wu, S. Preservation of mango fruit quality using fucoidan coatings. LWT Food Sci. Technol. 2021, 143, 111150. [Google Scholar] [CrossRef]
- Duan, Z.; Duan, W.; Li, F.; Li, Y.; Luo, P.; Liu, H. Effect of carboxymethylation on properties of fucoidan from Laminaria japonica: Antioxidant activity and preservative effect on strawberry during cold storage. Postharvest Biol. Technol. 2019, 151, 127–133. [Google Scholar] [CrossRef]
- Luo, P.; Li, F.; Liu, H.; Yang, X.; Duan, Z. Effect of fucoidan-based edible coating on antioxidant degradation kinetics in strawberry fruit during cold storage. J. Food Process. Preserv. 2020, 44, e14381. [Google Scholar] [CrossRef]
- Hurr, B.M.; Huber, D.J.; Vallejos, C.E.; Lee, E.; Sargent, S.A. Ethylene-induced overproduction of reactive oxygen species is responsible for the development of watersoaking in immature cucumber fruit. J. Plant Physiol. 2013, 170, 56–62. [Google Scholar] [CrossRef]
- Chen, X.J.; Wang, P.J.; Wei, M.X.; Lin, X.Y.; Gu, M.Y.; Fang, W.P.; Zheng, Y.C.; Zhao, F.; Jin, S.; Ye, N. Lipidomics analysis unravels changes from flavor precursors in different processing treatments of purple-leaf tea. J. Sci. Food Agric. 2022, 102, 3730–3741. [Google Scholar] [CrossRef]
- Gan, Z.; Yuan, X.; Shan, N.; Wan, C.; Chen, C.; Zhu, L.; Xu, Y.; Kai, W.; Zhai, X.; Chen, J. AcERF1b and AcERF073 positively regulate indole-3-acetic acid degradation by activating AcGH3.1 transcription during postharvest kiwifruit ripening. J. Agric. Food Chem. 2021, 69, 13859–13870. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Zhao, Z.; Qian, C.; Sui, Y.; Malik, A.A.; Chen, J. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal. Biochem. 2010, 399, 257–261. [Google Scholar] [CrossRef]
- Cao, S.F.; Hu, Z.C.; Wang, H.O. Effect of salicylic acid on the activities of anti-oxidant enzymes and phenylalanine ammonia-lyase in cucumber fruit in relation to chilling injury. J. Hortic. Sci. Biotechnol. 2009, 84, 125–130. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Han, T.; Li, L.; Xu, L. Effect of exogenous glycine betaine on chilling injury and chilling-resistance parameters in cucumber fruits stored at low temperature. Sci. Agric. Sin. 2008, 41, 2407–2412. [Google Scholar]
- Yu, K.B.; Zhou, L.; Xu, J.; Jiang, F.H.; Zhong, Z.W.; Zou, L.Q.; Liu, W. Carboxymethyl cellulose-based water barrier coating regulated postharvest quality and ROS metabolism of pakchoi (Brassica chinensis L.). Postharvest Biol. Technol. 2022, 185, 111804. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Ejaz, S.; Hussain, S.; Ercisli, S.; Saleem, M.S.; Sardar, H. Carboxymethyl cellulose coating delays chilling injury development and maintains eating quality of ‘Kinnow’ mandarin fruits during low temperature storage. Int. J. Biol. Macromol. 2021, 168, 77–85. [Google Scholar] [CrossRef]
- Ehteshami, S.; Abdollahi, F.; Ramezanian, A.; Dastjerdi, A.M.; Rahimzadeh, M. Enhanced chilling tolerance of pomegranate fruit by edible coatings combined with malic and oxalic acid treatments. Sci. Hortic. 2019, 250, 388–398. [Google Scholar] [CrossRef]
- Tian, J.X.; Xie, S.Y.; Zhang, P.; Wang, Q.; Li, J.K.; Xu, X.B. Attenuation of postharvest peel browning and chilling injury of banana fruit by Astragalus polysaccharides. Postharvest Biol. Technol. 2022, 184, 111783. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abushouk, A.I.; Bahbah, E.I.; Bungau, S.G.; Alyousif, M.S.; Aleya, L.; Alkahtani, S. Fucoidan protects against subacute diazinon-induced oxidative damage in cardiac, hepatic, and renal tissues. Environ. Sci. Pollut. Res. 2020, 27, 11554–11564. [Google Scholar] [CrossRef]
- Aleissa, M.S.; Alkahtani, S.; Abd Eldaim, M.A.; Ahmed, A.M.; Bungau, S.G.; Almutairi, B.; Bin-Jumah, M.; AlKahtane, A.A.; Alyousif, M.S.; Abdel-Daim, M.M. Fucoidan ameliorates oxidative stress, inflammation, DNA damage, and hepatorenal injuries in diabetic rats intoxicated with aflatoxin B-1. Oxidative Med. Cell. Longev. 2020, 2020, 9316751. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, A.G.; Palma, T.; Stanley, D.W. Membrane effects in postharvest physiology. Postharvest Biol. Technol. 1996, 7, 193–221. [Google Scholar] [CrossRef]
- Ge, W.; Kong, X.; Zhao, Y.; Wei, B.; Zhou, Q.; Ji, S. Insights into the metabolism of membrane lipid fatty acids associated with chilling injury in post-harvest bell peppers. Food Chem. 2019, 295, 26–35. [Google Scholar] [CrossRef]
- Ge, W.; Zhao, Y.; Kong, X.; Sun, H.; Luo, M.; Yao, M.; Wei, B.; Ji, S. Combining salicylic acid and trisodium phosphate alleviates chilling injury in bell pepper (Capsicum annuum L.) through enhancing fatty-acid desaturation efficiency and water retention. Food Chem. 2020, 327, 127057. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhu, Z.; Cheng, S.; Zhou, Q.; Zhou, X.; Kong, X.; Hu, M.; Yin, X.; Wei, B.; Ji, S. Methyl jasmonate alleviates chilling injury by regulating membrane lipid composition in green bell pepper. Sci. Hortic. 2020, 266, 109308. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Pareek, S.; Mani, S.; Dominguez-Avila, J.A.; Gonzalez-Aguilar, G.A. A melatonin treatment delays postharvest senescence, maintains quality, reduces chilling injury, and regulates antioxidant metabolism in mango fruit. J. Food Qual. 2022, 2022, 2379556. [Google Scholar] [CrossRef]
- Holthuis, J.C.M.; Menon, A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature 2014, 510, 48–57. [Google Scholar] [CrossRef]
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.; Yang, T.; Cao, M.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2018, 245, 659–666. [Google Scholar] [CrossRef]
- Yao, W.; Xu, T.; Farooq, S.U.; Jin, P.; Zheng, Y. Glycine betaine treatment alleviates chilling injury in zucchini fruit (cucurbita pepo L.) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biol. Technol. 2018, 144, 20–28. [Google Scholar] [CrossRef]
- Dong, C.; Cao, N.; Zhang, Z.; Shang, Q. Characterization of the fatty acid desaturase genes in cucumber: Structure, phylogeny, and expression patterns. PLoS ONE 2016, 11, e0149917. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, Q.; Zhou, X.; Wei, B.; Ji, S. Transcription factor CaNAC1 regulates low-temperature-induced phospholipid degradation in green bell pepper. J. Exp. Bot. 2020, 71, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yin, X.; Zhang, B.; Grierson, D.; Xu, C.; Chen, K. Transcriptomic and metabolic analyses provide new insights into chilling injury in peach fruit. Plant Cell Environ. 2017, 40, 1531–1551. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Luo, M.; Zhou, X.; Zhou, Q.; Sun, Y.; Ge, W.; Yao, M.; Ji, S. PuMYB21/PuMYB54 coordinate to activate PuPLDbeta1 transcription during peel browning of cold-stored “Nanguo” pears. Hortic. Res. 2020, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wu, M.; Zhou, Y.; Gong, Z.; Yu, W.; Zhang, Y.; Yang, Z. NAC-mediated membrane lipid remodeling negatively regulates fruit cold tolerance. Hortic. Res. 2022, 9, uhac039. [Google Scholar] [CrossRef] [PubMed]
- Welti, R.; Li, W.Q.; Li, M.Y.; Sang, Y.M.; Biesiada, H.; Zhou, H.E.; Rajashekar, C.B.; Williams, T.D.; Wang, X.M. Profiling membrane lipids in plant stress responses—Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, Y.; Lin, H.; Lin, Y.; Chen, Y.; Wang, H.; Shi, J.; Lin, Y. Lasiodiplodia theobromae (Pat.) Griff. & Maubl.-induced disease development and pericarp browning of harvested longan fruit in association with membrane lipids metabolism. Food Chem. 2018, 244, 93–101. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lin, D.; Yan, R.; Xu, Y.; Xing, M.; Liao, S.; Wan, C.; Chen, C.; Zhu, L.; Kai, W.; et al. Amelioration of Chilling Injury by Fucoidan in Cold-Stored Cucumber via Membrane Lipid Metabolism Regulation. Foods 2023, 12, 301. https://doi.org/10.3390/foods12020301
Zhang Y, Lin D, Yan R, Xu Y, Xing M, Liao S, Wan C, Chen C, Zhu L, Kai W, et al. Amelioration of Chilling Injury by Fucoidan in Cold-Stored Cucumber via Membrane Lipid Metabolism Regulation. Foods. 2023; 12(2):301. https://doi.org/10.3390/foods12020301
Chicago/Turabian StyleZhang, Yupei, Duo Lin, Ruyu Yan, Yunhe Xu, Mengying Xing, Shuyuan Liao, Chunpeng Wan, Chuying Chen, Liqin Zhu, Wenbin Kai, and et al. 2023. "Amelioration of Chilling Injury by Fucoidan in Cold-Stored Cucumber via Membrane Lipid Metabolism Regulation" Foods 12, no. 2: 301. https://doi.org/10.3390/foods12020301
APA StyleZhang, Y., Lin, D., Yan, R., Xu, Y., Xing, M., Liao, S., Wan, C., Chen, C., Zhu, L., Kai, W., Chen, J., & Gan, Z. (2023). Amelioration of Chilling Injury by Fucoidan in Cold-Stored Cucumber via Membrane Lipid Metabolism Regulation. Foods, 12(2), 301. https://doi.org/10.3390/foods12020301






