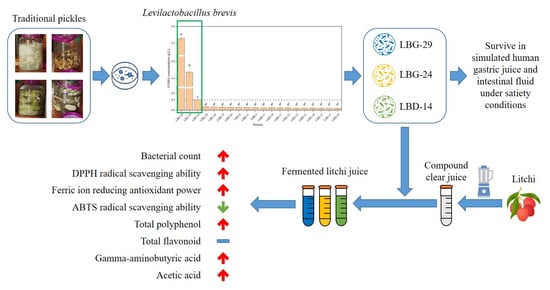
Gamma-Aminobutyric Acid-Producing Levilactobacillus brevis Strains as Probiotics in Litchi Juice Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Isolation and Identification
2.1.1. Isolation
2.1.2. Species Identification
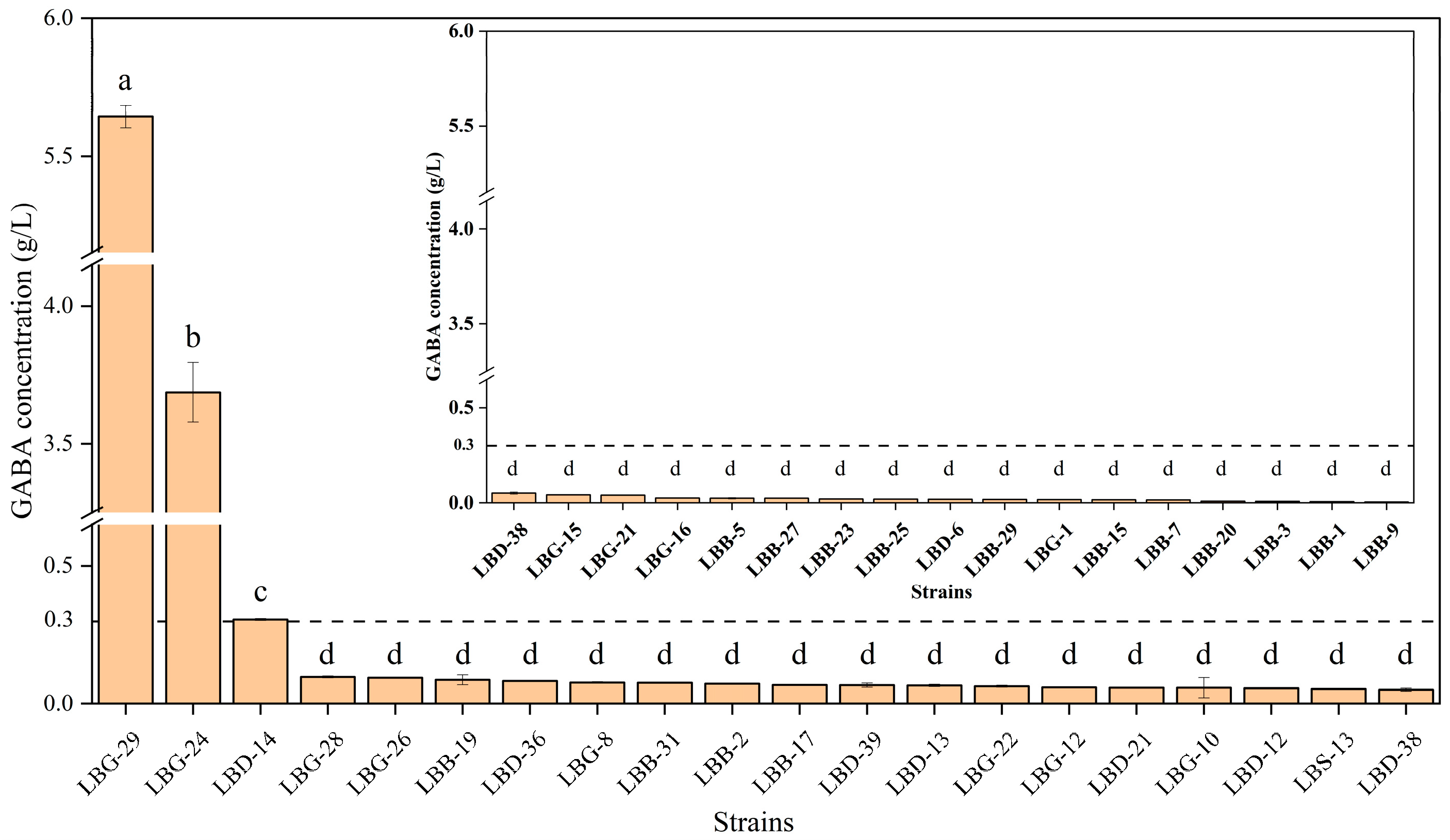
2.1.3. Strain Screening
2.1.4. GABA Measurement
2.2. Strain Characterisation
2.2.1. Physio-Biochemical Assays
2.2.2. Sugar Fermentation Assay
2.3. Safety Evaluations
2.3.1. Arginine Ammonia
2.3.2. Bile Salt Hydrolase
2.3.3. Haemolysis
2.3.4. Antibiotic Susceptibility
2.4. In Vitro Simulations of Tolerance
2.4.1. Gastric Acid
2.4.2. Bile Salt
2.4.3. Simulated Human Gastric Juice
2.4.4. Simulated Human Intestinal Fluid
2.5. Litchi Juice Fermentation
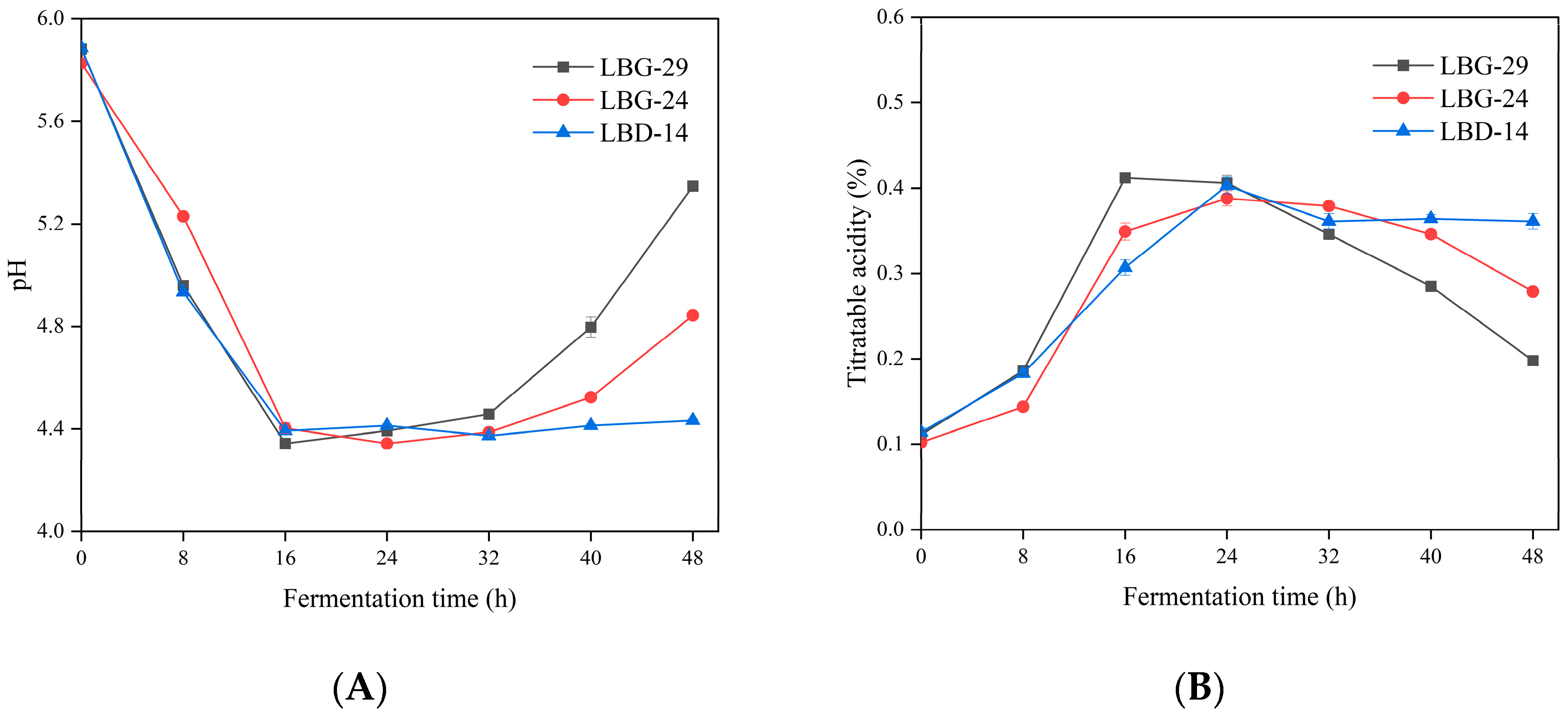
2.6. Acidity Measurements
2.7. Bacterial Count
2.8. Determination of Antioxidant Capacity
2.8.1. DPPH Radical Scavenging
2.8.2. Ferric Ion Reduction
2.8.3. ABTS Radical Scavenging
2.9. Polyphenol
2.10. Flavonoids
2.11. Acetic Acid
2.12. Statistical Analysis
3. Results
3.1. Strain Isolation and Identification
3.2. Physio-Biochemical Analysis
3.3. In Vitro Safety Evaluations
3.4. Gastric Acid and Bile Acid Tolerance
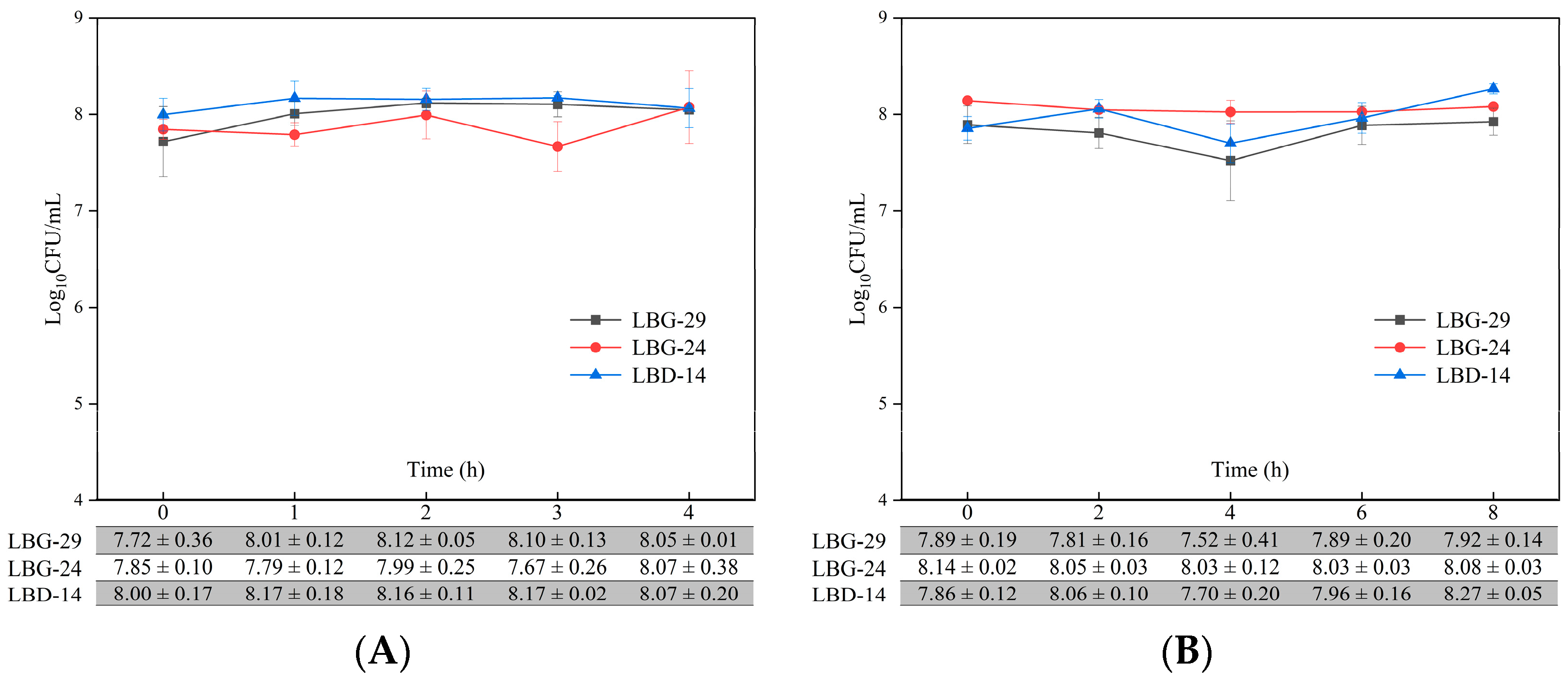
3.5. Simulated Human Gastric Juice and Intestinal Fluid Tolerance
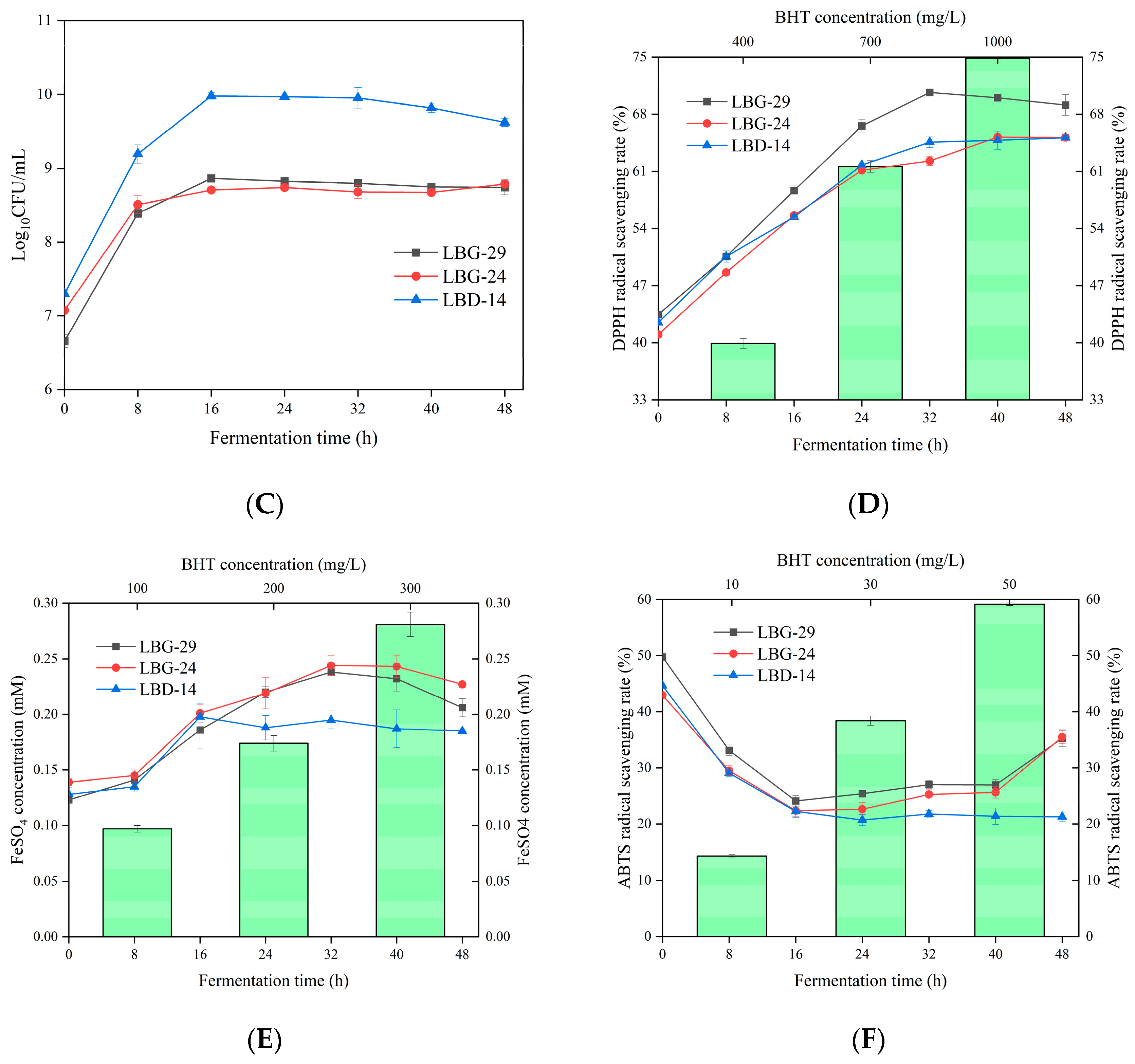
3.6. pH and Acidity during Fermentation
3.7. Viability during Fermentation
3.8. Antioxidative Capacity
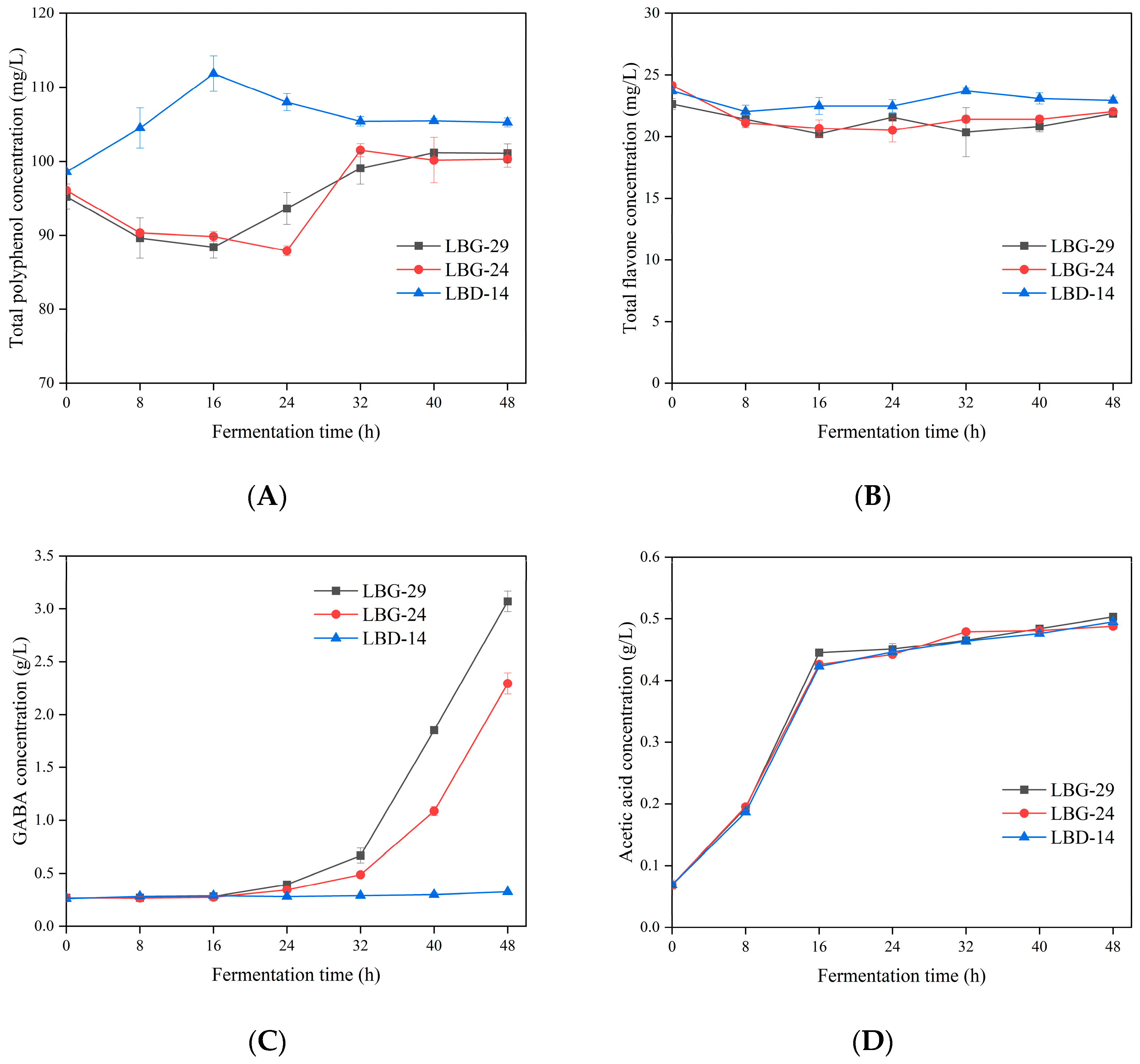
3.9. Polyphenol Production during Fermentation
3.10. Flavonoid Production during Fermentation
3.11. GABA
3.12. Acetic Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-amino-di(3-ethyl-benzothiazoline sulphonic acid-6)ammonium salt |
| BHT | 2,6-di-tert-butyl-4-methylphenol |
| CFU | colony-forming units |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FRAP | ferric ion-reducing antioxidant power |
| GABA | gamma-amino butyric acid |
| HPLC | high-performance liquid chromatography |
| LAB | lactic acid bacteria |
| L. brevis | Levilactobacillus brevis |
| MRS agar medium | de Man, Rogosa, and Sharpe agar medium |
| SD | standard deviation |
References
- Sarasa, S.B.; Mahendran, R.; Muthusamy, G.; Thankappan, B.; Selta, D.R.F.; Angayarkanni, J. A Brief Review on the Non-protein Amino Acid, Gamma-amino Butyric Acid (GABA): Its Production and Role in Microbes. Curr. Microbiol. 2020, 77, 534–544. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of Gamma-Aminobutyric Acid from Lactic Acid Bacteria: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, D.H.; Kang, H.J.; Shin, M.; Yang, S.-Y.; Yang, J.; Jung, Y.H. Enhanced production of γ-aminobutyric acid (GABA) using Lactobacillus plantarum EJ2014 with simple medium composition. LWT 2020, 137, 110443. [Google Scholar] [CrossRef]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef]
- Hasan, M.; Alabdallah, N.M.; Alharbi, B.M.; Waseem, M.; Yao, G.; Liu, X.-D.; El-Gawad, H.G.A.; El-Yazied, A.A.; Ibrahim, M.F.M.; Jahan, M.S.; et al. GABA: A Key Player in Drought Stress Resistance in Plants. Int. J. Mol. Sci. 2021, 22, 10136. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Jeong, A.-H.; Hwang, J.; Jo, K.; Kim, S.; Ahn, Y.; Suh, H.; Choi, H.-S. Fermented Gamma Aminobutyric Acid Improves Sleep Behaviors in Fruit Flies and Rodent Models. Int. J. Mol. Sci. 2021, 22, 3537. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-J.; Chang, C.-Y.; Lai, Y.-T.; Shyu, Y.-T. Increasing γ-Aminobutyric Acid Content in Vegetable Soybeans via High-Pressure Processing and Efficacy of Their Antidepressant-Like Activity in Mice. Foods 2020, 9, 1673. [Google Scholar] [CrossRef]
- Bruni, O.; Ferini-Strambi, L.; Giacomoni, E.; Pellegrino, P. Herbal Remedies and Their Possible Effect on the GABAergic System and Sleep. Nutrients 2021, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. Front. Neurosci. 2020, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Peerboom, C.; Wierenga, C.J. The postnatal GABA shift: A developmental perspective. Neurosci. Biobehav. Rev. 2021, 124, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Quillin, S.J.; Tran, P.; Prindle, A. Potential Roles for Gamma-Aminobutyric Acid Signaling in Bacterial Communities. Bioelectricity 2021, 3, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Dasagrandhi, C.; Park, S.-K.; Eom, S.-H.; Huh, M.-K.; Mok, J.-S.; Kim, Y.-M. Optimization of gamma-aminobutyric acid production using sea tangle extract by lactic acid bacterial fermentation. LWT 2018, 90, 636–642. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Li, S.; Yin, P.; Sheng, H.; Wang, T.; Zhang, Y.; Zhang, K.; Wang, Q.; Lu, S.; et al. Influence of GABA-producing yeasts on cheese quality, GABA content, and the volatilome. LWT 2022, 154, 112766. [Google Scholar] [CrossRef]
- Jitpakdee, J.; Kantachote, D.; Kanzaki, H.; Nitoda, T. Potential of lactic acid bacteria to produce functional fermented whey beverage with putative health promoting attributes. LWT 2022, 160, 113269. [Google Scholar] [CrossRef]
- Cai, L.; Wang, W.; Tong, J.; Fang, L.; He, X.; Xue, Q.; Li, Y. Changes of bioactive substances in lactic acid bacteria and yeasts fermented kiwifruit extract during the fermentation. LWT 2022, 164, 113629. [Google Scholar] [CrossRef]
- Abdelazez, A.; Alshehry, G.; Algarni, E.; Al Jumayi, H.; Abdel-Motaal, H.; Meng, X.-C. Postbiotic Gamma-Aminobutyric Acid and Camel Milk Intervention as Innovative Trends Against Hyperglycemia and Hyperlipidemia in Streptozotocin-Induced C57BL/6J Diabetic Mice. Front. Microbiol. 2022, 13, 943930. [Google Scholar] [CrossRef]
- Li, H.; Cao, Y. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 2010, 39, 1107–1116. [Google Scholar] [CrossRef]
- Sahab, N.R.; Subroto, E.; Balia, R.L.; Utama, G.L. γ-Aminobutyric acid found in fermented foods and beverages: Current trends. Heliyon 2020, 6, e05526. [Google Scholar] [CrossRef]
- Jin, Y.H.; Hong, J.H.; Lee, J.-H.; Yoon, H.; Pawluk, A.M.; Yun, S.J.; Mah, J.-H. Lactic acid fermented green tea with Levilactobacillus brevis capable of producing γ-aminobutyric acid. Fermentation 2021, 7, 110. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, P.; Pan, D.; Zeng, X.; Guo, Y.; Zhao, G. Effect of adzuki bean sprout fermented milk enriched in γ-aminobutyric acid on mild depression in a mouse model. J. Dairy Sci. 2021, 104, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Kanklai, J.; Somwong, T.C.; Rungsirivanich, P.; Thongwai, N. Screening of GABA-Producing Lactic Acid Bacteria from Thai Fermented Foods and Probiotic Potential of Levilactobacillus brevis F064A for GABA-Fermented Mulberry Juice Production. Microorganisms 2020, 9, 33. [Google Scholar] [CrossRef]
- Yu, L.; Han, X.; Cen, S.; Duan, H.; Feng, S.; Xue, Y.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; et al. Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol. Res. 2020, 233, 126409. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Gao, Y.; Simal-Gandara, J.; Farag, M.A.; Chen, W.; Yao, D.; Delmas, D.; Chen, Z.; Liu, K.; Hu, H.; et al. Litchi (Litchi chinensis Sonn.): A comprehensive review of phytochemistry, medicinal properties, and product development. Food Funct. 2021, 12, 9527–9548. [Google Scholar] [CrossRef]
- Meng, X.; Ouyang, G.C.; Liu, H.; Hou, B.H.; Huang, S.S.; Guo, M.F. Molecular screening and predation evaluation of the key predators of Conopomorpha sinensis Bradley (Lepidoptera: Gracilariidae) in litchi orchards. Bull. Èntomol. Res. 2014, 104, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liu, Y.; Zhang, R.; Bai, Y.; Dong, L.; Liu, L.; Jia, X.; Wang, G.; Zhang, M. Structural characterization and in vitro gastrointestinal digestion and fermentation of litchi polysaccharide. Int. J. Biol. Macromol. 2019, 140, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Kitadate, K.; Homma, K.; Roberts, A.; Maeda, T. Thirteen-week oral dose toxicity study of Oligonol containing oligomerized polyphenols extracted from lychee and green tea. Regul. Toxicol. Pharmacol. 2014, 68, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Li, M.; Wang, G.; Zhu, S. Application of ABA and GA3 alleviated browning of litchi (Litchi chinensis Sonn.) via different strategies. Postharvest Biol. Technol. 2021, 181, 111672. [Google Scholar] [CrossRef]
- Tan, S.; Tang, J.; Shi, W.; Wang, Z.; Xiang, Y.; Deng, T.; Gao, X.; Li, W.; Shi, S. Effects of three drying methods on polyphenol composition and antioxidant activities of Litchi chinensis Sonn. Food Sci. Biotechnol. 2019, 29, 351–358. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, K.; Wang, K.; Zhu, J.; Hu, Z. Nutrient components, health benefits, and safety of litchi (Litchi chinensis Sonn.): A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2139–2163. [Google Scholar] [CrossRef]
- Wen, J.; Ma, L.; Xu, Y.; Wu, J.; Yu, Y.; Peng, J.; Tang, D.; Zou, B.; Li, L. Effects of probiotic litchi juice on immunomodulatory function and gut microbiota in mice. Food Res. Int. 2020, 137, 109433. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.A.; de Oliveira Lima, L.C.; Nunes, C.A.; Dias, D.R.; Schwan, R.F. Chemical, physical-chemical, and sensory characteristics of lychee (Litchi chinensis Sonn) wines. J. Food Sci. 2011, 76, S330–S336. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Mei, L.-H.; Wu, H.; Lin, D.-Q. Biosynthesis of γ-aminobutyric acid (GABA) using immobilized whole cells of Lactobacillus brevis. World J. Microbiol. Biotechnol. 2006, 23, 865–871. [Google Scholar] [CrossRef]
- Zhao, L.; Ruan, S.; Yang, X.; Chen, Q.; Shi, K.; Lu, K.; He, L.; Liu, S.; Song, Y. Characterization of volatile aroma compounds in litchi (Heiye) wine and distilled spirit. Food Sci. Nutr. 2021, 9, 5914–5927. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.J. Taxonomic Identification and Antimicrobial Spectrum of a Highly Inhibitory Penicillium Strain. Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2014. [Google Scholar]
- Ling, D.; Dong, X. Classification, Identification and Experimental Methods of Lactic Acid Bacteria, 1st ed.; China Light Industry Press: Beijing, China, 1999; pp. 121–126. [Google Scholar]
- Gerhardt, P. Manual of Methods for General Bacteriology, 1st ed.; American Society for Microbiology: Washington, DC, USA, 1981; p. 511. [Google Scholar]
- Moser, S.A.; Savage, D.C. Bile Salt Hydrolase Activity and Resistance to Toxicity of Conjugated Bile Salts Are Unrelated Properties in Lactobacilli. Appl. Environ. Microbiol. 2001, 67, 3476–3480. [Google Scholar] [CrossRef] [PubMed]
- Pavli, F.; Gkana, E.; Adebambo, O.; Karatzas, K.-A.; Panagou, E.; Nychas, G.-J.E. In Vitro Screening of γ-Aminobutyric Acid and Autoinducer-2 Signalling in Lactic Acid Bacteria Exhibiting Probiotic Potential Isolated from Natural Black Conservolea Olives. Foods 2019, 8, 640. [Google Scholar] [CrossRef]
- Hu, D.; Wu, J.; Jin, L.; Yuan, L.; Li, J.; Chen, X.; Yao, J. Evaluation of Pediococcus pentosaceus strains as probiotic adjunct cultures for soybean milk post-fermentation. Food Res. Int. 2021, 148, 110570. [Google Scholar] [CrossRef] [PubMed]
- Argyri, A.A.; Zoumpopoulou, G.; Karatzas, K.-A.; Tsakalidou, E.; Nychas, G.-J.; Panagou, E.Z.; Tassou, C.C. Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef]
- Khalil, M.A.; El-Sheekh, M.M.; El-Adawi, H.I.; El-Deeb, N.M.; Hussein, M.Z. Efficacy of microencapsulated lactic acid bacteria in Helicobater pylori eradication therapy. J. Res. Med. Sci. 2015, 20, 950–957. [Google Scholar] [CrossRef]
- Cataldo, P.G.; Villena, J.; Elean, M.; de Giori, G.S.; Saavedra, L.; Hebert, E.M. Immunomodulatory Properties of a γ-Aminobutyric Acid-Enriched Strawberry Juice Produced by Levilactobacillus brevis CRL 2013. Front. Microbiol. 2020, 11, 610016. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Lan, H.; Wang, K.; Zhao, L.; Hu, Z. Enhanced production of γ-aminobutyric acid in litchi juice fermented by Lactobacillus plantarum HU-C2W. Food Biosci. 2021, 42, 101155. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Ren, Y.; Wang, Y.; Ren, Y.; Wang, X.; Yue, T.; Wang, Z.; Gao, Z. Preparation, model construction and efficacy lipid-lowering evaluation of kiwifruit juice fermented by probiotics. Food Biosci. 2022, 47, 101710. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Oh, Y.; Kim, T.; Moon, H.; Lee, S.; Lee, S.; Ji, G.; Hwang, K. Lactobacillus plantarum PMO 08 as a Probiotic Starter Culture for Plant-Based Fermented Beverages. Molecules 2020, 25, 5056. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Jacobo, A.; Rüfer, C.E.; Gervilla, R.; Guamis, B.; Roig-Sagués, A.X.; Saldo, J. Influence of ultra-high pressure homogenisation on antioxidant capacity, polyphenol and vitamin content of clear apple juice. Food Chem. 2011, 127, 447–454. [Google Scholar] [CrossRef]
- Qi, J.; Huang, H.; Wang, J.; Liu, N.; Chen, X.; Jiang, T.; Xu, H.; Lei, H. Insights into the improvement of bioactive phytochemicals, antioxidant activities and flavor profiles in Chinese wolfberry juice by select lactic acid bacteria. Food Biosci. 2021, 43, 101264. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Gao, T.; Wu, Y.; Sun, H.; Zhu, Q.; Liu, C.; Zhou, C.; Han, Y.; Tao, Y. Fermentation and storage characteristics of “Fuji” apple juice using Lactobacillus acidophilus, Lactobacillus casei and Lactobacillus plantarum: Microbial growth, metabolism of bioactives and in vitro bioactivities. Front. Nutr. 2022, 9, 833906. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Xiao, L.; Li, X.; Hu, M. Effect of fermentation parameters and their optimization on the phytochemical properties of lactic-acid-fermented mulberry juice. J. Food Meas. Charact. 2017, 11, 1462–1473. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Domingos-Lopes, M.F.P.; Stanton, C.; Ross, R.P.; Silva, C.C. Production of γ-aminobutyric acid (GABA) by Lactobacillus otakiensis and other Lactobacillus sp. isolated from traditional Pico cheese. Int. J. Dairy Technol. 2018, 71, 1012–1017. [Google Scholar] [CrossRef]
- Yogeswara, I.; Maneerat, S.; Haltrich, D. Glutamate Decarboxylase from Lactic Acid Bacteria—A Key Enzyme in GABA Synthesis. Microorganisms 2020, 8, 1923. [Google Scholar] [CrossRef] [PubMed]
- Hill, I.R.; Kenworthy, R.; Porter, P. The effect of dietary lactobacilli on in-vitro catabolic activities of the small-intestinal microflora of newly weaned pigs. J. Med. Microbiol. 1970, 3, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-B.; Lew, L.-C.; Yeo, S.-K.; Parvathy, S.N.; Liong, M.-T. Probiotics and the BSH-related cholesterol lowering mechanism: A Jekyll and Hyde scenario. Crit. Rev. Biotechnol. 2015, 35, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Zarzecka, U.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Starter cultures as a reservoir of antibiotic resistant microorganisms. LWT 2020, 127, 109424. [Google Scholar] [CrossRef]
- Rönkä, E.; Malinen, E.; Saarela, M.; Rinta-Koski, M.; Aarnikunnas, J.; Palva, A. Probiotic and milk technological properties of Lactobacillus brevis. Int. J. Food Microbiol. 2003, 83, 63–74. [Google Scholar] [CrossRef]
- Mathur, S.; Singh, R. Antibiotic resistance in food lactic acid bacteria—A review. Int. J. Food Microbiol. 2005, 105, 281–295. [Google Scholar] [CrossRef]
- Vecchione, A.; Celandroni, F.; Mazzantini, D.; Senesi, S.; Lupetti, A.; Ghelardi, E. Compositional quality and potential gastrointestinal behavior of probiotic products commercialized in Italy. Front. Med. 2018, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Chondrou, P.; Bontsidis, C.; Karolidou, K.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Galanis, A.; Plessas, S. Assessment of the probiotic potential of lactic acid bacteria isolated from kefir grains: Evaluation of adhesion and antiproliferative properties in in vitro experimental systems. Ann. Microbiol. 2019, 69, 751–763. [Google Scholar] [CrossRef]
- Ayyash, M.M.; Abdalla, A.K.; AlKalbani, N.S.; Baig, M.A.; Turner, M.S.; Liu, S.-Q.; Shah, N.P. Invited review: Characterization of new probiotics from dairy and nondairy products-insights into acid tolerance, bile metabolism and tolerance, and adhesion capability. J. Dairy Sci. 2021, 104, 8363–8379. [Google Scholar] [CrossRef]
- Lei, S.; Li, X.; Liu, L.; Zheng, M.; Chang, Q.; Zhang, Y.; Zeng, H. Effect of lotus seed resistant starch on tolerance of mice fecal microbiota to bile salt. Int. J. Biol. Macromol. 2020, 151, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Horáčková, Š.; Plocková, M.; Demnerová, K. Importance of microbial defence systems to bile salts and mechanisms of serum cholesterol reduction. Biotechnol. Adv. 2018, 36, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Adams, M.C. In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int. J. Food Microbiol. 2004, 91, 253–260. [Google Scholar] [CrossRef]
- Sharma, V.; Mishra, H.N. Fermentation of vegetable juice mixture by probiotic lactic acid bacteria. Nutrafoods 2013, 12, 17–22. [Google Scholar] [CrossRef]
- Kim, N.J.; Jang, H.L.; Yoon, K.Y. Potato juice fermented with Lactobacillus casei as a probiotic functional beverage. Food Sci. Biotechnol. 2012, 21, 1301–1307. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Abu-Ghannam, N. Kinetic studies for the preparation of probiotic cabbage juice: Impact on phytochemicals and bioactivity. Ind. Crop. Prod. 2013, 50, 212–218. [Google Scholar] [CrossRef]
- Cataldo, P.G.; Villegas, J.M.; de Giori, G.S.; Saavedra, L.; Hebert, E.M. Enhancement of γ-aminobutyric acid (GABA) production by Lactobacillus brevis CRL 2013 based on carbohydrate fermentation. Int. J. Food Microbiol. 2020, 333, 108792. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Verón, H.E.; Cano, P.G.; Fabersani, E.; Sanz, Y.; Isla, M.I.; Espinar, M.T.F.; Ponce, J.V.G.; Torres Castaños, S. Cactus Pear (Opuntia Ficus-Indica) Juice Fermented with Autochthonous Lactobacillus Plantarum S-811. Food Funct. 2019, 10, 1085–1097. [Google Scholar] [CrossRef]
- Cele, N.P.; Akinola, S.A.; Shoko, T.; Manhevi, V.E.; Remize, F.; Sivakumar, D. The Bioaccessibility and Antioxidant Activities of Fermented Mango Cultivar Juices after Simulated In Vitro Digestion. Foods 2022, 11, 2702. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Song, Y.; Liang, Y.; Li, Y.; Chang, Y.; Ma, R.; Cao, X.; Wang, S. Dynamics of physicochemical properties, functional compounds and antioxidant capacity during spontaneous fermentation of Lycium ruthenicum Murr. (Qinghai-Tibet Plateau) natural vinegar. Foods 2022, 11, 1344. [Google Scholar] [CrossRef]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT 2020, 122, 109064. [Google Scholar] [CrossRef]
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, W.; Pan, X.; Lao, F.; Liao, X.; Shi, Y.; Wu, J. Improvement of antioxidant properties of jujube puree by biotransformation of polyphenols via Streptococcus thermophilus fermentation. Food Chem. X 2022, 13, 100214. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Bora, J.; Sharma, V. Growth studies of potentially probiotic lactic acid bacteria (Lactobacillus plantarum, Lactobacillus acidophilus, and Lactobacillus casei) in carrot and beetroot juice substrates. J. Food Process. Preserv. 2019, 43, e14214. [Google Scholar] [CrossRef]
- González-Herrera, S.M.; Simental-Mendía, L.E.; López, M.G.; Rocha-Guzmán, N.E.; Rutiaga-Quiñones, O.M.; Rodríguez-Herrera, R.; Gamboa-Gómez, C.I. Effect of agave fructans on the production of short chain fatty acid in mice. Food Sci. Biotechnol. 2019, 28, 1493–1498. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]

| Strain | Source | Blast Identification | Preservation Institution | Preservation Number | Accession Number |
|---|---|---|---|---|---|
| LBG-29 | Pickled cucumber | L. brevis | CCTCC (China Center for Type Culture Collection) | M 2022686 | ON287036 |
| LBG-24 | Pickled cucumber | L. brevis | - | - | ON287037 |
| LBD–14 | Pickled bell pepper | L. brevis | - | - | ON287034 |
| Assay | LBG-29 | LBG-24 | LBD–14 |
|---|---|---|---|
| Carbohydrate metabolism | |||
| Methyl red | − | − | − |
| V-P | − | − | − |
| Esculin hydrolysis | + | − | − |
| Hydrolysed hippurate | − | − | − |
| Starch hydrolysis | − | − | − |
| Sugar fermentation | The results are shown in Table 3 | ||
| Protein and amino acid metabolism | |||
| Indole | − | − | − |
| Hydrogen sulphide | − | − | − |
| Urease production | − | − | − |
| Gelatin liquefaction | − | − | − |
| Enzymes | |||
| Nitrate reduction | − | − | − |
| Sugar | LBG-29 | LBG-24 | LBD–14 |
|---|---|---|---|
| Amygdalin | + | − | + |
| DL-Arabinose | +++ | − | +++ |
| D-(+)-Cellobiose | ++ | − | ++ |
| Dulcitol | + | − | + |
| Erythritol | +++ | +++ | +++ |
| Esculin hydrate | ++ | − | ++ |
| D-Fructose | +++ | +++ | +++ |
| Glucose | +++ | +++ | +++ |
| Glycerine | + | − | + |
| Sodium hippurate | ++ | − | ++ |
| myo-Inositol | + | − | + |
| Inulin | + | − | + |
| Lactose | + | − | + |
| D-(+)-Maltose monohydrate | ++ | ++ | +++ |
| D(+)-Mannose | ++ | − | ++ |
| D-(−)-Ribose | +++ | +++ | +++ |
| L-(+)-Ribose | +++ | − | +++ |
| D-Sorbitol | + | − | + |
| Stachyose | ++ | − | ++ |
| D-Raffinose | + | − | + |
| Mannitol | + | − | + |
| D-(+)-Melezitose monohydrate | + | − | + |
| D-(+)-Melibiose monohydrate | +++ | +++ | +++ |
| L-Rhamnose monohydrate | ++ | − | ++ |
| D(−)-Salicin | + | − | + |
| L(−)-Sorbose | ++ | − | ++ |
| Soluble starch | + | + | + |
| Sucrose | + | − | + |
| D-(+)-Trehalose anhydrous | + | − | + |
| D(+)-Xylose | +++ | +++ | +++ |
| D-Gluconic acid sodium salt | +++ | ++ | +++ |
| Xylan | +++ | +++ | +++ |
| Arbutin | + | − | + |
| Maltotriose | ++ | − | ++ |
| (+)-Arabinogalactan | + | − | + |
| Dextrin | + | − | + |
| Isomalto-oligosaccharide | +++ | +++ | +++ |
| Fructo-oligosaccharide | + | − | + |
| Maltodextrin | ++ | − | ++ |
| Pectin | +++ | − | +++ |
| Strain Code | Levilactobacillus brevis LBG-29 | Levilactobacillus brevis LBG-24 | Levilactobacillus brevis LBD–14 |
|---|---|---|---|
| Deamination of arginine | − | − | − |
| Bile salt hydrolase activity | − | − | − |
| Haemolysis | − | − | − |
| Antibiotics | LBG-29 | LBG-24 | LBD–14 |
|---|---|---|---|
| Vancomycin (0.016–256 mg/L) | >32 (+) | >32 (+) | >32 (+) |
| Erythromycin (0.016–256 mg/L) | 1.0 (−) | 0.19 (−) | 0.50 (−) |
| Tetracycline (0.016–256 mg/L) | 5 (−) | 1.5 (−) | 5 (−) |
| Gentamicin (0.016–256 mg/L) | 6 (−) | 5 (−) | 5 (−) |
| Ampicillin (0.016–256 mg/L) | 1.000 (−) | 0.125 (−) | 0.750 (−) |
| Ciprofloxacin (0.002–32 mg/L) | >32 (+) | >32 (+) | >32 (+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Wu, J.; Hu, D.; Li, J.; Zhu, W.; Yuan, L.; Chen, X.; Yao, J. Gamma-Aminobutyric Acid-Producing Levilactobacillus brevis Strains as Probiotics in Litchi Juice Fermentation. Foods 2023, 12, 302. https://doi.org/10.3390/foods12020302
Jin Y, Wu J, Hu D, Li J, Zhu W, Yuan L, Chen X, Yao J. Gamma-Aminobutyric Acid-Producing Levilactobacillus brevis Strains as Probiotics in Litchi Juice Fermentation. Foods. 2023; 12(2):302. https://doi.org/10.3390/foods12020302
Chicago/Turabian StyleJin, Yiwen, Jinyong Wu, Dan Hu, Jun Li, Weiwei Zhu, Lixia Yuan, Xiangsong Chen, and Jianming Yao. 2023. "Gamma-Aminobutyric Acid-Producing Levilactobacillus brevis Strains as Probiotics in Litchi Juice Fermentation" Foods 12, no. 2: 302. https://doi.org/10.3390/foods12020302
APA StyleJin, Y., Wu, J., Hu, D., Li, J., Zhu, W., Yuan, L., Chen, X., & Yao, J. (2023). Gamma-Aminobutyric Acid-Producing Levilactobacillus brevis Strains as Probiotics in Litchi Juice Fermentation. Foods, 12(2), 302. https://doi.org/10.3390/foods12020302