Subcritical Water Extraction for Valorisation of Almond Skin from Almond Industrial Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material Preparation
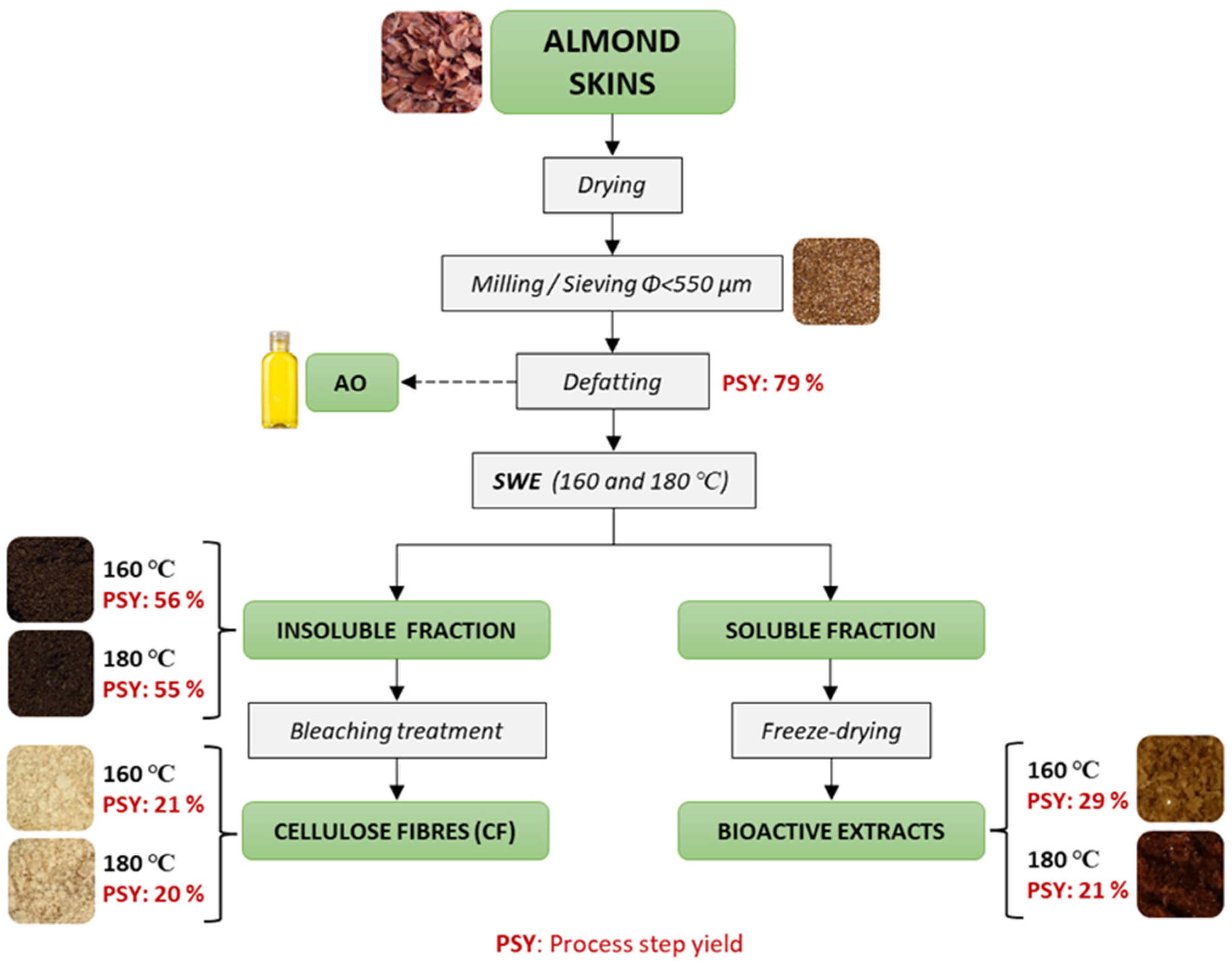
2.3. Obtaining AS Fractions by Applying SWE
2.4. Bleaching Process of the Extraction Residue
2.5. Characterisation of Cellulosic Residues
2.5.1. Chemical Composition
2.5.2. Thermogravimetric Analysis (TGA)
2.6. Characterisation of SWE Extracts
2.6.1. Determination of Total Phenolic Content (TPC)
2.6.2. Radical Scavenging Activity (DPPH)
2.6.3. Antibacterial Activity
2.6.4. Protein Content
2.7. Statistical Analysis
3. Results and Discussion
3.1. Yields of the Process Steps
3.2. Antioxidant and Antibacterial Properties of the SWE Extracts
3.3. Bleaching of the Extraction Residues
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef]
- González, J.F.; Gañán, J.; Ramiro, A.; González-García, C.M.; Encinar, J.M.; Sabio, E.; Román, S. Almond Residues Gasification Plant for Generation of Electric Power. Preliminary Study. Fuel Proces. Technol. 2006, 87, 149–155. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; Genovese, T.; Mazzon, E.; Wickham, M.S.J.; Paterniti, I.; Cuzzocrea, S. Natural Almond Skin Reduced Oxidative Stress and Inflammation in an Experimental Model of Inflammatory Bowel Disease. Int. Immunopharmacol. 2011, 11, 915–924. [Google Scholar] [CrossRef]
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The Importance of Almond (Prunus amygdalus L.) and Its by-Products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Bolling, B.W. Almond Polyphenols: Methods of Analysis, Contribution to Food Quality, and Health Promotion: Almond Polyphenol. Compr. Rev. Food Sci. Food Saf. 2017, 16, 346–368. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolome, B.; Gómez-Cordovés, C. Almond (Prunus dulcis (Mill.) D.A. Webb) Skins as a Potential Source of Bioactive Polyphenols. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Arena, A.; Tomaino, A.; Wickham, M.S.J. Antimicrobial Potential of Polyphenols Extracted from Almond Skins: Antimicrobials from Almond Skins. Lett. Appl. Microbiol. 2010, 51, 83–89. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) Skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Polyphenolic Content and Biological Properties of Avola Almond (Prunus dulcis Mill. D.A. Webb) Skin and Its Industrial Byproducts. Ind. Crops Prod. 2016, 83, 283–293. [Google Scholar] [CrossRef]
- Garrido, I.; Urpi-Sarda, M.; Monagas, M.; Gómez-Cordovés, C.; Martín-álvarez, P.J.; Llorach, R.; Bartolomé, B.; Andrés-Lacueva, C. Targeted Analysis of Conjugated and Microbial-Derived Phenolic Metabolites in Human Urine After Consumption of an Almond Skin Phenolic Extract. J. Nutr. 2010, 140, 1799–1807. [Google Scholar] [CrossRef]
- García-Pérez, P.; Barreal, M.E.; Rojo-De Dios, L.; Cameselle-Teijeiro, J.F.; Gallego, P.P. Bioactive Natural Products From the Genus Kalanchoe as Cancer Chemopreventive Agents: A Review. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 61, pp. 49–84. ISBN 978-0-444-64183-0. [Google Scholar]
- Bisignano, C.; Mandalari, G.; Smeriglio, A.; Trombetta, D.; Pizzo, M.; Pennisi, R.; Sciortino, M. Almond Skin Extracts Abrogate HSV-1 Replication by Blocking Virus Binding to the Cell. Viruses 2017, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Berryman, C.E.; Preston, A.G.; Karmally, W.; Deckelbaum, R.J.; Kris-Etherton, P.M. Effects of Almond Consumption on the Reduction of LDL-Cholesterol: A Discussion of Potential Mechanisms and Future Research Directions. Nutr. Rev. 2011, 69, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Kalita, S.; Khandelwal, S.; Madan, J.; Pandya, H.; Sesikeran, B.; Krishnaswamy, K. Almonds and Cardiovascular Health: A Review. Nutrients 2018, 10, 468. [Google Scholar] [CrossRef]
- Chen, C.-Y.O.; Milbury, P.E.; Blumberg, J.B. Polyphenols in Almond Skins after Blanching Modulate Plasma Biomarkers of Oxidative Stress in Healthy Humans. Antioxidants 2019, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Rao, P.; Ni, L. Prebiotic Effects of Almonds and Almond Skins on Intestinal Microbiota in Healthy Adult Humans. Anaerobe 2014, 26, 1–6. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Rich, G.T.; Curto, R.L.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Sajia, A.; Parker, M.L.; Waldron, K.W.; et al. Polyphenol and nutrient release from skin of almonds during simulated human digestion. Food Chem. 2010, 122, 1083–1088. [Google Scholar] [CrossRef]
- Mandalari, G.; Vardakou, M.; Faulks, R.; Bisignano, C.; Martorana, M.; Smeriglio, A.; Trombetta, D. Food Matrix Effects of Polyphenol Bioaccessibility from Almond Skin during Simulated Human Digestion. Nutrients 2016, 8, 568. [Google Scholar] [CrossRef]
- Mandalari, G.; Faulks, R.M.; Bisignano, C.; Waldron, K.W.; Narbad, A.; Wickham, M.S.J. In Vitro Evaluation of the Prebiotic Properties of Almond Skins (Amygdalus communis L.). FEMS Microbiol. Lett. 2010, 304, 116–122. [Google Scholar] [CrossRef]
- Pasqualone, A.; Laddomada, B.; Spina, A.; Todaro, A.; Guzmàn, C.; Summo, C.; Mita, G.; Giannone, V. Almond By-Products: Extraction and Characterization of Phenolic Compounds and Evaluation of Their Potential Use in Composite Dough with Wheat Flour. LWT 2018, 89, 299–306. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-Assisted Extraction of Phenolic Compounds from Almond Skin Byproducts (Prunus amygdalus): A Multivariate Analysis Approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef]
- Harrison, K.; Were, L. Effect of Gamma Irradiation on Total Phenolic Content Yield and Antioxidant Capacity of Almond Skin Extracts. Food Chem. 2007, 102, 932–937. [Google Scholar] [CrossRef]
- Gabaston, J.; Leborgne, C.; Valls, J.; Renouf, E.; Richard, T.; Waffo-Teguo, P.; Mérillon, J.-M. Subcritical Water Extraction of Stilbenes from Grapevine By-Products: A New Green Chemistry Approach. Ind. Crops Prod. 2018, 126, 272–279. [Google Scholar] [CrossRef]
- Ruthes, A.C.; Martínez-Abad, A.; Tan, H.-T.; Bulone, V.; Vilaplana, F. Sequential Fractionation of Feruloylated Hemicelluloses and Oligosaccharides from Wheat Bran Using Subcritical Water and Xylanolytic Enzymes. Green Chem. 2017, 19, 1919–1931. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent Advances in the Extraction of Bioactive Compounds with Subcritical Water: A Review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Hanim, S.S.; Norsyabilah, R.; Suhaila, M.H.N.; Noraishah, A.; Kartina, A.K.S. Effects of Temperature, Time and Pressure on the Hemicelluloses Yield Extracted Using Subcritical Water Extraction. Procedia Eng. 2012, 42, 562–565. [Google Scholar] [CrossRef]
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Subcritical Water Extraction of Phenolic and Antioxidant Constituents from Pistachio (Pistacia vera L.) Hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the Extraction of Phenolic Antioxidants from Chestnut Shells by Subcritical Water Extraction Using Response Surface Methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef]
- Vladic, J.; Nastic, N.; Stanojkovic, T.; Zizak, Z.; Cakarevic, J.; Popovic, L.; Vidovic, S. Subcritical Water for Recovery of Polyphenols from Comfrey Root and Biological Activities of Extracts. ACSi 2019, 66, 473–783. [Google Scholar] [CrossRef]
- Vladić, J.; Janković, T.; Živković, J.; Tomić, M.; Zdunić, G.; Šavikin, K.; Vidović, S. Comparative Study of Subcritical Water and Microwave-Assisted Extraction Techniques Impact on the Phenolic Compounds and 5-Hydroxymethylfurfural Content in Pomegranate Peel. Plant Foods Hum. Nutr. 2020, 75, 553–560. [Google Scholar] [CrossRef]
- Requena, R.; Jiménez-Quero, A.; Vargas, M.; Moriana, R.; Chiralt, A.; Vilaplana, F. Integral Fractionation of Rice Husks into Bioactive Arabinoxylans, Cellulose Nanocrystals, and Silica Particles. ACS Sustain. Chem. Eng. 2019, 7, 6275–6286. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Influence of the Cellulose Purification Process on the Properties of Aerogels Obtained from Rice Straw. Carbohydr. Polym. 2023, 312, 120805. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Active Poly (Lactic Acid) Films with Rice Straw Aqueous Extracts for Meat Preservation Purposes. Food Bioprocess. Technol. 2023, 16, 2635–2650. [Google Scholar] [CrossRef]
- Li, L.; Lee, S.; Lee, H.L.; Youn, H.J. Hydrogen Peroxide Bleaching of Hardwood Kraft Pulp with Adsorbed Birch Xylan and Its Effect on Paper Properties. BioRes 2011, 6, 721–736. [Google Scholar] [CrossRef]
- Sluiter, A. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP); Technical Report; National Renewable Energy Laboratory: Golden, CO, USA, 7 July 2005; p. 12.
- Sluiter, A. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); Technical Report, Version 07-08-2011; National Renewable Energy Laboratory: Golden, CO, USA, 2008; p. 18.
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Application of Ultrasound Pre-Treatment for Enhancing Extraction of Bioactive Compounds from Rice Straw. Foods 2020, 9, 1657. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Tomaino, A.; Arcoraci, T.; Martorana, M.; Turco, V.L.; Cacciola, F.; Rich, G.T.; Bisignano, C.; Saija, A.; Dugo, P.; et al. Characterization of Polyphenols, Lipids and Dietary Fibre from Almond Skins (Amygdalus communis L.). J. Food Compos. Anal. 2010, 23, 166–174. [Google Scholar] [CrossRef]
- Ouzir, M.; Bernoussi, S.E.; Tabyaoui, M.; Taghzouti, K. Almond Oil: A Comprehensive Review of Chemical Composition, Extraction Methods, Preservation Conditions, Potential Health Benefits, and Safety. Comp. Rev. Food Sci. Food Saf. 2021, 20, 3344–3387. [Google Scholar] [CrossRef]
- Çalhan, S.D.; Meryemoğlu, B.; Eroğlu, P.; Saçlı, B.; Kalderis, D. Subcritical Water Extraction of Onosma Mutabilis: Process Optimization and Chemical Profile of the Extracts. Molecules 2023, 28, 2314. [Google Scholar] [CrossRef]
- Yabalak, E.; Gizir, A.M. Treatment of Agrochemical Wastewater by Subcritical Water Oxidation Method: Chemical Composition and Ion Analysis of Treated and Untreated Samples. J. Environ. Sci. Health Part A 2020, 55, 1424–1435. [Google Scholar] [CrossRef]
- Yabalak, E. Water Recovery from Textile Bath Wastewater Using Combined Subcritical Water Oxidation and Nanofiltration. J. Clean. Prod. 2021, 290, 125207. [Google Scholar] [CrossRef]
- Spiegel, M.; Cel, K.; Sroka, Z. The Mechanistic Insights into the Role of pH and Solvent on Antiradical and Prooxidant Properties of Polyphenols—Nine Compounds Case Study. Food Chem. 2023, 407, 134677. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; Del Castillo, M.D.; Ibáñez, E.; Herrero, M. Neoformation of Antioxidants in Glycation Model Systems Treated under Subcritical Water Extraction Conditions. Food Res. Int. 2010, 43, 1123–1129. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; Del Castillo, M.D.; Ibáñez, E.; Herrero, M. Facts about the Formation of New Antioxidants in Natural Samples after Subcritical Water Extraction. Food Res. Int. 2010, 43, 2341–2348. [Google Scholar] [CrossRef]
- Singh, R.D.; Nadar, C.G.; Muir, J.; Arora, A. Green and Clean Process to Obtain Low Degree of Polymerisation Xylooligosaccharides from Almond Shell. J. Clean. Prod. 2019, 241, 118237. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S. Isolation and Characterisation of Microcrystalline Cellulose and Cellulose Nanocrystals from Coffee Husk and Comparative Study with Rice Husk. Carbohydr. Polym. 2018, 11, 205–215. [Google Scholar] [CrossRef]
- Mansaray, G.; Ghaly, A.E. Thermal degradation of rice husks in nitrogen atmosphere. Bioresour. Technol. 1998, 65, 13–20. [Google Scholar] [CrossRef]
- Theng, D.; Arbat, G.; Delgado-Aguilar, M.; Ngo, B.; Labonne, L.; Evon, P.; Mutjé, P. Comparison between Two Different Pretreatment Technologies of Rice Straw Fibers Prior to Fiberboard Manufacturing: Twin-Screw Extrusion and Digestion plus Defibration. Ind. Crops Prod. 2017, 107, 184–197. [Google Scholar] [CrossRef]
- Malayil, S.; Surendran, A.N.; Kate, K.; Satyavolu, J. Impact of Acid Hydrolysis on Composition, Morphology and Xylose Recovery from Almond Biomass (Skin and Shell). Bioresour. Technol. Rep. 2022, 19, 101150. [Google Scholar] [CrossRef]
- Mohammed, D.; Freije, A.; Abdulhussain, H.; Khonji, A.; Hasan, M.; Ferraris, C.; Gasparri, C.; Aziz Aljar, M.A.; Ali Redha, A.; Giacosa, A.; et al. Analysis of the Antioxidant Activity, Lipid Profile, and Minerals of the Skin and Seed of Hazelnuts (Corylus avellana L.), Pistachios (Pistacia vera) and Almonds (Prunus dulcis)—A Comparative Analysis. Appl. Chem 2023, 3, 110–118. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A. Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef]
- Abraham, K.; Gürtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and Risk Assessment of 5-Hydroxymethylfurfural in Food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Polyphenols and Antioxidant Properties of Almond Skins: Influence of Industrial Processing. J. Food Sci. 2008, 73, C106–C115. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ahmedna, M.; Goktepe, I.; Dai, J. Peanut Skin Procyanidins: Composition and Antioxidant Activities as Affected by Processing. J. Food Compos. Anal. 2006, 19, 364–371. [Google Scholar] [CrossRef]
- Lawag, I.L.; Nolden, E.S.; Schaper, A.A.M.; Lim, L.Y.; Locher, C. A Modified Folin-Ciocalteu Assay for the Determination of Total Phenolics Content in Honey. Appl. Sci. 2023, 13, 2135. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. ISBN 978-0-12-182200-2. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mohan, V.; Wibisono, R.; De Hoop, L.; Summers, G.; Fletcher, G.C. Identifying Suitable Listeria Innocua Strains as Surrogates for Listeria Monocytogenes for Horticultural Products. Front. Microbiol. 2019, 10, 2281. [Google Scholar] [CrossRef]
- Moon, R.J.; Schueneman, G.T.; Simonsen, J. Overview of Cellulose Nanomaterials, Their Capabilities and Applications. JOM 2016, 68, 2383–2394. [Google Scholar] [CrossRef]
- Potůček, F.; Milichovský, M. Kraft Pulp Bleaching with Hydrogen Peroxide and Peracetic Acid. Chem. Pap. 2000, 54, 406–411. [Google Scholar]
- Liu, Y.; Hu, H. X-Ray Diffraction Study of Bamboo Fibers Treated with NaOH. Fibers Polym. 2008, 9, 735–739. [Google Scholar] [CrossRef]
- Yue, Y.; Zhou, C.; French, A.D.; Xia, G.; Han, G.; Wang, Q.; Wu, Q. Comparative Properties of Cellulose Nano-Crystals from Native and Mercerized Cotton Fibers. Cellulose 2012, 19, 1173–1187. [Google Scholar] [CrossRef]
- Budtova, T.; Navard, P. Cellulose in NaOH-Water Based Solvents: A Review. Cellulose 2016, 23, 5–55. [Google Scholar] [CrossRef]




| E-160 | E-180 | |
|---|---|---|
| TPC1 (mg GAE.g−1 dried extract) | 100.9 ± 0.5 b | 161 ± 4 a |
| TPC2 (mg GAE.g−1 DAS) | 29.6 ± 0.15 b | 33.8 ± 0.9 a |
| EC50 (mg extract.mg−1 DPPH) | 1.490 ± 0.003 a | 1.063 ± 0.012 b |
| EC50 (mg DAS.mg−1 DPPH) | 5.13 ± 0.01 a | 5.06 ± 0.06 b |
| MIC L. innocua (mg.mL−1) | 90 | 34 |
| MIC E. coli (mg.mL−1) | 90 | 48 |
| Ash (g.100 g−1 solid extract) | 13.1 ± 0.1 b | 15.5 ± 0.7 a |
| Sample | Cellulose (%wt.) | Hemicellulose (%wt.) | Klason Lignin (%wt.) | Ash (%wt.) |
|---|---|---|---|---|
| DAS | 9.8 ± 0.3 | 11.6 ± 0.5 | 17.4 ± 0.2 | 5.1 ± 0.1 |
| R-160 | 18.6 ± 0.9 b | 6.4 ± 0.3 a | 45.2 ± 0.5 b | 2.3 ± 0.1 a |
| R-180 | 20.9 ± 0.2 a | 5.1 ± 0.1 b | 51.2 ± 0.3 a | 2.7 ± 0.6 a |
| BR-160-1 | 35.0 ± 3.0 a,1 | 13.4 ± 0.9 b,1 | 22.0 ± 3.0 a,2 | 15.0 ± 1.0 c,1 |
| BR-160-2 | 40.0 ± 4.0 a,2 | 17.5 ± 1.9 a,1 | 18.9 ± 1.9 b,2 | 17.0 ± 4.0 bc,1 |
| BR-160-3 | 40.0 ± 4.0 a,2 | 15.0 ± 3.0 ab,1 | 15.0 ± 2.0 c,2 | 18.0 ± 4.0 b,2 |
| BR-160-4 | 40.0 ± 3.0 a,2 | 13.6 ± 0.7 ab,1 | 13.6 ± 0.1 c,2 | 25.0 ± 3.0 a,1 |
| BR-180-1 | 35.6 ± 1.8 b,1 | 9.3 ± 1.2 b,2 | 33.0 ± 2.0 a,1 | 10.4 ± 0.1 c,2 |
| BR-180-2 | 52.0 ± 2.0 a,1 | 13.5 ± 0.5 a,2 | 22.5 ± 0.7 b,1 | 17.0 ± 5.0 b,1 |
| BR-180-3 | 49.9 ± 0.8 a,1 | 10.0 ± 2.0 b,1 | 22.0 ± 4.0 bc,1 | 26.3 ± 0.5 a,1 |
| BR-180-4 | 49.6 ± 0.6 a,1 | 13.9 ± 1.2 a,1 | 16.7 ± 0.7 c,1 | 22.0 ± 7.0 ab,1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, P.A.V.; Martín-Pérez, L.; Gil-Guillén, I.; González-Martínez, C.; Chiralt, A. Subcritical Water Extraction for Valorisation of Almond Skin from Almond Industrial Processing. Foods 2023, 12, 3759. https://doi.org/10.3390/foods12203759
Freitas PAV, Martín-Pérez L, Gil-Guillén I, González-Martínez C, Chiralt A. Subcritical Water Extraction for Valorisation of Almond Skin from Almond Industrial Processing. Foods. 2023; 12(20):3759. https://doi.org/10.3390/foods12203759
Chicago/Turabian StyleFreitas, Pedro A. V., Laia Martín-Pérez, Irene Gil-Guillén, Chelo González-Martínez, and Amparo Chiralt. 2023. "Subcritical Water Extraction for Valorisation of Almond Skin from Almond Industrial Processing" Foods 12, no. 20: 3759. https://doi.org/10.3390/foods12203759






