Effects of Amylopectins from Five Different Sources on Disulfide Bond Formation in Alkali-Soluble Glutenin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation and Isolation of the Different Amylopectins
2.2.2. The Purification of the Different Amylopectins
2.2.3. Preparation of the ASG
2.2.4. Mixture of the Different Amylopectins and ASG
2.2.5. Retrogradation of the Different Amylopectin–ASG Mixtures
2.2.6. Determination of the Disulfide Bonds
2.2.7. Molecular Weight Distribution Profiles
2.2.8. Chain Length Distribution Profiles
2.2.9. FTIR Spectroscopy
2.2.10. 13C Solid-State NMR Spectroscopy
2.2.11. X-ray Powder Diffraction (XRD) Analysis
2.2.12. Statistical Analysis
3. Results and Discussion
3.1. The Effects of the ASG+Amylopectins on Disulfide Bond Formation
3.2. The Average Molecular Weight (Mw) and Chain Length Distribution
3.3. Fourier Transform Infrared Spectroscopy Analysis
3.4. 13C Solid-State NMR Spectra of Different Amylopectins Mixed with and without ASG
3.5. X-ray Diffraction of the Different Amylopectins Mixed with ASG
3.6. The Possible Mechanism of Disulfide Bond Formation When ASG Is Mixed and Co-Retrograded with Different Amylopectins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandner, S.; Becker, T.; Jekle, M. Classification of starch-gluten networks into a viscoelastic liquid or solid, based on rheological aspects—A review. Int. J. Biol. Macromol. 2019, 136, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, Y.; Jia, F.; Chen, D.; Zhang, X.; Wang, Q.; Wang, J. Effect of extrusion temperature on the protein aggregation of wheat gluten with the addition of peanut oil during extrusion. Int. J. Biol. Macromol. 2021, 166, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, S.; Sun, B.; Wang, F.; Huang, J.; Wang, X.; Bao, Q. Effects of thermal properties and behavior of wheat starch and gluten on their interaction: A review. Int. J. Biol. Macromol. 2021, 177, 474–484. [Google Scholar] [CrossRef]
- Lindsay, M.P.; Skerritt, J.H. The glutenin macropolymer of wheat flour doughs: Structure–function perspectives. Trends Food Sci. Tech. 1999, 10, 247–253. [Google Scholar] [CrossRef]
- Carceller, J.L.; Aussenac, T. Size characterisation of glutenin polymers by HPSEC-MALLS. J. Cereal Sci. 2001, 33, 131–142. [Google Scholar] [CrossRef]
- Ma, F.; Dang, Y.; Xu, S. Interaction between gluten proteins and their mixtures with water-extractable arabinoxylan of wheat by rheological, molecular anisotropy and CP/MAS 13C NMR measurements. Eur. Food Res. Technol. 2016, 242, 1177–1185. [Google Scholar] [CrossRef]
- Gianibelli, M.C.; Larroque, O.R.; MacRitchie, F.; Wrigley, C.W. Biochemical, genetic, and molecular characterization of wheat endosperm proteins. Cereal Chem. 2001, 78, 635–646. [Google Scholar] [CrossRef]
- Payne, P.I. Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Ann. Rev. Plant Physiol. 1987, 38, 141–153. [Google Scholar] [CrossRef]
- Wellner, N.; Mills, E.C.; Brownsey, G.; Wilson, R.H.; Brown, N.; Freeman, J.; Halford, N.G.; Shewry, P.R.; Belton, P.S. Changes in protein secondary structure during gluten deformation studied by dynamic Fourier transform infrared spectroscopy. Biomacromolecules 2005, 6, 255–261. [Google Scholar] [CrossRef]
- Liu, J.; Luo, D.; Li, X.; Xu, B.; Zhang, X.; Liu, J. Effects of inulin on the structure and emulsifying properties of protein components in dough. Food Chem. 2016, 210, 235–241. [Google Scholar] [CrossRef]
- Zhang, D.; Mu, T.; Sun, H. Effects of starch from five different botanical sources on the rheological and structural properties of starch–gluten model doughs. Food Res. Int. 2018, 103, 156–162. [Google Scholar] [CrossRef]
- Li, J.; Yadav, M.P.; Li, J. Effect of different hydrocolloids on gluten proteins, starch and dough microstructure. J. Cereal Sci. 2019, 87, 85–90. [Google Scholar] [CrossRef]
- Jane, J.L. Mechanism of starch gelatinization in neutral salt solutions. Starch-Stärke 1993, 45, 161–166. [Google Scholar] [CrossRef]
- Green, M.M.; Blankenhorn, G.; Hart, H. Which starch fraction is water-soluble, amylose or amylopectin? J. Chem. Educ. 1975, 52, 729. [Google Scholar] [CrossRef]
- Lian, X.; Sun, H.; Li, L.; Wu, H.; Zhang, N. Characterizing the chemical features of lipid and protein in sweet potato and maize starches. Starch-Stärke 2014, 66, 361–368. [Google Scholar]
- Yazar, G.; Duvarci, O.C.; Tavman, S.; Kokini, J.L. LAOS behavior of the two main gluten fractions: Gliadin and glutenin. J. Cereal Sci. 2017, 77, 201–210. [Google Scholar] [CrossRef]
- He, Z.; Cheng, H.N.; Nam, S. Comparison of the wood bonding performance of water-and alkali-soluble cottonseed protein fractions. J. Adhes. Sci. Technol. 2021, 35, 1500–1517. [Google Scholar] [CrossRef]
- Zhu, J.; Li, L.; Zhao, L.; Song, L.; Li, X. Effects of freeze–thaw cycles on the structural and thermal properties of wheat gluten with variations in the high molecular weight glutenin subunit at the Glu-B1 locus. J. Cereal Sci. 2019, 87, 266–272. [Google Scholar] [CrossRef]
- Ruan, Q.; Chen, Y.; Kong, X.; Hua, Y. Comparative studies on sulfhydryl determination of soy protein using two aromatic disulfide reagents and two fluorescent reagents. J. Agr. Food Chem. 2013, 61, 2661–2668. [Google Scholar] [CrossRef]
- Wei, B.; Cai, C.; Jin, Z.; Tian, Y. High-pressure homogenization induced degradation of amylopectin in a gelatinized state. Starch-Stärke 2016, 68, 734–741. [Google Scholar] [CrossRef]
- Chen, C.; Lu, K.; Hu, X.; Liu, Y.; Cui, S.W.; Miao, M. Biofabrication, structure and characterization of an amylopectin-based cyclic glucan. Food Funct. 2020, 11, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhang, L.; Liu, Q.; Liu, W.; Hu, H. Rheological behaviors and physicochemical changes of doughs reconstituted from potato starch with different sizes and gluten. Food Res. Int. 2021, 145, 110397. [Google Scholar] [CrossRef] [PubMed]
- Köhler, P.; Belitz, H.D.; Wieser, H. Disulphide bonds in wheat gluten: Further cystine peptides from high molecular weight (HMW) and low molecular weight (LMW) subunits of glutenin and from γ-gliadins. Z. Für Lebensm. Unters. Und Forsch. 1993, 196, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of soy protein to wheat gluten ratio on the physicochemical properties of extruded meat analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- Wang, P.; Zou, M.; Tian, M.; Gu, Z.; Yang, R. The impact of heating on the unfolding and polymerization process of frozen-stored gluten. Food Hydrocolloid. 2018, 85, 195–203. [Google Scholar] [CrossRef]
- You, S.; Fiedorowicz, M.; Lim, S.T. Molecular characterization of wheat amylopectins by multiangle laser light scattering analysis. Cereal Chem. 1999, 76, 116–121. [Google Scholar] [CrossRef]
- Mua, J.P.; Jackson, D.S. Fine structure of corn amylose and amylopectin fractions with various molecular weights. J. Agr. Food Chem. 1997, 45, 3840–3847. [Google Scholar] [CrossRef]
- Mufumbo, R.; Baguma, Y.; Kashub, S.; Nuwamanya, E.; Rubaihayo, P.; Mukasa, S.; Hamaker, B.; Kyamanywa, S. Amylopectin molecular structure and functional properties of starch from three Ugandan cassava varieties. J. Plant Breed. Crop Sci. 2011, 3, 195–202. [Google Scholar]
- Wang, H.; Yang, Q.; Ferdinand, U.; Gong, X.; Qu, Y.; Gao, W.; Ivanistau, A.; Feng, B.; Liu, M. Isolation and characterization of starch from light yellow, orange, and purple sweet potatoes. Int. J. Biol. Macromol. 2020, 160, 660–668. [Google Scholar] [CrossRef]
- Lu, H.; Xiong, L.; Li, M.; Chen, H.; Xiao, J.; Wang, S.; Qiu, L.; Bian, X.; Sun, C.; Sun, Q. Separation and characterization of linear glucans debranched from normal corn, potato and sweet potato starches. Food Hydrocolloid. 2019, 89, 196–206. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Perera, C.O.; Waterhouse, G.I.N. Application of FT-IR and Raman spectroscopy for the study of biopolymers in breads fortified with fibre and polyphenols. Food Res. Int. 2013, 50, 574–585. [Google Scholar] [CrossRef]
- Ogawa, K.; Yamazaki, I.; Yoshimura, T.; Ono, S.; Rengakuji, S.; Nakamura, Y.; Shimasaki, C. Studies on the retrogradation and structural properties of waxy corn starch. B. Chem. Soc. Jpn. 1998, 71, 1095–1100. [Google Scholar] [CrossRef]
- Liu, M.; Wu, P.; Ding, Y.; Chen, G.; Li, S. Two-dimensional (2D) ATR− FTIR spectroscopic study on water diffusion in cured epoxy resins. Macromolecules 2002, 35, 5500–5507. [Google Scholar] [CrossRef]
- Jian, X.; Hu, Y.; Zhou, W.; Xiao, L. Self-healing polyurethane based on disulfide bond and hydrogen bond. Polym. Advan. Technol. 2018, 29, 463–469. [Google Scholar] [CrossRef]
- Guo, J.; Lian, X.; Kang, H.; Gao, K.; Li, L. Effects of glutenin in wheat gluten on retrogradation of wheat starch. Eur. Food Res. Technol. 2016, 242, 1485–1494. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, F.L.; Lin, W.J.; Li, Z.F.; Yang, J.Y.; Park, K.H.; Ni, L.; Liu, P. Gluten-starch interactions in wheat gluten during carboxylic acid deamidation upon hydrothermal treatment. Food Chem. 2019, 283, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Huang, P.; Caughey, W.S. Protein secondary structures in water from second-derivative amide I infrared spectra. Biochemistry 1990, 29, 3303–3308. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, D.; Foster, T.J.; Liu, Y.; Wang, Y.; Nirasawa, S.; Tatsumi, E.; Cheng, Y. Konjac glucomannan-induced changes in thiol/disulphide exchange and gluten conformation upon dough mixing. Food Chem. 2014, 143, 163–169. [Google Scholar] [CrossRef]
- Czekus, B.; Pećinar, I.; Petrović, I.; Paunović, N.; Savić, S.; Jovanović, Z.; Stikić, R. Raman and Fourier transform infrared spectroscopy application to the Puno and Titicaca cvs. of quinoa seed microstructure and perisperm characterization. J. Cereal Sci. 2019, 87, 25–30. [Google Scholar] [CrossRef]
- Calucci, L.; Forte, C.; Galleschi, L.; Geppi, M.; Ghiringhelli, S. 13C and 1H solid state NMR investigation of hydration effects on gluten dynamics. Int. J. Biol. Macromol. 2003, 32, 179–189. [Google Scholar] [CrossRef]
- Belton, P.S.; Shewry, P.R.; Tatham, A.S. 13C Solid state nuclear magnetic resonance study of wheat gluten. J. Cereal Sci. 1985, 3, 305–317. [Google Scholar] [CrossRef]
- Alberti, E.; Gilbert, S.M.; Tatham, A.S.; Shewry, P.R.; Gil, A.M. Study of high molecular weight wheat glutenin subunit 1Dx5 by 13C and 1H solid-state NMR spectroscopy. I. Role of covalent crosslinking. Biopolymers. 2002, 67, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Belton, P.S.; Colquhoun, I.J.; Grant, A.; Wellner, N.; Field, J.M.; Shewry, P.R.; Tatham, A.S. FTIR and NMR studies on the hydration of a high-Mr subunit of glutenin. Int. J. Biol. Macromol. 1995, 17, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Belton, P.S.; Duce, S.L.; Tatham, A.S. 13C solution state and solid state nmr of wheat gluten. Int. J. Biol. Macromol. 1987, 9, 357–362. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Müller, D. Secondary structure of peptides: 15. 13C nmr CP/MAS study of solid elastin and proline-containing copolyesters. Int. J. Biol. Macromol. 1984, 6, 145–151. [Google Scholar] [CrossRef]
- Hizukuri, S. Relationship between the distribution of the chain length of amylopectin and the crystalline structure of starch granules. Carbohyd. Res. 1985, 141, 295–306. [Google Scholar] [CrossRef]
- Li, Y.; Kijac, A.Z.; Sligar, S.G.; Rienstra, C.M. Structural analysis of nanoscale self-assembled discoidal lipid bilayers by solid-state NMR spectroscopy. Biophys. J. 2006, 91, 3819–3828. [Google Scholar] [CrossRef]
- Postal, W.S.; Vogel, E.J.; Young, C.M.; Greenaway, F.T. The binding of copper (II) and zinc (II) to oxidized glutathione. J. Inorg. Biochem. 1985, 25, 25–33. [Google Scholar] [CrossRef]
- Tilley, K.A.; Benjamin, R.E.; Bagorogoza, K.E.; Okot-Kotber, B.M.; Prakash, O.; Kwen, H. Tyrosine cross-links: Molecular basis of gluten structure and function. J. Agr. Food Chem. 2001, 49, 2627–2632. [Google Scholar] [CrossRef]
- He, W.; Wei, C. Progress in C-type starches from different plant sources. Food Hydrocolloid. 2017, 73, 162–175. [Google Scholar] [CrossRef]
- Wang, D.; He, Z.; Yang, L.; Wang, H.; Lian, X.; Zhu, W. Retrogradation of sweet potato amylose and amylopectin with narrow molecular weight distribution. Int. J. Food Sci. Tech. 2022, 57, 1954–1964. [Google Scholar] [CrossRef]
- Srichuwong, S.; Sunarti, T.C.; Mishima, T.; Isono, N.; Hisamatsu, M. Starches from different botanical sources I: Contribution of amylopectin fine structure to thermal properties and enzyme digestibility. Carbohyd. Polym. 2005, 60, 529–538. [Google Scholar] [CrossRef]
- Jane, J. Structural features of starch granules II. In Starch, Chemistry and Technology, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Academic Press: New York, NY, USA, 2009; pp. 193–236. [Google Scholar]
- Peng, P.; Wang, X.; Zou, X.; Zhang, X.; Hu, X. Dynamic behaviors of protein and starch and interactions associated with glutenin composition in wheat dough matrices during sequential thermo-mechanical treatments. Food Res. Int. 2022, 154, 110986. [Google Scholar] [CrossRef] [PubMed]
- Darby, N.J.; Freedman, R.B.; Creighton, T.E. Dissecting the mechanism of protein disulfide isomerase: Catalysis of disulfide bond formation in a model peptide. Biochemistry 1994, 33, 7937–7947. [Google Scholar] [CrossRef]
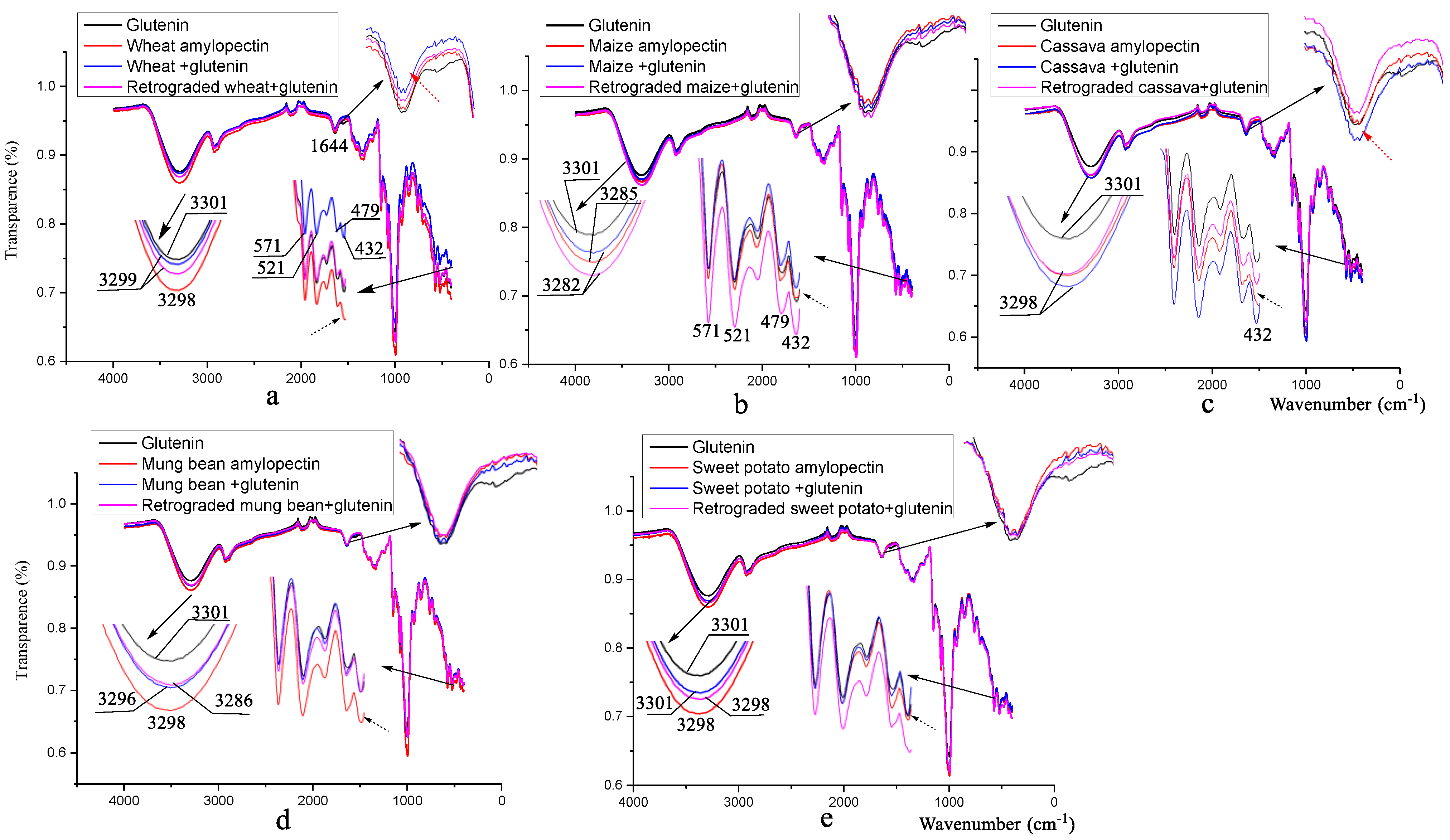

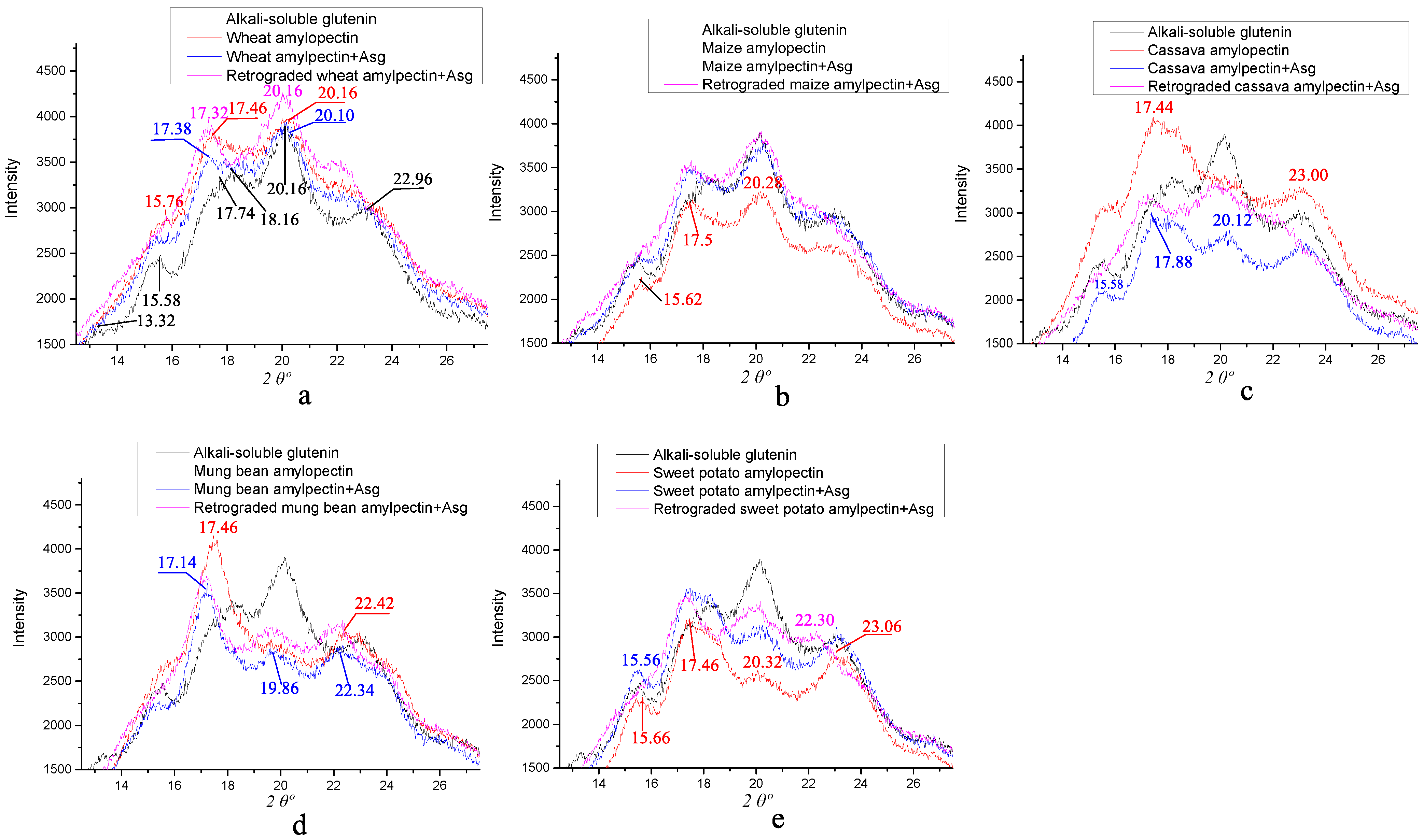
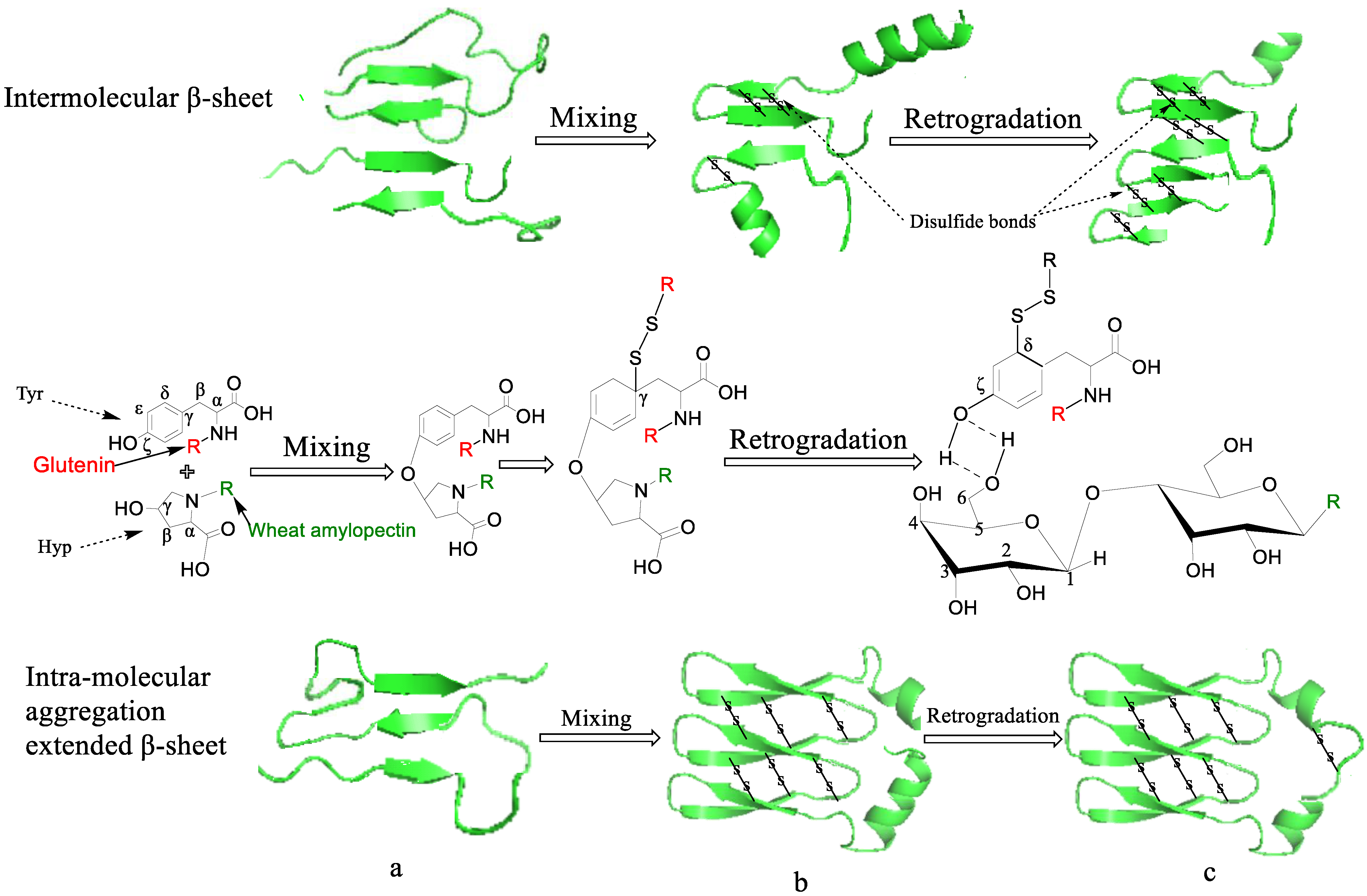
| Samples | Control | Wheat | Maize | Cassava | Mung Bean | Sweet Potato |
|---|---|---|---|---|---|---|
| Before retrogradation | 0.04 ± 0.00 a | 0.31 ± 0.02 b,** | 0.24 ± 0.02 c,** | 0.08 ± 0.00 d,* | 0.18 ± 0.03 e | 0.29 ± 0.11 b |
| After retrogradation | 0.03 ± 0.00 a | 0.55 ± 0.03 b,** | 0.16 ± 0.03 c,* | 0.26 ± 0.05 d | 0.07 ± 0.01 e | 0.19 ± 0.04 f |
| Molecular Characteristics | Amylopectins | ||||
|---|---|---|---|---|---|
| Wheat | Maize | Cassava | Mung Bean | Sweet Potato | |
| Retention time (min) | 12.84 (51%) 16.52 (40%) 19.45 (9%) | 13.34 (47%) 17.05 (27%) 19.45 (26%) | 12.75 (39%) 16.50 (35%) 19.50 (26%) | 16.90 (81%) 19.50 (19%) | 12.96 (82%) 19.64 (18%) |
| Mn (g/mol) | 1387006 18672 828 | 326426 12381 942 | 1501812 16784 682 | 19894 611 | 62126 713 |
| Mw (g/mol) | 3503982 63486 1041 | 1211463 22550 1314 | 3881679 61698 910 | 695605 854 | 2044753 1112 |
| Mp (g/mol) | 3509755 38910 1085 | 1337876 21017 1411 | 3938537 40091 1055 | 24461 1014 | 3047294 853 |
| Polydispersity | 2.53 3.40 1.26 | 3.71 1.82 1.39 | 2.58 3.68 1.33 | 34.97 1.40 | 32.91 1.56 |
| Chain Length (Glucose Number) | Amylopectins | ||||
|---|---|---|---|---|---|
| Wheat | Maize | Cassava | Mung Bean | Sweet Potato | |
| 1 | 3.6 | 10.89 | 3.55 | 5.57 | 4.41 |
| 2 | 20.04 | 9.48 | 18.66 | 30.56 | 18.07 |
| 3 | 6.96 | 14.07 | 11.78 | 5.56 | 12.93 |
| 4 | 2.08 | 16.68 | 15.01 | 4.53 | 3.13 |
| 5 | 11.09 | 14.06 | 10.47 | 7.33 | 14.78 |
| 6 | 8.34 | 15.13 | 15.89 | 4.84 | 12.64 |
| 7 | 10.11 | 9.96 | 11.68 | 4.68 | 14.92 |
| 8 | 8.15 | 5.11 | 6.75 | 4.28 | 10.32 |
| 9 | 7.19 | 2.47 | 2.93 | 4.07 | 5.16 |
| 10 | 5.57 | 1.3 | 1.57 | 3.77 | 2.01 |
| 11 | 4.35 | 0.51 | 0.85 | 3.54 | 1.06 |
| 12 | 3.33 | 0.25 | 0.42 | 3.19 | 0.41 |
| 13 | 2.73 | 0.11 | 0.28 | 2.86 | 0.17 |
| 14 | 1.91 | 0.16 | 2.65 | ||
| 15 | 1.39 | 2.17 | |||
| 16 | 1.03 | 1.91 | |||
| 17 | 0.74 | 1.63 | |||
| 18 | 0.55 | 1.39 | |||
| 19 | 0.39 | 1.17 | |||
| 20 | 0.28 | 0.98 | |||
| 21 | 0.18 | 0.82 | |||
| 22 | 0.69 | ||||
| 23 | 0.56 | ||||
| 24 | 0.45 | ||||
| 25 | 0.35 | ||||
| 26 | 0.26 | ||||
| 27 | 0.19 | ||||
| Samples | α-Helix Content (%) | Intermolecular β-Sheet Content (%) | Intra-Molecular Aggregation Extended β-Sheet Content (%) | β-Turn Content (%) | Random Coils Content (%) |
|---|---|---|---|---|---|
| ASG (Asg) | 0.00 | 50.62 | 47.14 | 0.00 | 2.24 |
| Wheat amylopectin + Asg | 1.17 | 36.71 | 59.82 | 2.31 | 0.00 |
| Retrograded wheat amylopectin + Asg | 0.15 | 37.48 | 59.39 | 2.97 | 0.00 |
| Maize amylopectin + Asg | 0.74 | 42.87 | 52.02 | 4.38 | 0.00 |
| Retrograded maize amylopectin + Asg | 0.93 | 36.89 | 59.53 | 2.65 | 0.00 |
| Cassava amylopectin + Asg | 0.00 | 38.73 | 59.46 | 1.81 | 0.00 |
| Retrograded cassava amylopectin + Asg | 0.00 | 37.90 | 60.25 | 1.86 | 0.00 |
| Mung bean amylopectin + Asg | 0.53 | 36.91 | 60.47 | 2.09 | 0.00 |
| Retrograded mung bean amylopectin + Asg | 0.76 | 43.50 | 48.32 | 2.80 | 4.62 |
| Sweet potato amylopectin + Asg | 0.00 | 40.70 | 55.71 | 3.59 | 0.00 |
| Retrograded sweet potato amylopectin + Asg | 0.11 | 38.46 | 58.77 | 2.66 | 0.00 |
| Samples | Chemical Shift and Assignments (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| Carbonyl Groups | Protein Aromatic Moieties | C1 of Oligosaccharide or Starch | C4 of Oligosaccharide or Starch | C2, 3, 5 of Oligosaccharide or Starch | C6 of Oligosaccharide or Starch | Alkyl Groups in Protein Side Chains | |
| ASG | 174.1, 172.2 | 132.6, 131.4, 128.5 | 103.4, 95.1 | 82.1 | 73.0 | 62.7 | 31.9 |
| Wheat amylopectin | 173.8, 171.5 | nd | 103.4, 94.8 | 82.0 | 73.2 | 62.5 | 32.6, 31.5 |
| Wheat + glutenin | 174.6, 171.3 | 132.5, 128.3 | 103.4, 95.1 | 82.2 | 73.1 | 62.6 | 32.0 |
| Wheat + glutenin + retrogradation | 173.2, 171.3 | 131.2, 128.9 | 103.3, 95.1 | 82.5 | 73.1 | 62.4 | 32.3 |
| Maize amylopectin | 173.3, 171.3 | nd | 103.3, 95.0 | 82.3 | 73.0 | 62.7 | 32.2 |
| Maize +glutenin | 173.3, 171.2 | 131.7, 129.0 | 103.2, 94.9 | 82.4 | 73.1 | 62.5 | 31.9 |
| Maize + glutenin + retrogradation | 173.6, 171.9 | 130.8, 129.6 | 103.4, 95.0 | 82.7 | 73.1 | 62.6 | 32.1 |
| Cassava amylopectin | Ignorable | nd | 103.2, 95.1 | 82.7 | 73.1 | 62.6 | nd |
| Cassava +glutenin | 173.0, 171.0 | nd | 103.3, 94.8 | 82.4 | 73.0 | 62.7 | 32.1 |
| Cassava + glutenin + retrogradation | 173.8, 172.2 | 131.7 | 103.4 | 82.0 | 73.2 | 62.4 | 31.8 |
| Mung bean amylopectin | 174.3, 171.6 | nd | 101.3, 95.3 | 82.0 | 73.1 | 62.6 | nd |
| Mung bean + glutenin | 175.3, 173.9, 171.3 | nd | 100.9, 95.0 | 82.3 | 73.0 | 62.6 | nd |
| Mung bean + glutenin + retrogradation | 171.8 | nd | 101.9, 94.9 | 82.3 | 73.1 | 62.4 | nd |
| Sweet potato amylopectin | 172.7 | nd | 102.0 | 82.3 | 73.0 | 62.7 | nd |
| Sweet potato + glutenin | 175.4, 173.4 172.3 | nd | 103.4, 94.9 | 82.4 | 73.0 | 62.7 | nd |
| Sweet potato + glutenin + retrogradation | Ignorable | nd | 103.3 | 82.1 | 73.2 | 62.5 | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhao, J.; Guo, J.; Lian, X.; Wang, H. Effects of Amylopectins from Five Different Sources on Disulfide Bond Formation in Alkali-Soluble Glutenin. Foods 2023, 12, 414. https://doi.org/10.3390/foods12020414
Zhou Y, Zhao J, Guo J, Lian X, Wang H. Effects of Amylopectins from Five Different Sources on Disulfide Bond Formation in Alkali-Soluble Glutenin. Foods. 2023; 12(2):414. https://doi.org/10.3390/foods12020414
Chicago/Turabian StyleZhou, Yu, Jinjin Zhao, Junjie Guo, Xijun Lian, and Huaiwen Wang. 2023. "Effects of Amylopectins from Five Different Sources on Disulfide Bond Formation in Alkali-Soluble Glutenin" Foods 12, no. 2: 414. https://doi.org/10.3390/foods12020414
APA StyleZhou, Y., Zhao, J., Guo, J., Lian, X., & Wang, H. (2023). Effects of Amylopectins from Five Different Sources on Disulfide Bond Formation in Alkali-Soluble Glutenin. Foods, 12(2), 414. https://doi.org/10.3390/foods12020414






