Bay Leaves Extracts as Active Additive for Food Protective Coatings
Abstract
:1. Introduction
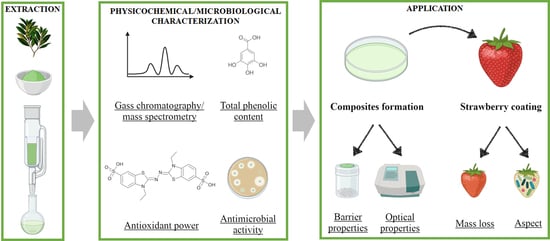
2. Materials and Methods
2.1. Materials
2.2. Bay Leaves Extraction Process
2.3. Bay Leaves Extract Characterization
2.4. Edible Films Preparation and Characterization
2.5. Fruit Protection Tests
2.6. Statistical Analysis
3. Results and Discussion
3.1. BLE Extraction and Characterization
3.2. Properties of BLE Containing Edible Films
3.3. Protection/Coating of Fresh Fruit
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseini, H.; Jafari, S.M. Introducing nano/microencapsulated bioactive ingredients for extending the shelf-life of food products. Adv. Colloid Interfac. 2020, 282, 102210. [Google Scholar] [CrossRef] [PubMed]
- Trinh, B.M.; Smith, M.; Mekonnen, T.H. A nanomaterial-stabilized starch-beeswax Pickering emulsion coating to extend produce shelf-life. Chem. Eng. J. 2022, 431, 133905. [Google Scholar] [CrossRef]
- Pott, D.M.; de Abreu e Lima, F.; Soria, C.; Willmitzer, L.; Fernie, A.R.; Nikoloski, Z.; Osorio, S.; Vallarino, J.G. Metabolic reconfiguration of strawberry physiology in response to postharvest practices. Food Chem. 2020, 321, 126747. [Google Scholar] [CrossRef]
- Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Sánchez, A.; Santana, R.V.; Mariniello, L. Effect of Mesoporous Silica Nanoparticles on The Physicochemical Properties of Pectin Packaging Material for Strawberry Wrapping. Nanomaterials 2020, 10, 52. [Google Scholar] [CrossRef]
- Salehi, F. Edible Coating of Fruits and Vegetables Using Natural Gums: A Review. Int. J. Fruit Sci. 2020, 20, S570–S589. [Google Scholar] [CrossRef]
- Bamdad, F.; Goli, A.H.; Kadivar, M. Preparation and Characterization of Proteinous Film from Lentil (Lens culinaris): Edible Film from Lentil (Lens culinaris). Food Res. Int. 2006, 39, 106–111. [Google Scholar] [CrossRef]
- Youseftabar-Miri, N.; Sedaghat, N.; Khoshnoudi-Nia, S. Effect of Active Edible Coating on Quality Properties of Green-Raisin and Ranking the Samples Using Fuzzy Approach. J. Food Meas. Charact. 2021, 15, 46–58. [Google Scholar] [CrossRef]
- Yousuf, B.; Wu, S.; Siddiqui, M.W. Incorporating essential oils or compounds derived thereof into edible coatings: Effect on quality and shelf life of fresh/fresh-cut produce. Trends Food Sci. Tech. 2021, 108, 245–257. [Google Scholar] [CrossRef]
- Chiralt, A.; Menzel, C.; Hernandez-García, E.; Collazo, S.; González-Martínez, C. Use of By-Products in Edible Coatings and Biodegradable Packaging Materials for Food Preservation. In Sustainability of the Food System: Sovereignty, Waste, and Nutrients Bioavailability; Betoret, N., Betoret, E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 101–127. [Google Scholar] [CrossRef]
- Gao, X.; Lv, S.; Wu, Y.; Li, J.; Zhang, W.; Meng, W.; Wang, C.; Meng, Q. Volatile Components of Essential Oils Extracted from Pu-Erh Ripe Tea by Different Extraction Methods. Int. J. Food Prop. 2017, 20, S240–S253. [Google Scholar] [CrossRef]
- Batool, S.; Khera, R.A.; Hanif, M.A.; Ayub, M.A. Bay Leaf. In Medicinal Plants of South Asia; Hanif, M.A., Khan, M.M., Nawaz, H., Byrne, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 63–74. [Google Scholar] [CrossRef]
- Rincón, E.; Zuliani, A.; Jiménez-Quero, A.; Vilaplana, F.; Luque, R.; Serrano, L.; Balu, A.M. Combined Extraction/Purification-Catalytic Microwave-Assisted Conversion of Laurus nobilis L. Pruning Waste Polysaccharides into Methyl Levulinate. ACS Sustain. Chem. Eng. 2020, 8, 11016–11023. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, N.; Upadhyay, A.; Sethi, S.; Singh, A. Edible Coating as Postharvest Management Strategy for Shelf-Life Extension of Fresh Tomato (Solanum lycopersicum L.): An Overview. J. Food Sci. 2022, 87, 2256–2290. [Google Scholar] [CrossRef] [PubMed]
- Rincón, E.; Serrano, L.; Balu, A.M.; Aguilar, J.J.; Luque, R.; García, A. Effect of bay leaves essential oil concentration on the properties of biodegradable carboxymethyl cellulose-based edible films. Materials 2019, 12, 2356. [Google Scholar] [CrossRef]
- García, A.; Spigno, G.; Labidi, J. Antioxidant and biocide behaviour of lignin fractions from apple tree pruning residues. Ind. Crops Prod. 2017, 104, 242–252. [Google Scholar] [CrossRef]
- Koudehi, M.F.; Ardalan, A.A.; Zibaseresht, R. Chemical constituents of an Iranian grown Capsicum annuum and their cytotoxic activities evaluation. Org. Med. Chem. Int. J. 2020, 9, 148–154. [Google Scholar] [CrossRef]
- Diba, K.; Shoar, M.G.; Shabatkhori, M.; Khorshivand, Z. Antifungal activity of alcoholic extract of Peganum harmala seeds. J. Med. Plant Res. 2011, 5, 5550–5554. [Google Scholar]
- Șachir, E.E.; Pușcașu, C.G.; Caraiane, A.; Raftu, G.; Badea, F.C.; Mociu, M.; Albu, C.M.; Sachelarie, L.; Liliana Hurjui, L.; Bartok-Nicolae, C. Studies Regarding the Antibacterial Effect of Plant Extracts Obtained from Epilobium parviflorum Schreb. App. Sci. 2022, 12, 2751. [Google Scholar] [CrossRef]
- Chau, T.P.; Veeraragavan, G.R.; Narayanan, M.; Chinnathambi, A.; Alharbi, S.A.; Subramani, B.; Brindhadevi, K.; Pimpimon, T.; Pikulkaew, S. Green synthesis of Zirconium nanoparticles using Punica granatum (pomegranate) peel extract and their antimicrobial and antioxidant potency. Environ. Res. 2022, 209, 112771. [Google Scholar] [CrossRef]
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and Antimicrobial Activity of the Essential Oils of Two Origanum Species. J. Agric. Food. Chem. 2001, 49, 4168–4170. [Google Scholar] [CrossRef]
- Jannatyha, N.; Shojaee-Aliabadi, S.; Moslehishad, M.; Moradi, E. Comparing mechanical, barrier and antimicrobial properties of nanocellulose/CMC and nanochitosan/CMC composite films. Int. J. Biol. Macromol. 2020, 164, 2323–2328. [Google Scholar] [CrossRef]
- Vecchi, C.F.; Cesar, G.B.; Souza, P.R.; de Caetano, W.; Bruschi, M.L. Mucoadhesive polymeric films comprising polyvinyl alcohol, polyvinylpyrrolidone, and poloxamer 407 for pharmaceutical applications. Pharm. Dev. Technol. 2020, 26, 138–149. [Google Scholar] [CrossRef]
- Mehalaine, S.; Chenchouni, H. Quantifying how climatic factors influence essential oil yield in wild-growing plants. Arab. J. Geosci. 2021, 14, 1257. [Google Scholar] [CrossRef]
- Muñiz-Márquez, D.B.; Wong-Paz, J.E.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Aguilar, C.N. Bioactive compounds from bay leaves (Laurus nobilis) extracted by microwave technology. Z. Naturforschung C J. Biosci. 2018, 73, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of essential oil and its biological activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S. Food spoilage: Microorganisms and their prevention. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Kawsar, S.M.; Kabir, A.K.; Bhuiyan, M.M.; Manik, M.M. Synthesis and Characterization of Methyl 4, 6-O-Enzylidene-α-D-Glucopyranoside Derivatives. J. Bangladesh Chem. Soc. 2012, 25, 101–109. [Google Scholar] [CrossRef]
- Rozman, V.; Kalinovic, I.; Korunic, Z. Toxicity of naturally occurring compounds of Lamiaceae and Lauraceae to three stored-product insects. J. Stored Prod. Res. 2007, 43, 349–355. [Google Scholar] [CrossRef]
- Liu, T.T.; Yang, T.S. Antimicrobial impact of the components of essential oil of Litsea cubeba from Taiwan and antimicrobial activity of the oil in food systems. Int. J. Food Microbiol. 2012, 156, 68–75. [Google Scholar] [CrossRef]
- Oriola, A.O.; Oyedeji, A.O. Essential Oils and Their Compounds as Potential Anti-Influenza Agents. Molecules 2022, 27, 7797. [Google Scholar] [CrossRef]
- Rossi, P.G.; Bao, L.; Luciani, A.; Panighi, J.; Desjobert, J.M.; Costa, J.; Casanova, J.; Bolla, J.M.; Berti, L. (E)-methylisoeugenol and elemicin: Antibacterial components of Daucus carota L. essential oil against Campylobacter jejuni. J. Agric. Food Chem. 2007, 55, 7332–7336. [Google Scholar] [CrossRef]
- Rajani, P.; Rajasekaran, C.; Vasanthakumari, M.M.; Olsson, S.B.; Ravikanth, G.; Uma Shaanker, R. Inhibition of plant pathogenic fungi by endophytic Trichoderma spp. through mycoparasitism and volatile organic compounds. Microbiol. Res. 2021, 242, 126595. [Google Scholar] [CrossRef]
- Valadbeigi, T.; Rashki, S. GC-MS analysis and anticancer effect against MCF-7 and HT-29 cell lines and antioxidant, antimicrobial and wound healing activities of plant-derived. J. Basic Res. Med. Sci. 2015, 2, 1–11. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Nasr, S.B.; El Beyrouthy, M.; Mnif, W. Phytochemical composition and antioxidant activity of Tunisian Laurus nobilis. Pak. J. Pharm. Sci. 2018, 31, 2397–2402. [Google Scholar] [PubMed]
- Jeyakani, M.; Rajalakshmi, M. Antioxidant activity, total phenolic content of essential oils, and extract determined from natural leaves, in-vitro leaves, and callus sources of Melaleuca alternifolia-a comparative study. Asian J. Pharm. Clin. Res. 2021, 14, 141–143. [Google Scholar] [CrossRef]
- Muñiz-Márquez, D.B.; Rodríguez, R.; Balagurusamy, N.; Carrillo, M.L.; Belmares, R.; Contreras, J.C.; Nevárez, G.V.; Aguilar, C.N. Phenolic content and antioxidant capacity of extracts of Laurus nobilis L., Coriandrum sativum L. and Amaranthus hybridus L. CyTA—J. Food 2014, 12, 271–276. [Google Scholar] [CrossRef]
- Ramos, C.; Teixeira, B.; Batista, I.; Matos, O.; Serrano, C.; Neng, N.R.; Nogueira, J.M.F.; Nunes, M.L.; Marques, A. Antioxidant and antibacterial activity of essential oil and extracts of bay laurel Laurus nobilis Linnaeus (Lauraceae) from Portugal. Nat. Prod. Res. 2012, 26, 518–529. [Google Scholar] [CrossRef]
- Btissam, R.; Fatima, E.; Mohamed, N. In vitro Study of Antibacterial Activity of Hydro-Alcohol Moroccan Plants Extracts. Pharmacogn. J. 2018, 10, 519–526. [Google Scholar] [CrossRef]
- Erkmen, O.; Özcan, M.M. Antimicrobial effects of Turkish propolis, pollen, and laurel on spoilage and pathogenic food-related microorganisms. J. Med. Food 2008, 11, 587–592. [Google Scholar] [CrossRef]
- Peixoto, L.R.; Rosalen, P.L.; Ferreira, G.L.S.; Freires, I.A.; de Carvalho, F.G.; Castellano, L.R.; de Castro, R.D. Antifungal activity, mode of action and anti-biofilm effects of Laurus nobilis Linnaeus essential oil against Candida spp. Arch. Oral Biol. 2017, 73, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.A.; Shin, G.H.; Kim, J.T. Multifunctional poly(vinyl alcohol) films using cellulose nanocrystals/oregano and cellulose nanocrystals/cinnamon Pickering emulsions: Effect of oil type and concentration. Int. J. Biol. Macromol. 2022, 194, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and Antimicrobial Properties of Starch-PVA Blend Films as Affected by the Incorporation of Natural Antimicrobial Agents. Foods 2015, 5, 3. [Google Scholar] [CrossRef]
- Hashimoto, A.; Sakamoto, K. UV-blocking film for food storage using titanium dioxide. Food Sci. Technol. Res. 2011, 17, 199–202. [Google Scholar] [CrossRef]
- Fernández, N.J.; Damiani, N.; Podaza, E.A.; Martucci, J.F.; Fasce, D.; Quiroz, F.; Meretta, P.E.; Quintana, S.; Eguaras, M.J.; Gende, L.B. Laurus nobilis L. extracts against Paenibacillus larvae: Antimicrobial activity, antioxidant capacity, hygienic behavior and colony strength. Saudi J. Biol. Sci. 2019, 26, 906–912. [Google Scholar] [CrossRef]
- Moustafa, H.; El-Sayed, S.M.; Youssef, A.M. Synergistic impact of cumin essential oil on enhancing of UV-blocking and antibacterial activity of biodegradable poly(butylene adipate-co-terephthalate)/clay platelets nanocomposites. J. Thermoplast. Compos. 2023, 36, 96–117. [Google Scholar] [CrossRef]
- Hindi, S.S.Z.; Albureikan, M.O.; Al-Ghamdi, A.A.; Alhummiany, H.; Ansari, M.S. Synthesis, characterization and biodegradation of gum Arabic-based bioplastic membranes. Nanosci. Nanotechnol. Res. 2017, 4, 32–42. [Google Scholar] [CrossRef]



| RT (min) | Compound | Type | Structure | RA * (%) | Bioactivity | Refs. |
|---|---|---|---|---|---|---|
| 1.07 | Methyl-4,6-O-benzylidene-hexopyranose | CD |  | 8.08 | Antimicrobial | [27] |
| 1.17 | 1,8-cineole | ME |  | 17.65 | Insecticidal | [28] |
| 1.28 | Isopulegol | MA |  | 5.17 | Antibacterial | [29] |
| 1.45 | α-terpineol | MA |  | 4.98 | Antibacterial | [30] |
| 1.53 | Methyleugenol | Ph |  | 5.44 | Antibacterial | [30] |
| 1.88 | Elemicin | Ph |  | 2.87 | Antibacterial | [31] |
| 1.65 | β-acorenol | SA |  | 3.38 | Antimicrobial | [32] |
| 2.17 | β-caryophyllene | S |  | 1.32 | Antimicrobial | [30] |
| 2.45 | Cedrandiol | SA |  | 0.89 | Antimicrobial | [33] |
| Composite | WVP (g·Pa−1·m−1·h−1·10−6) | ST (%/mm) | UVblock (%) |
|---|---|---|---|
| CMC0 | 2.89 ± 0.19 | 29.42 ± 0.01 | 37.22 ± 2.63 |
| CMC5 | 1.68 ± 0.39 | 28.05 ± 0.03 (*) | 99.94 ± 0.00 (*) |
| CMC10 | 1.58 ± 0.30 (*) | 30.46 ± 0.00 (*) | 99.93 ± 0.00 (*) |
| CMC15 | 1.52 ± 0.32 (*) | 26.26 ± 0.01 (*) | 99.91 ± 0.00 (*) |
| GA0 | 10.53 ± 1.39 | 23.03 ± 0.01 | 8.25 ± 0.45 |
| GA5 | 2.64 ± 1.47 (*) | 28.21 ± 0.03 (*) | 99.94 ± 0.00 (*) |
| GA10 | 6.28 ± 2.83 | 22.38 ± 0.03 (*) | 99.93 ± 0.00 (*) |
| GA15 | 2.75 ± 1.63 | 31.55 ± 0.02 (*) | 99.92 ± 0.00 (*) |
| PVP0 | 5.55 ± 1.05 | 27.01 ± 0.33 | 17.63 ± 3.07 |
| PVP5 | 4.31 ± 1.16 | 25.28 ± 0.26 | 99.94± 0.00 (*) |
| PVP10 | 3.14 ± 1.21 | 27.26 ± 0.09 | 99.93 ± 0.00 (*) |
| PVP15 | 1.96 ± 0.71 | 28.99 ± 1.03 | 99.92 ± 0.00 (*) |
| PVA0 | 1.64 ± 0.30 | 39.12 ± 0.01 | 47.99 ± 5.95 |
| PVA5 | 0.86 ± 0.41 | 41.38 ± 0.01 (*) | 99.94 ± 0.00 (*) |
| PVA10 | 0.43 ± 0.20 | 37.61 ± 0.01 (*) | 99.92 ± 0.00 (*) |
| PVA15 | 0.58 ± 0.26 | 36.08 ± 0.00 (*) | 99.92 ± 0.00 (*) |
| Coating | Mass Loss (%) | |||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 5 | Day 6 | |
| UF | 5.22 ± 0.37 | 11.23 ± 0.97 | 34.54 ± 1.78 | 46.72 ± 1.96 |
| CMC0 | 3.64 ± 0.43 (*) | 10.45 ± 0.38 | 23.38 ± 1.75 (*) | 32.17 ± 2.34 (*) |
| CMC15 | 3.32 ± 0.23 | 9.42 ± 0.18 | 22.11 ± 0.29 (*) | 27.82 ± 0.41 (*) |
| GA0 | 5.02 ± 0.40 | 10.04 ± 0.42 | 36.89 ± 1.89 | 48.01 ± 1.64 |
| GA15 | 3.48 ± 0.54 (*) | 8.07 ± 0.31 (*) | 27.23 ± 1.20 (*) | 37.21 ± 0.91 (*) |
| PVP0 | 4.67 ± 0.16 | 10.76 ± 0.51 | 25.30 ± 0.67 (*) | 29.13 ± 0.36 (*) |
| PVP15 | 5.65 ± 0.47 | 11.12 ± 0.66 | 26.60 ± 0.31 (*) | 30.39 ± 0.17 (*) |
| PVA0 | 2.52 ± 0.56 (*) | 6.05 ± 0.88 (*) | 18.92 ± 0.54 (*) | 26.69 ± 0.48 (*) |
| PVA15 | 3.87 ± 0.22 | 8.25 ± 0.05 (*) | 19.60 ± 0.19 (*) | 22.13 ± 0.77 (*) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Ortiz, M.; Serrano, L.; Romero, A.A.; García, A. Bay Leaves Extracts as Active Additive for Food Protective Coatings. Foods 2023, 12, 3741. https://doi.org/10.3390/foods12203741
Peña-Ortiz M, Serrano L, Romero AA, García A. Bay Leaves Extracts as Active Additive for Food Protective Coatings. Foods. 2023; 12(20):3741. https://doi.org/10.3390/foods12203741
Chicago/Turabian StylePeña-Ortiz, Manuel, Luis Serrano, Antonio A. Romero, and Araceli García. 2023. "Bay Leaves Extracts as Active Additive for Food Protective Coatings" Foods 12, no. 20: 3741. https://doi.org/10.3390/foods12203741
APA StylePeña-Ortiz, M., Serrano, L., Romero, A. A., & García, A. (2023). Bay Leaves Extracts as Active Additive for Food Protective Coatings. Foods, 12(20), 3741. https://doi.org/10.3390/foods12203741