Effect of Beetroot Powder Incorporation on Functional Properties and Shelf Life of Biscuits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
Preparation of Beetroot Powder (BP)
2.3. Physical Properties of Beetroot Powder and Spelt Flour
2.3.1. Bulk Density
2.3.2. Water-Binding Capacity
2.3.3. Oil-Binding Capacity
2.3.4. Swelling Capacity
2.3.5. Hydration Capacity
2.4. Preparation of Biscuits (BPB)
2.5. Determining the Color of Biscuits
2.6. Determination of Water Activity (aw) of Starting Raw Materials and Biscuits
2.7. Proximate Composition of Beetroot Powder, Spelt Flour, and Biscuits
2.8. Spectrophotometric Determination of Betalain, Polyphenol, and Flavonoid Content
2.8.1. Extract Preparation
2.8.2. Determination of Total Betalains
2.8.3. Determination of Total Polyphenols Content
2.8.4. Determination of Total Flavonoid Content
2.9. Estimation of Antioxidant Determination
2.9.1. DPPH Radical Scavenging Assay
2.9.2. Ferric Reducing Antioxidant Power (FRAP)
2.10. Qualitative and Quantitative Determination of Polyphenols by HPLC-UV-Vis Method
2.11. Determination of Microbiological Correctness
2.12. Statistical Analysis
3. Results and Discussions
3.1. Physicochemical Properties of Beetroot Powder, Spelt Flour, and Beetroot–Enriched Biscuits
3.2. Changes in the Total Content of Betalains, Polyphenols, and Flavonoids
3.2.1. Total Betalains
3.2.2. Total Polyphenols and Flavonoid Content
3.3. Antioxidative Determination during Storage
3.3.1. DPPH Assay
3.3.2. FRAP Assay
3.4. Changes in Qualitative and Quantitative Phenolic Composition during Storage
3.5. Changes in Visual Color Parameters
3.6. Estimation of Biscuits Shelf Life Based on Microbiological Safety and Water Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bianchi, F.; Pünsch, M.; Venir, E. Effect of Processing and Storage on the Quality of Beetroot and Apple Mixed Juice. Foods 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Sahni, P.; Shere, D.M. Physico-chemical and sensory characteristics of beet root pomace powder incorporated fibre rich cookies. Int. J. Food Ferment. Technol. 2016, 6, 309–315. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Vidal, D.; Fito, P. Functional foods development: Trends and technologies. Trends Food Sci. Technol. 2011, 22, 498–508. [Google Scholar] [CrossRef]
- Hu, M. Oxidative stability and shelf life of low-moisture foods. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Elsevier Inc.: London, UK, 2016; pp. 313–371. [Google Scholar]
- Ćetković, G.; Šeregelj, V.; Brandolini, A.; Čanadanović-Brunet, J.; Tumbas Šaponjac, V.; Vulić, J.; Šovljanski, O.; Četojević-Simin, D.; Škrobot, D.; Mandić, L.A.; et al. Composition, texture, sensorial quality, and biological activity after in vitro digestion of durum wheat pasta enriched with carrot waste extract encapsulates. Int. J. Food Sci. Nutr. 2021, 73, 638–649. [Google Scholar] [CrossRef]
- Zlatanović, S.; Kalušević, A.; Micić, D.; Lalić-Petronijević, J.; Tomić, N.; Ostojić, S.; Gorjanović, S. Functionality and storability of cookies fortified at the industrial scale with up to 75% of apple pomace flour produced by dehydration. Foods 2019, 8, 561. [Google Scholar] [CrossRef] [Green Version]
- Jazić, M.; Kukrić, Z.; Vulić, J.; Četojević-Simić, D. Polyphenolic composition, antioxidant and antiproliferative effects of wild and cultivated blackberries (Rubus fruticosus L.) pomace. Int. J. Food Sci. Technol. 2019, 54, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of apple pomace flour obtained industrially by dehydration as a source of biomolecules with antioxidant, antidiabetic and antiobesity effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef]
- Kohajdova, Z.; Karovičova, J.; Laukova, M. Influence of carrot pomace powder on the rheological characteristics of wheat flour dough and on wheat rolls quality. Acta Sci. Pol. Technol. Aliment. 2012, 11, 381–387. [Google Scholar]
- Raczyk, M.; Kruszewski, B.; Zachariasz, E. Effect of tomato, beetroot and carrot juice addition on physicochemical, antioxidant and texture properties of wheat bread. Antioxidants 2020, 11, 2178. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.V.; Jagus, R.J.; Agüero, M.V. Evaluation and characterization of nutritional, microbiological and sensory properties of beet greens. Act. Sci. Nutr. Health 2017, 1, 37–45. [Google Scholar]
- Krejcova, A.; Cernohorsky, T.; Meixner, D. Elemental analysis of instant soups and seasoning mixtures by ICP-OES. Food Chem. 2007, 105, 242–247. [Google Scholar] [CrossRef]
- Pinki, P.A. Sensory and nutritional evaluation of value added cakes formulated by incorporating beetroot powder. Int. J. Food Sci. Nutr. 2014, 3, 145–148. [Google Scholar]
- Singh, J.P.; Kaur, A.; Shevkani, K.; Singh, N.; Singh, B. Physicochemical characterization of corn extrudates prepared with varying levels of beetroot (Beta vulgaris) at different extrusion temperatures. Int. J. Food Sci. Technol. 2016, 51, 911–919. [Google Scholar] [CrossRef]
- Kavalcova, P.; Bystricka, J.; Tomas, J.; Karovicova, J.; Kovarovic, J.; Lenkova, M. The content of total polyphenol and antioxidant activity in red beetroot. J. Food Sci. Technol. 2015, 9, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Houshialsadat, Z.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr. Metab. 2020, 17, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshehry, G.; Abdelazez, A.; Abdelmotaal, H.; Abdel-Aleem, W. Investigating antioxidant and antibacterial activity of functional cookies enriched with beetroot during storage. Czech J. Food Sci. 2021, 39, 479–486. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Schiffler, B.; Bernhardt, R. Effect of fermentation on the functional properties of sorghum flour. Food Chem. 2005, 92, 1–5. [Google Scholar] [CrossRef]
- Rana, S.; Gupta, S.; Rana, A.; Bhushan, S. Functional properties, phenolic constituents and antioxidant potential of industrial apple pomace for utilization as active food ingredient. Food Sci. Hum. Wellness 2015, 4, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J.B.A.; Monredon, F.D.; Dysseler, P.; Guillon, F.; Amadò, R.; Thibault, J.F. Hydration properties of dietary fibre and resistant starch. Food Sci. Technol. 2000, 33, 72–79. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Lee, S.C.; Prosky, L.; DeVries, J.W. Determination of total, soluble, and insoluble, dietary fiber in foods—Enzymatic10 gravimetric method, MES-TRIS buffer: Collaborative study. J. Assoc. Off. Anal. Chem. 1992, 75, 395–416. [Google Scholar]
- Jaćimović, S.; Popović-Djordjević, J.; Sarić, B.; Krstić, A.; Mickovski-Stefanović, V.; Pantelić, N. Antioxidant activity and multi-elemental analysis of dark chocolate. Foods 2022, 11, 1445. [Google Scholar] [CrossRef] [PubMed]
- Von Elbe, J.H. Betalains. Curr. Protoc. Food Anal. Chem. 2003, 2001, F3.1.1–F3.1.7. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1955, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regulation on general and specific food hygiene requirements at any stage of production, processing and transportation. In Official Gazette of the Republic of Serbia; No 62/2018; National Assembly of the Republic of Serbia: Belgrade, Serbia, 2018.
- Guide for the Application of Microbiological Criteria for Food, 1st ed.; Republic of Serbia, Ministry of Agriculture, Trade, Forestry and Water Management: Belgrade, Serbia, 2011.
- Kohajdova, Z.; Karovicova, J.; Magala, M.; Laukova, M. Utilisation of beetroot powder for bakery applications. Chem. Pap. 2018, 72, 1507–1515. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fiber in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef]
- Bchir, B.; Rabetafka, N.; Paquot, M.; Blecker, C. Efect of pear, apple and date fibers from cooked fruit by-products on dough performance and bread quality. Food Bioproc. Technol. 2014, 7, 1114–1127. [Google Scholar] [CrossRef]
- Mora, Y.N.; Contreras, J.C.; Aguilar, C.N.; Meléndez, P.; Dela Garya, H.; Rodríguez, R. Chemical composition and functional properties form diferent sources of dietary fiber. Am. J. Food Nutr. 2013, 1, 27–33. [Google Scholar]
- Petek, M.; Toth, N.; Pecina, M.; Karazija, T.; Lazarević, B.; Palčić, I. Beetroot mineral composition affected by mineral and organic fertilization. PLoS ONE 2019, 14, e0221767. [Google Scholar] [CrossRef]
- Bhavani, U.; Mokenapalli, S.; Vellanki, B. Comparative studies and quality evaluation of beetroot powder by different drying methods. Int. J. Innov. Res. Sci. Eng. Technol. 2022, 8, 273–279. [Google Scholar]
- Nazni, P.; Thara, D. Optimization of beetroot peel osmotic dehydration process using response surface methodology. Int. J. Curr. Res. 2011, 3, 27–32. [Google Scholar]
- Weickert, M.O.; Pfeiffer, A.F. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Food Sci. Technol. 2006, 44, 2365–2376. [Google Scholar] [CrossRef] [Green Version]
- Besbes, E.; Jury, V.; Monteau, J.Y.; Le Bail, A. Effect of baking conditions and storage with crust on the moisture profile, local textural properties and staling kinetics of pan bread. LWT Food Sci. Technol. 2014, 58, 658–666. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, A. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [Green Version]
- Pap, N.; Fidelis, M.; Azevedo, L.; Araújo, M.; Carmo, V.; Wang, D.; Mocan, A.; Penha, E.; Pereira, R.; Xavier-Santos, D.; et al. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Marak, N.; Malemnganbi, C.C.; Marak, C.R.; Mishra, L.K. Functional and antioxidant properties of cookies incorporated with foxtail millet and ginger powder. J. Food Sci. Technol. 2019, 56, 5087–5096. [Google Scholar] [CrossRef]
- Shafi, A.; Ahmad, F.; Mohammed, Z.H. Effect of the addition of banana peel flour on the shelf life and antioxidant properties of cookies. ACS Food Sci. Technol. 2022, 2, 1355–1363. [Google Scholar] [CrossRef]
- Dundar, A.N. Total phenolic and antioxidant bioaccessibilities of cookies enriched with bee pollen. J. Food Process. Preserv. 2021, 46, e16085. [Google Scholar] [CrossRef]
- Aziz, M.; Yasmin, I.; Batool, R.; Khan, W.A.; Naz, S.; Ashraf, F.; Azam, M.; Khaliq, A.; Iqubal, R. Exploring the effect of apricot addition on nutritional, antioxidant, textural and sensory characteristics of cookies apricot supplemented functional cookies. Ital. J. Food Sci. 2020, 32, 831–844. [Google Scholar]
- Najjar, Z.; Kizhakkayil, J.; Shakoor, H.; Platat, C.; Stathopoulos, C.; Ranasinghe, M. Antioxidant potential of cookies formulated with date seed powder. Foods 2022, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Panche, N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurminah, M.; Sinaga, H.; Sahrina Gultomm, N. The effect of the additional of Moringa leaves flour (Moringa oleifera) on the physicochemical properties of cup cake from composite flour. IOP Conf. Ser. Earth Environ. Sci. 2019, 305, 12031. [Google Scholar] [CrossRef]
- Ju, H.; Ho Lee, J. Quality and antioxidant properties of wheat cookies supplemented with maqui berry powder. J. Korean J. Food Preserv. 2021, 28, 480–488. [Google Scholar]
- Choi, J.E.; Lee, J.H. Quality and antioxidant attributes of cookies supplemented with cranberry powder. Korean J. Food Sci. Technol. 2015, 47, 132–135. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.S.; Kim, S.K.; Mo, E.K. Quality characteristics of cookies with acai berry (Euterpe oleracea Mart.) powder added. Korean J. Food Preserv. 2014, 21, 661–667. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Liu, Y.; Yoon, L.; Park, H. Comparison of quality characteristics and antioxidative activities of cookies containing blueberry powder and different types of egg yolk. J. Korean Soc. Food Sci. Nutr. 2014, 43, 999–1008. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food. Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Dvořáková, M.; Guido, L.F.; Dostálek, P.; Skulilová, Z.; Moreira, M.M.; Barros, A.A. Antioxidant properties of free, soluble ester and insoluble-bound phenolic compounds in different barley varieties and corresponding malts. J. Inst. Brew. 2008, 114, 27–33. [Google Scholar] [CrossRef]
- Hidalgo, A.; Yilmaz, V.A.; Brandolini, A. Influence of water biscuit processing and kernel puffing on the phenolic acid content and the antioxidant activity of einkorn and bread wheat. J. Food Sci. Technol. 2016, 53, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.M.; Rabalski, I. Effect of baking on free and bound phenolic acids in wholegrain bakery products. J. Cereal Sci. 2013, 57, 312–318. [Google Scholar] [CrossRef]
- Fares, C.; Platani, C.; Baiano, A.; Menga, V. Effect of processing and cooking on phenolic acid profile and antioxidant capacity of durum wheat pasta enriched with debranning fractions of wheat. Food Chem. 2010, 119, 1023–1029. [Google Scholar] [CrossRef]
- Georgiev, V.; Weber, J.; Kneschke, E.M.; Denev, P. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant. Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Castro-Lópeza, C.; Sánchez-Alejoa, E.J.; Saucedo-Pompab, S.; Rojasa, R.; Aranda-Ruiza, J.; Martínez-Avilaa, G.C.G. Fluctuations in phenolic content, ascorbic acid and total carotenoids and antioxidant activity of fruit beverages during storage. Heliyon 2016, 2, e00152. [Google Scholar] [CrossRef] [Green Version]
- Purlis, E.; Salvadori, V.O. Bread browning kinetics during baking. J. Food Eng. 2007, 80, 1107–1115. [Google Scholar] [CrossRef]
- Kayın, N.; Atalay, D.; Akcay, T.T.; Erge, H.S. Color stability and change in bioactive compounds of red beet juice concentrate stored at different temperatures. J. Food Sci. Technol. 2019, 56, 5097–5106. [Google Scholar] [CrossRef]
- Uthumporn, U.; Woo, W.L.; Tajul, A.Y.; Fazilah, A. Physico-chemical and nutritional evaluation of cookies with different levels of eggplant flour substitution. J. Food Sci. 2015, 13, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Impact of thermal treatment on colour and pigment pattern of red beet (Beta vulgaris L.) preparations. J. Food Sci. 2004, 69, 491–498. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Mennella, C.; Barba, F.; Russo, M.; Russo, G.L.; Krome, K.; Fogliano, V. Characterization of coloured compounds obtained by enzymatic extraction of bakery products. Food Chem. Toxicol. 2003, 41, 1367–1374. [Google Scholar] [CrossRef]
- Nyam, K.L.; Sod-Ying, L.; Chin-Ping, T.; Kamariah, L. Functional properties of roselle (Hibiscus sabdariffa L.) seed and its application as bakery product. J. Food Sci. Technol. 2014, 51, 3830–3837. [Google Scholar] [CrossRef] [PubMed]
- Pitalua, E.; Jimenez, M.; Vernon-Carter, E.J.; Beristain, C.I. Antioxidative activity of microcapsules with beetroot juice using gum Arabic as wall material. Food Bioprod. Process. 2010, 88, 253–258. [Google Scholar] [CrossRef]
- Marriott, N.G.; Schilling, M.W.; Gravani, R.B. Principles of Food Sanitation, 6th ed.; Springer: Cham, Switzerland, 2018; p. 437. [Google Scholar]
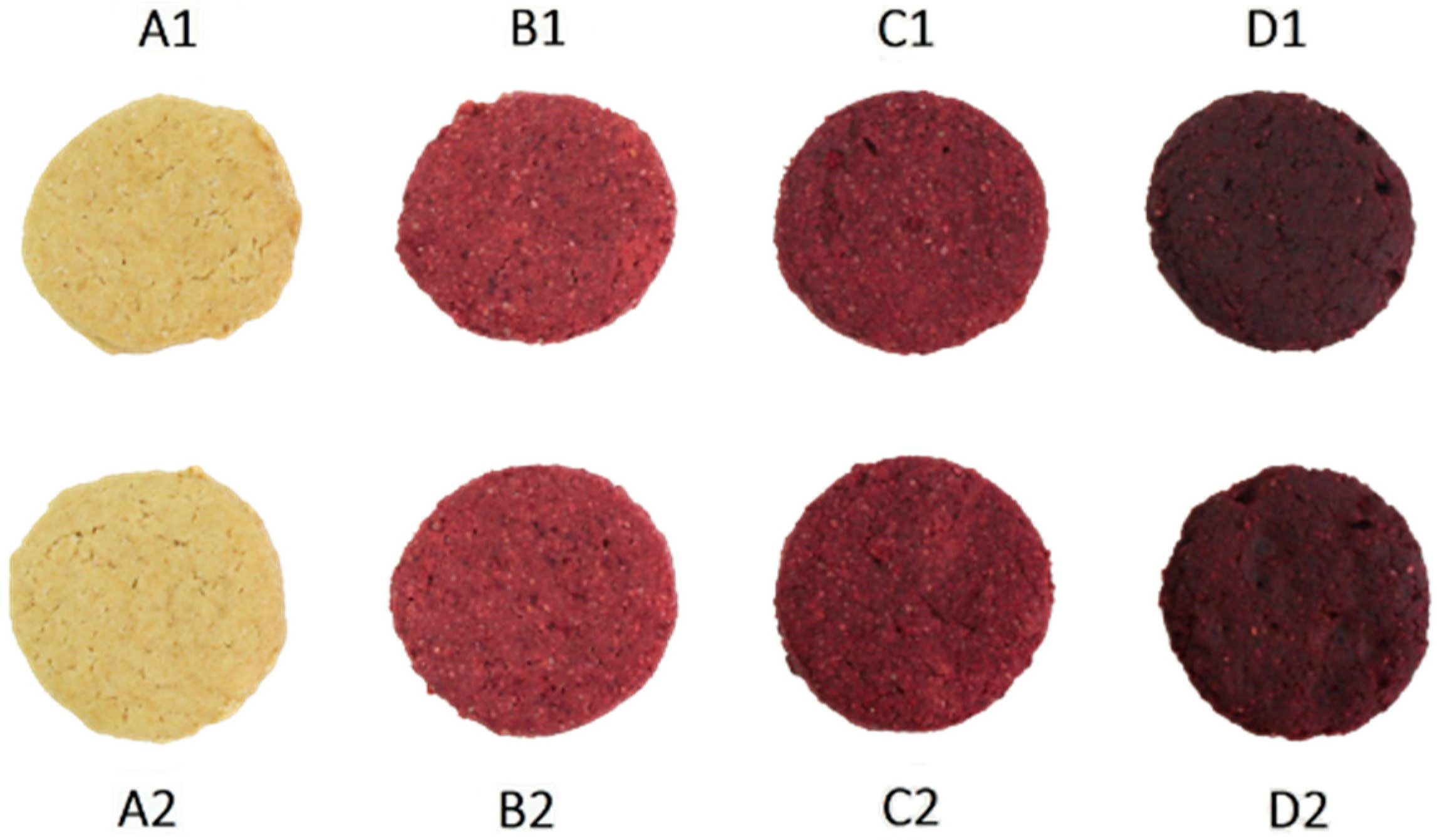




| Sample Designation | Spelt Flour (g) | Amount of Beetroot Powder (g) | Mass Fraction of Beetroot Powder (%) | |
|---|---|---|---|---|
| 150 °C | 170 °C | |||
| A1 | A2 | 150 | 0 | 0 |
| B1 | B2 | 105 | 45 | 15 |
| C1 | C2 | 90 | 60 | 20 |
| D1 | D2 | 75 | 75 | 25 |
| Bulk Density [g/mL] | Water-binding Capacity [g/g] | Oil-binding Capacity [g/g] | Swelling Capacity [mL/g] | Hydration Capacity, [g/mL] | |
|---|---|---|---|---|---|
| Beetroot Powder (BP) | 14.00 ± 0.61 A | 2.00 ± 0.12 A | 3.56 ± 0.21 A | 32.50 ± 1.02 A | 11.00 ± 0.44 A |
| Spelt flour (SF) | 17.00 ± 0.69 B | 7.12 ± 0.22 B | 3.42 ± 0.19 A | 33.00 ± 1.23 A | 11.00 ± 0.53 A |
| Proximate Composition (%) | BP | SF | A1 | B1 | C1 | D1 |
|---|---|---|---|---|---|---|
| Fat | 0.59 ± 0.12 A | 2.41 ± 0.13 B | 25.62 ± 0.35 C | 25.55 ± 0.38 C | 25.01 ± 0.34 C | 25.10 ± 0.30 C |
| Total carbohydrate | 77.41 ± 0.60 A | 71.89 ± 0.19 B | 59.95 ± 0.69 C | 58.39 ± 0.62 C | 59.11 ± 0.64 C | 59.18 ± 0.79 C |
| Total dietary fiber | 19.90 ± 0.55 A | 4.03 ± 0.21 B | 3.36 ± 0.23 B | 6.85 ± 0.20 C | 7.10 ± 0.21 C | 7.60 ± 0.41 C |
| Insoluble dietary fiber | 14.80 ± 0.26 A | 3.08 ± 0.32 B | 2.64 ± 0.14 B | 5.06 ± 0.29 C | 5.30 ± 0.28 C | 5.60 ± 0.39 C |
| Soluble dietary fiber | 5.10 ± 0.29 A | 0.95 ± 0.14 B | 0.71 ± 0.10 B | 1.79 ± 0.16 C | 1.80 ± 0.19 C | 2.04 ± 0.25 C |
| Total sugar | 57.06 ± 1.2 A | 1.58 ± 0.19 B | 22.19 ± 0.32 B | 30.63 ± 0.33 C | 33.69 ± 0.35 D | 35.89 ± 0.53 D |
| Sucrose | 51.82 ± 0.5 A | 0.51 ± 0.08 B | 20.96 ± 0.18 C | 28.97 ± 0.22 D | 31.97 ± 0.20 E | 33.86 ± 0.20 F |
| Glucose | 3.46 ± 0.42 A | 0.82 ± 0.11 B | 0.85 ± 0.09 B | 1.14 ± 0.06 B | 1.19 ± 0.09 B | 1.42 ± 0.21 B |
| Fructose | 1.78 ± 0.30 A | 0.25 ± 0.04 B | 0.38 ± 0.05 B | 0.53 ± 0.05 B | 0.53 ± 0.06 B | 0.61 ± 0.13 B |
| Protein | 11.4 ± 0.20 A | 13.50 ± 0.11 B | 8.92 ± 0.12 C | 9.17 ± 0.08 C | 9.06 ± 0.13 C | 8.94 ± 0.1 C |
| Ash | 3.80 ± 0.18 A | 1.40 ± 0.07 B | 2.54 ± 0.10 C | 2.75 ± 0.08 CD | 2.92 ± 0.11 D | 2.95 ± 0.14 D |
| Moisture | 6.80 ± 0.18 A | 10.80 ± 0.18 B | 2.96 ± 0.14 C | 4.15 ± 0.06 D | 3.91 ± 0.07 D | 3.84 ± 0.18 D |
| Total Betacyanins (mgbeanin/100g) | Total Betaxanthins (mg vulgaxantin I/100g) | |||||
|---|---|---|---|---|---|---|
| Start of Storage | 3 Months | 6 Months | Start of Storage | 3 Months | 6 Months | |
| B1 | 3.19 ± 0.00 A;1 | 1.47 ± 0.01 B;1 | 1.27 ± 0.02 C;1 | 2.23 ± 0.03 A;1 | 1.41 ± 0.34 B;1 | 1.01 ± 0.00 C;1 |
| C1 | 3.43 ± 0.01 A;1 | 2.28 ± 0.00 B;2 | 1.13 ± 0.01 C;2 | 3.31 ± 0.71 A;2 | 2.11 ± 0.02 B;2 | 0.97 ± 0.01 C;1 |
| D1 | 3.88 ± 0.05 A;2 | 3.24 ± 0.045 B;3 | 1.23 ± 0.01 C;12 | 3.28 ± 0.06 A;2 | 2.74 ± 0.05 B;3 | 0.95 ± 0.02 C;1 |
| B2 | 1.78 ± 0.01 A;1 | 1.27 ± 0.01 B;1 | 1.15 ± 0.01 C;1 | 1.65 ± 0.037 A;1 | 1.18 ± 0.03 B;1 | 0.92 ± 0.19 C;1 |
| C2 | 1.90 ± 0.05 A;1 | 1.36 ± 0.04 B;12 | 1.61 ± 0.02 C;2 | 1.49 ± 0.036 A;2 | 1.06 ± 0.02 B;2 | 1.11 ± 0.02 C;2 |
| D2 | 2.30 ± 0.01 A;2 | 1.64 ± 0.01 B;2 | 1.11 ± 0.01 C;1 | 2.18 ± 0.035 A;3 | 1.56 ± 0.02 B;3 | 1.10 ± 0.02 C;2 |
| BP | 12.79 ± 0.10 | 10.59 ± 0.16 | 8.14 ± 0.12 | 9.17 ± 0.54 | 8.88 ± 0.26 | 6.34 ± 0.18 |
| Beetroot Powder | Spelt Flour | |||||
|---|---|---|---|---|---|---|
| Polyhenolic Composition mg/100 g | Start of Storage | 3 Months | 6 Months | Start of Storage | 3 Months | 6 Months |
| Gallic acid | 106.36 ± 5.01 | 79.76 ± 3.87 | 78.29 ± 1.12 | 4.28 ± 0.19 | 4.00 ± 0.19 | 3.10 ± 0.16 |
| Protocatechuic acid | 88.04 ± 3.98 | 49.17 ± 2.34 | 32.76 ± 1.01 | 4.47 ± 0.18 | 4.33 ± 0.20 | 3.62 ± 0.20 |
| Epicatechin | 17.57 ± 0.69 | 18.71 ± 0.78 | 16.92 ± 0.54 | n.d. | n.d. | n.d. |
| Catechin | 45.28 ± 2.11 | 68.05 ± 2.98 | 48.19 ± 2.01 | n.d. | n.d. | n.d. |
| p-Coumaric acid | 4.66 ± 0.19 | 2.63 ± 0.09 | 4.22 ± 0.08 | n.d. | n.d. | n.d. |
| Caffeic acid | 11.47 ± 0.43 | 4.75 ± 0.21 | 6.80 ± 0.31 | 0.58 ± 0.02 | 0.28 ± 0.01 | 0.29 ± 0.01 |
| Chlorogenic acid | 3.93 ± 0.13 | 6.28 ± 0.28 | 5.63 ± 0.21 | 0.20 ± 0.01 | 0.19 ± 0.01 | 0.05 ± 0.01 |
| Vanillic acid | 43.62 ± 1.79 | 30.01 ± 1.43 | 14.04 ± 0.77 | 25.92 ± 1.12 | 23.95 ± 1.13 | 20.52 ± 1.11 |
| Total | 320.93 A | 259.37 B | 206.85 C | 35.44 A | 32.74 B | 30.45 C |
| Polyhenolic Composition mg/100 g | A1 | A2 | B1 | B2 | C1 | C2 | D1 | D2 |
|---|---|---|---|---|---|---|---|---|
| Start of storage | ||||||||
| Gallic acid | 2.76 ± 1.11 A,1 | 2.97 ± 0.12 A;1 | 22.19 ± 1.06 A;2 | 15.15 ± 0.69 A;2 | 25.16 ± 1.20 A;3 | 31.02 ± 1.45 A;3 | 32.04 ± 1.56 A;4 | 41.51 ± 2.16 A;4 |
| Protocatechuic acid | 3.33 ± 0.09 A;1 | 5.11 ± 0.28 A;1 | 21.11 ± 1.01 A;2 | 13.51 ± 0.64 A;2 | 18.16 ± 0.83 A;3 | 29.73 ± 1.45 A;3 | 24.86 ± 1.12 A;4 | 40.36 ± 2.06 A;4 |
| Epicatechin | n.d. | n.d. | n.d. | 0.71 ± 0.02 1 | 2.20 ± 0.10 1 | 2.07 ± 0.08 2 | 3.56 ± 0.14 2 | n.d. |
| Catechin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| p-Coumaric acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Caffeic acid | 0.14 ± 0.01 A;1 | 0.19 ± 0.01 A;1 | 1.37 ± 0.05 A;2 | 0.85 ± 0.03 A;2 | 1.27 ± 0.09 A;2 | 2.32 ± 0.06 A;3 | 6.29 ± 0.29 A;3 | 2.71 ± 0.09 A;4 |
| Chlorogenic acid | n.d | 0.14 ± 0.01 A;1 | 5.29 ± 0.21 A;1 | 1.45 ± 0.06 A;2 | 4.28 ± 0.19 A;2 | 4.22 ± 0.15 A;3 | 2.25 ± 0.09 A;3 | 4.33 ± 0.20 A;3 |
| Vanillic acid | 13.54 ± 0.61 A;1 | 11.74 ± 0.43 A;1 | 24.45 ± 1.23 A;2 | 11.12 ± 0.51 A;1 | 25.85 ± 1.11 A;2 | 18.03 ± 0.90 A;2 | 20.83 ± 0.98 A;3 | 21.22 ± 0.82 A;3 |
| Total | 19.77 | 20.15 | 74.40 | 42.80 | 76.92 | 87.40 | 89.82 | 110.13 |
| 3 months | ||||||||
| Gallic acid | 4.26 ± 0.17 B;1 | 5.52 ± 0.23 B;1 | 18.34 ± 0.89 B;2 | 20.00 ± 0.99 B;2 | 23.73 ± 1.16 B;3 | 23.69 ± 1.01 B;3 | 23.36 ± 1.13 B;3 | 24.02 ± 1.95 B;3 |
| Protocatechuic acid | 7.86 ± 0.42 B;1 | 7.15 ± 0.27 B;1 | 10.88 ± 0.51 B;2 | 14.79 ± 0.07 B;2 | 16.68 ± 0.76 B;3 | 13.81 ± 0.56 B;2 | 19.97 ± 1.09 B;4 | 19.38 ± 0.87 B;3 |
| Epicatechin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Catechin | n.d. | n.d. | 1.54 ± 0.06 A;1 | 0.18 ± 0.01 A;1 | 7.46 ± 0.28 A;2 | 4.88 ± 0.21A;2 | 13.09 ± 0.54 A;3 | 4.48 ± 0.21 A;2 |
| p-Coumaric acid | n.d. | n.d. | 0.60 ± 0.03 1 | n.d. | 0.77 ± 0.02 2 | n.d. | 0.47 ± 0.01 3 | n.d. |
| Caffeic acid | 0.97 ± 0.03 B;1 | 0.14 ± 0.01 B;1 | 1.97 ± 0.08 B;2 | 1.71 ± 1.07 B;2 | 0.29 ± 0.01 B;3 | 2.45 ± 0.09 B;3 | 2.36 ± 0.11 | 2.32 ± 0.07 B;3 |
| Chlorogenic acid | 0.22 ± 0.01 A;1 | 1.11 ± 0.04 B;1 | 1.74 ± 0.05 B;2 | 4.75 ± 0.19 B;2 | 1.41 ± 0.04 B;3 | 3.26 ± 0.14 B;3 | 1.82 ± 0.08 B;4 | 4.51 ± 0.20 B;2 |
| Vanillic acid | 6.24 ± 0.29 B;1 | 6.24 ± 0.61 B;1 | 18.02 ± 0.86 B;2 | 18.00 ± 0.84 B;2 | 19.19 ± 0.93 B;2 | 20.60 ± 0.91 B;3 | 25.86 ± 1.17 B;3 | 24.68 ± 1.15 B;4 |
| Total | 19.56 | 20.17 | 53.09 | 59.44 | 69.53 | 68.69 | 86.93 | 79.39 |
| 6 months | ||||||||
| Gallic acid | 2.41 ± 0.11 C;1 | 2.67 ± 0.21 C;1 | 21.16 ± 0.79 C;2 | 23.45 ± 0.86 C;2 | 23.79 ± 1.15 C;3 | 28.39 ± 1.03 C;3 | 26.59 ± 1.09 C;4 | 32.26 ± 1.84 C;4 |
| Protocatechuic acid | 3.26 ± 0.13 C;1 | 3.76 ± 0.28 C;1 | 17.32 ± 0.48 C;2 | 16.09 ± 0.06 C;2 | 20.00 ± 0.66 C;3 | 25.79 ± 0.58 C;3 | 20.35 ± 1.07 C;3 | 28.39 ± 0.44 C;3 |
| Epicatechin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Catechin | 0.86 ± 0.02 1 | 1.36 ± 0.08 1 | 2.19 ± 0.04 B;2 | 1.21 ± 0.02 B;2 | 2.26 ± 0.06 B;2 | 1.93 ± 0.11 B;3 | 7.67 ± 0.10 B;3 | 6.88 ± 0.43 B;4 |
| p-Coumaric acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Caffeic acid | 1.82 ± 0.06 C;1 | 0.30 ± 0.02 C;1 | 2.20 ± 0.06 C;2;3 | 2.47 ± 1.05 C;2 | 2.05 ± 0.04 C;2 | 2.79 ± 0.08 C;3 | 2.36 ± 0.11 C;3 | 2.60 ± 0.06 C;2;3 |
| Chlorogenic acid | 0.11 ± 0.01 B;1 | 0.11 ± 0.01 A;1 | 5.87 ± 0.15 C;2 | 2.98 ± 0.17 C;2 | 6.60 ± 0.11 C;3 | 3.08 ± 0.13 C;2 | 5.23 ± 0.10 C;4 | 3.96 ± 0.20 C;3 |
| Vanillic acid | 11.23 ± 0.54 C;1 | 9.98 ± 0.88 C;1 | 10.84 ± 0.45 C;1 | 8.27 ± 0.64 C;2 | 12.97 ± 0.44 C;2 | 8.19 ± 0.41 C;2 | 14.98 ± 0.98 C;3 | 3.91 ± 0.24 C;3 |
| Total | 19.69 | 18.18 | 59.59 | 54.51 | 67.75 | 70.18 | 78.14 | 78.13 |
| Start of Storage | 3 Months | 6 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | |
| A1 | 53.91 ± 0.05 A;3 | 9.05 ± 0.35 A;1 | 25.48 ± 0.96 A;5 | 55.91 ± 1.58 B;4 | 9.45 ± 0.38 A;1 | 24.73 ± 0.54 A;3 | 56.74 ± 1.34 C;4 | 9.22 ± 0.42 A;1 | 24.32 ± 0.40 A;3 |
| B1 | 26.80 ± 0.36 A;1 | 23.70 ± 0.88 A;4 | 10.62 ± 0.40 A;2 | 27.72 ± 1.13 A;3 | 24.00 ± 1.55 A;2 | 10.49 ± 1.01 A;2 | 30.55 ± 1.32 B;3 | 26.60 ± 1.29 B;2 | 11.59 ± 1.11 B;2 |
| C1 | 26.57 ± 1.39 A;1 | 24.49 ± +0.87 A;4 | 11.51 ± 0.62 C;3 | 26.23 ± 0.90 A;2,3 | 26.12 ± 0.74 B;3 | 8.54 ± 0.46 A;1 | 27.46 ± 2.12 A;1,2 | 26.08 ± 3.29 B;2 | 9.80 ± 0.95 B;1 |
| D1 | 25.88 ± 0.48 A;1 | 22.13 ± 2.17 A;3 | 9.94 ± 0.76 B;1 | 25.47 ± 1.30 A;2 | 24.36 ± 0.69 B;2 | 9.14 ± 0.56 A;1 | 27.16 ± 0.89 B;1,2 | 24.49 ± 0.84 B;2 | 9.97 ± 0.63 B;1 |
| A2 | 53.14 ± 0.33 A;3 | 10.04 ± 0.49 B;2 | 27.14 ± 1.17 B;6 | 56.82 ± 1.03 C;4 | 9.13 ± 0.53 A;1 | 24.41 ± 0.47 A;3 | 55.61 ± 0.83 B;4 | 9.49 ± 0.37 A;1 | 26.09 ± 2.04 B;3 |
| B2 | 28.83 ± 0.76 B;2 | 23.99 ± 0.52 A;4 | 13.42 ± 0.58 B;4 | 26.67 ± 0.78 A;2,3 | 26.33 ± 0.64 B;3 | 10.68 ± 0.77 A;2 | 28.44 ± 1.27 B;2 | 26.81 ± 1.54 B;2 | 10.88 ± 1.75 A;2 |
| C2 | 25.99 ± 0.98 A;1 | 22.22 ± 1.21 A;3 | 10.73 ± 1.13 A;2 | 25.22 ± 1.82 A;2 | 24.80 ± 1.66 B;2 | 10.21 ± 1.59 A;2 | 26.58 ± 1.26 A;1,2 | 25.01 ± 2.45 C;2 | 10.14 ± 1.54 A;2 |
| D2 | 25.21 ± 0.51 B;1 | 21.32 ± 1.62 A;3 | 9.05 ± 0.98 A;1 | 24.55 ± 0.83 A;1 | 24.76 ± 0.95 B;2 | 9.49 ± 1.25 AB;1,2 | 25.88 ± 0.58 B;1 | 25.75 ± 0.87 B;2 | 10.69 ± 1.22 B;2 |
| Start of Storage | 3 Months | |
|---|---|---|
| aw | aw | |
| A1 | 0.429 ± 0.006 | 0.355 ± 0.000 |
| A2 | 0.327 ± 0.000 | 0.356 ± 0.001 |
| B1 | 0.559 ± 0.001 | 0.349 ± 0.001 |
| B2 | 0.387 ± 0.002 | 0.350 ± 0.000 |
| C1 | 0.501 ± 0.004 | 0.347 ± 0.003 |
| C2 | 0.415 ± 0.005 | 0.344 ± 0.001 |
| D1 | 0.450 ± 0.002 | 0.335 ± 0.004 |
| D2 | 0.379 ± 0.001 | 0.341 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitrevski, J.; Pantelić, N.Đ.; Dodevska, M.S.; Kojić, J.S.; Vulić, J.J.; Zlatanović, S.; Gorjanović, S.; Laličić-Petronijević, J.; Marjanović, S.; Antić, V.V. Effect of Beetroot Powder Incorporation on Functional Properties and Shelf Life of Biscuits. Foods 2023, 12, 322. https://doi.org/10.3390/foods12020322
Mitrevski J, Pantelić NĐ, Dodevska MS, Kojić JS, Vulić JJ, Zlatanović S, Gorjanović S, Laličić-Petronijević J, Marjanović S, Antić VV. Effect of Beetroot Powder Incorporation on Functional Properties and Shelf Life of Biscuits. Foods. 2023; 12(2):322. https://doi.org/10.3390/foods12020322
Chicago/Turabian StyleMitrevski, Jasmina, Nebojša Đ. Pantelić, Margarita S. Dodevska, Jovana S. Kojić, Jelena J. Vulić, Snežana Zlatanović, Stanislava Gorjanović, Jovanka Laličić-Petronijević, Sonja Marjanović, and Vesna V. Antić. 2023. "Effect of Beetroot Powder Incorporation on Functional Properties and Shelf Life of Biscuits" Foods 12, no. 2: 322. https://doi.org/10.3390/foods12020322
APA StyleMitrevski, J., Pantelić, N. Đ., Dodevska, M. S., Kojić, J. S., Vulić, J. J., Zlatanović, S., Gorjanović, S., Laličić-Petronijević, J., Marjanović, S., & Antić, V. V. (2023). Effect of Beetroot Powder Incorporation on Functional Properties and Shelf Life of Biscuits. Foods, 12(2), 322. https://doi.org/10.3390/foods12020322









