Tart Cherry (Prunus cerasus L.) Pit Extracts Protect Human Skin Cells against Oxidative Stress: Unlocking Sustainable Uses for Food Industry Byproducts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction Process and Colorimetric Characterization of the Extracts
2.3. Preparation of Cherry Pit Extracts for Metabolomic Analyses and In Vitro Tests
2.4. Metabolomic Analyses of CP Extracts
2.5. UV–Visible Absorption Spectra
2.6. Cell Lines, Cultures, and Treatments
2.7. Cell Viability Assays
2.8. Assessment of Intracellular ROS
2.9. RNA Isolation and Real Time-PCR (RT-PCR) Analyses
2.10. Statistical Analyses
3. Results and Discussions
3.1. Chemical Analyses of CP Extracts
3.2. Metabolomic Analysis of CP Extracts
3.3. UV–Visible Spectra of CP Extracts
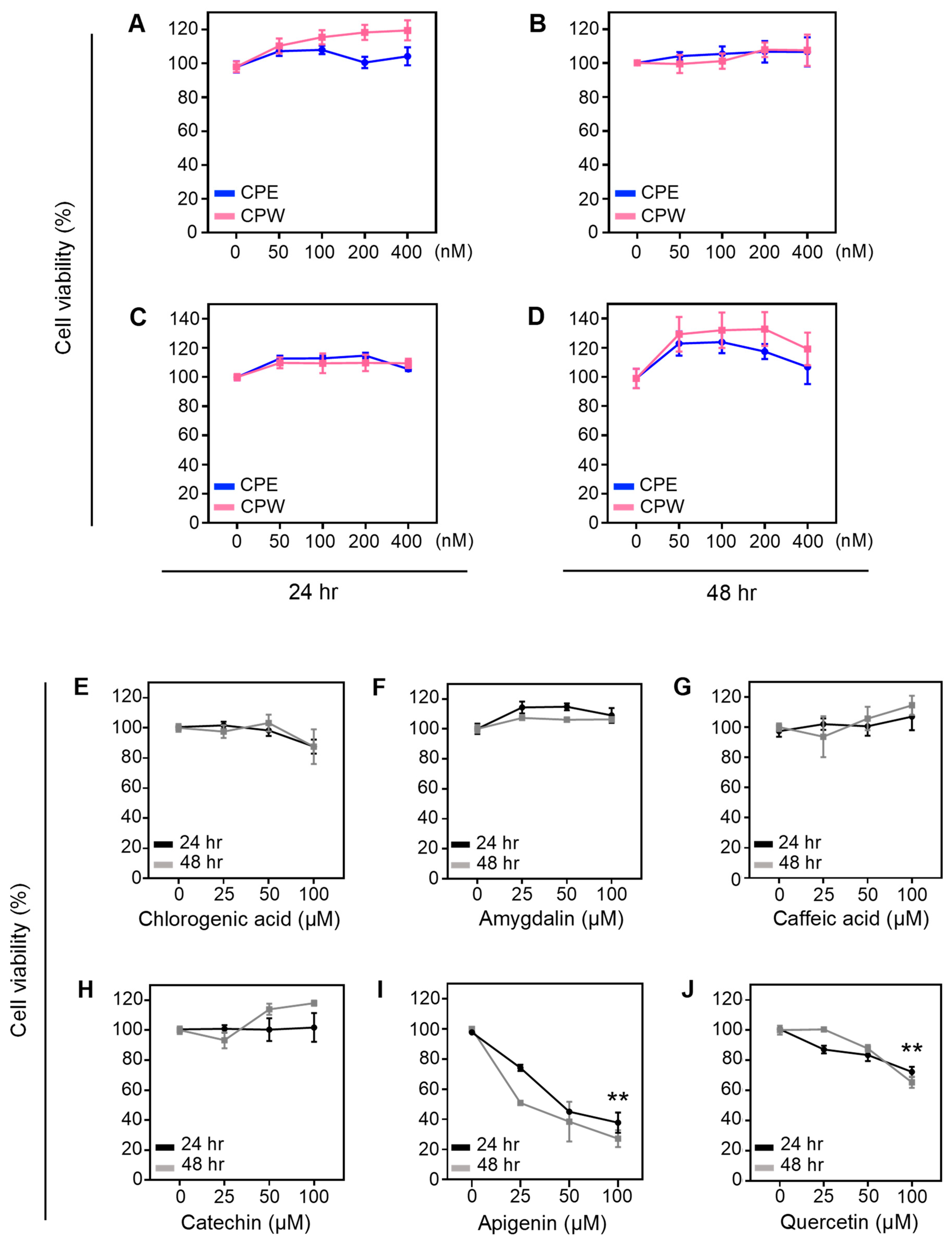
3.4. Effect of CP Extracts on Cell Viability
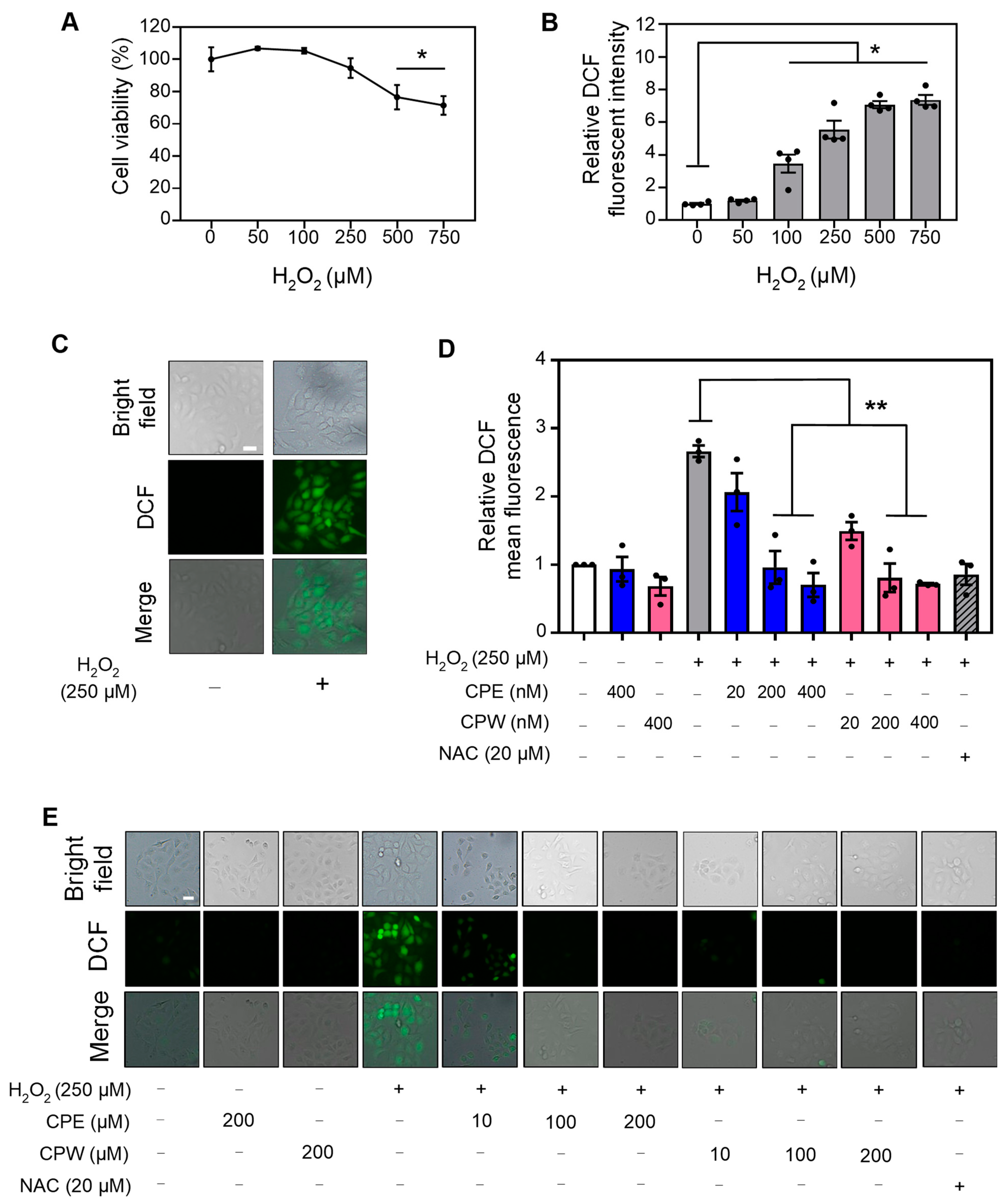
3.5. CP Extracts Reduced H2O2-Induced ROS Levels
3.6. CP Extracts Reduced LPS-Induced ROS Levels
3.7. CP Extracts Regulate Antioxidant Gene Expression
4. Conclusions
5. Future Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture’s National Agricultural Statistics Services (USDA NASS). US Sweet Cherry Production, 10 June 2022. Available online: https://www.nass.usda.gov/Statistics_by_State/Washington/Publications/Current_News_Release/2022/CH06_1.pdf (accessed on 9 March 2023).
- United States Department of Agriculture National Agricultural Statistics Service Great Lakes Region. 2022 Michigan Cherry Production Forecast, 10 June 2022. Available online: https://www.nass.usda.gov/Statistics_by_State/Michigan/Publications/Current_News_Release/2022/nr2234mi.pdf (accessed on 15 August 2023).
- Yılmaz, F.M.; Görgüç, A.; Karaaslan, M.; Vardin, H.; Ersus Bilek, S.; Uygun, Ö.; Bircan, C. Sour Cherry By-products: Compositions, Functional Properties and Recovery Potentials—A Review. Crit. Rev. Food. Sci. Nutr. 2018, 59, 3549–3563. [Google Scholar] [CrossRef]
- Bajerska, J.; Mildner-Szkudlarz, S.; Górnaś, P.; Seglina, D. The Effects of Muffins Enriched with Sour Cherry Pomace on Acceptability, Glycemic Response, Satiety and Energy Intake: A Randomized Crossover Trial. J. Sci. Food. Agric. 2016, 96, 2486–2493. [Google Scholar] [CrossRef]
- Popa, V.; Misca, C.; Bordean, D.; Raba, D.N.; Stef, D.; Dumbrava, D. Characterization of sour cherries (Prunus cerasus) kernel oil cultivars from Banat. J. Agroaliment. Process. Technol. 2011, 17, 398–401. [Google Scholar]
- Kućić, D.; Kopćić, N.; Briśki, F. Biodegradation of Agro-Industrial Waste. Chem. Biochem. Eng. Q. 2017, 31, 369–374. [Google Scholar] [CrossRef]
- Korlesky, N.M.; Stolp, L.J.; Kodali, D.R.; Goldschmidt, R.; Byrdwell, W.C. Extraction and Characterization of Montmorency Sour Cherry (Prunus cerasus L.) Pit Oil. J. Am. Oil Chem. Soc. 2016, 93, 995–1005. [Google Scholar] [CrossRef]
- Pollard, Z.A.; Goldfarb, J.L. Valorization of Cherry Pits: Great Lakes Agro-Industrial Waste to Mediate Great Lakes Water Quality. Environ. Pollut. 2021, 270, 116073. [Google Scholar] [CrossRef] [PubMed]
- Sezer, D.B.; Ahmed, J.; Sumnu, G.; Sahin, S. Green Processing of Sour Cherry (Prunus cerasus L.) Pomace: Process Optimization for the Modification of Dietary Fibers and Property Measurements. Food Meas. 2021, 15, 3015–3025. [Google Scholar] [CrossRef]
- Khan, A.Q.; Agha, M.V.; Sheikhan, K.S.A.M.; Younis, S.M.; Al Tamimi, M.; Alam, M.; Uddin, S.; Buddenkotte, J.; Steinhoff, M. Targeting Deregulated Oxidative Stress in Skin Inflammatory Diseases: An update on clinical importance. Biomed. Pharmacother. 2022, 154, 113601. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Oehrl, W.; Elsner, P.; Thiele, J.J. The Role of H2O2 as a Mediator of UVB-Induced Apoptosis in Keratinocytes. Free Radic. Res. 2003, 37, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Rodust, P.M.; Stockfleth, E.; Ulrich, C.; Leverkus, M.; Eberle, J. UV—Induced Squamous Cell Carcinoma—A Role for Antiapoptotic Signaling Pathways. Br. J. Dermatol. 2009, 161, 107–155. [Google Scholar] [CrossRef] [PubMed]
- Pleńkowska, J.; Gabig-Cimińska, M.; Mozolewski, P. Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 161, 6206. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.J.P.O.; De Oliveria, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-inflammatory Effect of Strawberry Extract Against LPS-induced Stress in RAW 264.7 Macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Agulló-Chazarra, L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A.; Micol, V.; Herranz-López, M.; Barrajón-Catalán, E. Sweet Cherry Byproducts Processed by Green Extraction Techniques as a Source of Bioactive Compounds with Antiaging Properties. Antioxidants 2020, 9, 418. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-Collagenase, Anti-Elastase and Anti-Oxidant Activities of Extracts from 21 Plants. BMC Complement Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Ziaullah, R.; Vasantha, H.P. Application of NMR Spectroscopy in Plant Polyphenols Associated with Human Health. In Applications of NMR Spectroscopy; ur-Rahman, A., Choudhary, M.I., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2015; Volume 2, pp. 3–92. [Google Scholar] [CrossRef]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant Activity of Phenolic Compounds: From in vitro results to in vivo evidence. Crit. Rev. Food. Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sanchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for Human Disease: An Update on Plant-Derived Compounds Antibacterial Activity. Microbiol. Res. 2017, 96, 44–68. [Google Scholar] [CrossRef]
- Peyrol, J.; Meyer, G.; Obert, P.; Dangles, O.; Pechere, L.; Amiot, M.J.; Riva, C. Involvement of Bilitranslocase and Beta-Glucuronidase in the Vascular Protection by Hydroxytyrosol and its Glucuronide Metabolites in Oxidative Stress Conditions. J. Nutr. Biochem. 2017, 51, 8–15. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Booren, A.M.; Gray, J.I. Antioxidant Polyphenols from Tart Cherries (Prunus cerasus). J. Agric. Food. Chem. 1999, 47, 840–844. [Google Scholar] [CrossRef]
- Dulyanska, Y.; Cruz-Lopes, L.P.; Esteves, B.; Ferreira, J.V.; Domingos, I.; Lima, M.J.; Correia, P.M.R.; Ferreira, M.; Fragata, A.; Barroca, M.J.; et al. Extraction of Phenolic Compounds from Cherry Seeds: A Preliminary Study. Agronomy 2022, 12, 1227. [Google Scholar] [CrossRef]
- Bagheri, S.; Alinejad, M.; Nejad, M.; Aliakbarian, B. Creating Incremental Revenue from Industry Cherry Wastes. Chem. Eng. Trans. 2021, 86, 553–558. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The Phenolic Constituents of Prunus domestica. I.—The Quantitative Analysis of Phenolic Constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Paini, M.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Binello, A.; Cravotto, G. Influence of Ethanol/Water Ratio in Ultrasound and High-Pressure/High-Temperature Phenolic Compound Extraction from Agri-Food Waste. Int. J. Food Sci. Technol. 2016, 51, 349–358. [Google Scholar] [CrossRef]
- Makori, S.I.; Mu, T.-H.; Sun, H.-N. Total Polyphenol Content, Antioxidant Activity, and Individual Phenolic Composition of Different Edible Parts of 4 Sweet Potato Cultivars. Nat. Prod. Commun. 2020, 15, 1–12. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Casazza, A.A.; Perego, P. Valorization of Olive Oil Solid Waste Using High Pressure–High Temperature Reactor. Food Chem. 2011, 128, 704–710. [Google Scholar] [CrossRef]
- Borowiec, K.; Stachniuk, A.; Szwajgier, D.; Trzpil, A. Polyphenols Composition and the Biological Effects of Six Selected Small Dark Fruits. Food Chem. 2022, 391, 133281. [Google Scholar] [CrossRef] [PubMed]
- Bursal, E.; Köksal, E.; Gülçin, İ.; Gökhan, B.; Gören, A.C. Antioxidant Activity and Polyphenol Content of Cherry Stem (Cerasus Avium L.) Determined by LC-MS/MS. Food Res. Int. 2013, 51, 66–74. [Google Scholar] [CrossRef]
- Hu, T.; Subbiah, V.; Wu, H.; Bk, A.; Rauf, A.; Alhumaydhi, F.A.; Suleria, H.A.R. Determination and Characterization of Phenolic Compounds from Australia-Grown Sweet Cherries (Prunus avium L.) and Their Potential Antioxidants Properties. ASC Omega 2021, 6, 34687–34699. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Moghe, G.D.; Leong, B.J.; Hurney, S.; Jones, A.D.; Last, R.L. Evolutionary routes to biochemical innovation revealed by integrative analysis of a plant-defense related specialized metabolic pathway. Elife 2017, 6, e28468. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; LaRocca, C.A.; Bernat, J.D.; Lindsey, J.S. Digital Database of Absorption Spectra of Diverse Flavonoids Enables Structural Comparisons and Quantitative Evaluations. J. Nat. Prod. 2023, 86, 1087–1119. [Google Scholar] [CrossRef] [PubMed]
- Patle, T.K.; Shrivas, K.; Kurrey, R.; Upadhyay, S.; Jangde, R.; Chauhan, R. Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV-vis and FTIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 1118717. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Parra, M.R.; Stoub, H.; Gallo, K.A.; Doseff, A.I. Apigenin by Targeting hnRNPA2 Sensitizes Triple-Negative Breast Cancer Spheroids to Doxorubicin-Induced Apoptosis and Regulates Expression of ABCC4 and ABCG2 Drug Efflux Transporters. Biochem. Pharma. 2020, 182, 114259. [Google Scholar] [CrossRef] [PubMed]
- Casazza, A.A.; Aliakbarian, B.; De Faveri, D.; Fiori, L.; Perego, P. Antioxidants from Winemaking Wastes: A study on extraction parameters using Response Surface Methodology. J. Food Biochem. 2012, 36, 28–37. [Google Scholar] [CrossRef]
- Rosero, J.C.; Cruz, S.; Osorio, C.; Hurtado, N. Analysis of Phenolic Composition of Byproducts (seeds and peels) of Avocado (persea americana mill.) Cultivated in Colombia. Molecules 2014, 24, 3209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Tang, G.Y.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Liu, Q.; Mao, Q.Q.; Shang, A.; Li, H.B. Phenolic Profiles and Antioxidant Activities of 30 Tea Infusions from Green, Black, Oolong, White, Yellow, and Dark Teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef]
- Dulyanska, Y.; Luísa, C.-L.; Esteves, B.; Ferreira, J.; Domingos, I.; Lima, M.J.; Correia, P.; Ferreira, M.; Fragata, A.; Barroca, M.; et al. Evaluation of the antioxidant activity of extracts obtained from cherry seeds. J. Hyg. Eng. Des. 2022, 40, 221–226. [Google Scholar]
- Chaovanalikit, A.; Wrolstad, R.E. Anthocyanin and Polyphenolic Composition of Fresh and Processed Cherries. J. Food Sci. 2004, 69, 73–83. [Google Scholar] [CrossRef]
- Peixoto, J.; Álvarez-Rivera, G.; Alves, R.C.; Costa, A.S.G.; Andrade, N.; Moreira, A.; Cifuentes, A.; Martel, F.; Oliveira, M.B.P.P.; Ibáñez, E. Cherry Stem Infusions: Antioxidant Potential and Phenolic Profile by UHPLC-ESI-QTOF-MS. Food Funct. 2020, 11, 3471–3482. [Google Scholar] [CrossRef]
- Md Salehan, N.A.; Ziad Sulaiman, A.; Azilah, A. Effect of tempretaure and sonication on the extraction of gacclic acid from Labisia Pumila (Kacip Fatimah). ARPN J. Eng. Appl. Sci. 2016, 11, 2193–2198. [Google Scholar]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Demiray, S.; Piccirillo, C.; Rodrigues, C.L.; Pintado, M.E.; Castro, P.M.L. Extraction of Valuable Compounds from Ginja Cherry By-Products: Effect of the Solvent and Antioxidant Properties. Waste Biomass Valorization 2011, 2, 365–371. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Seymour, E.M.; Llanes, D.E.U.; Kaufman, P.B.; Bolling, S.F. Chemical Profile and Antioxidants Capabilities of Tart Cherry Products. Food Chem. 2009, 115, 20–25. [Google Scholar] [CrossRef]
- Abascal, K.; Ganora, L.; Yarnell, E. The Effect of Freeze-drying and its Implications for Botanical Medicine: A Review. Phytother. Res. 2005, 9, 655–660. [Google Scholar] [CrossRef]
- Bak, I.; Lekli, I.; Juhasz, B.; Varga, E.; Varga, B.; Gesztelyi, R.; Szendrei, L.; Tosaki, A. Isolation and Analysis of Bioactive Constituents of Sour Cherry (Prunus cerasus) Seed Kernel: An Emerging Functional Food. J. Med. Food 2010, 13, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Navarra, G.; Moschetti, M.; Guarrasi, V.; Mangione, M.R.; Militello, V.; Leone, M. Simultaneous Determination of Caffeine and Chlorogenic Acids in Green Coffee by UV/Vis Spectroscopy. J. Chem. 2017, 2017, 6435086. [Google Scholar] [CrossRef]
- Cha, J.W.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Zheng, J.; Kim, S.M.; Hyun, C.L.; Ahn, Y.S.; Hyun, J.W. The Polyphenol Chlorogenic Acid Attenuates UVB-Mediated Oxidative Stress in Human HaCat Kertinocytes. Biomol. Ther. 2014, 22, 136–142. [Google Scholar] [CrossRef]
- Balupillai, A.; Nagarajan, R.P.; Ramasamy, K.; Govindasamy, K.; Muthusamy, G. Caffeic Acid Prevents UVB Radiation Induced Photocarcinogenesis Through Regulation of PTEN Signaling in Human Dermal Fibroblasts and Mouse Skin. Toxicol. Appl. Pharmaco. 2018, 352, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.B.; Chiang, H.S.; Fang, J.Y.; Chen, S.K.; Huang, C.C.; Hung, C.F. (+)-Catechin Prevents Ultraviolet B-Induced Human Keratinocyte Death via Inhibition of JNK Phosphorylation. Life Sci. 2006, 79, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, B.; Dybowski, M.P.; Klimek, K.; Typek, R.; Miazga-Karska, M.; Dos Santos Szewczyk, K. The Anti-Acne Potential and Chemical Composition of Two Cultivated Cotoneaster Species. Cells 2022, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.G.; Lee, G.B.; Vinayagam, R.; Do, G.S.; Oh, S.Y.; Yang, S.J.; Kwon, J.B.; Singh, M. Anti-Inflammatory, Antioxidative, and Nitric Oxide-Scavenging Activities of a Quercetin Nanosuspension with Polyethylene Glycol in LPS-Induced RAW 264.7 Macrophages. Molecules 2022, 27, 7432. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Sahoo, D.; Agrahari, K.; Khan, A.; Mukhopadhyay, P.; Chanda, D.; Yadav, N.P. Anti-Inflammatory, Anti-Proliferative and Anti-Psoriatic Potential of Apigenin in RAW 264.7 Cells, HaCaT Cells and Psoriasis like Dermatitis in BALB/c Mice. Life Sci. 2023, 328, 121909. [Google Scholar] [CrossRef]
- Kim, D.W.; Jung, D.H.; Sung, J.; Min, I.S.; Lee, S.J. Tart Cherry Extract Containing Chlorogenic Acid, Quercetin, and Kaempferol Inhibits the Mitochondrial Apoptotic Cell Death Elicited by Airborne PM(10) in Human Epidermal Keratinocytes. Antioxidants 2021, 10, 443. [Google Scholar] [CrossRef]
- Martinelli, I.; Tomassoni, D.; Bellitto, V.; Roy, P.; Micioni Di Bonaventura, M.V.; Amenta, F.; Amantini, C.; Cifani, C.; Tayebati, S.K. Anti-Inflammatory and Antioxidant Properties of Tart Cherry Consumption in the Heart of Obese Rats. Biology 2022, 11, 646. [Google Scholar] [CrossRef]
- Duarte, S.; Arango, D.; Parihar, A.; Hamel, P.; Yasmeen, R.; Doseff, A.I. Apigenin Protects Endothelial Cells from Lipopolysaccharide (LPS)-Induced Inflammation by Decreasing Caspase-3 Activation and Modulating Mitochondrial Function. Int. J. Mol. Sci. 2013, 14, 17664–17679. [Google Scholar] [CrossRef]
- Guo, P.; Qi, Y.; Zhu, C.; Wang, Q. Purification and Identification of Antioxidants Peptides from Chinese Cherry (Prunus pseudocerasus Lindl.) Seeds. J. Funct. Foods 2015, 19, 394–403. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; Alem, C.; Ennassir, J.; Benlyas, M.; Mbark, A.N.; Zegzouti, Y.F. Phytochemical Compositions and Antioxidants Capacity of Three Date (Phoenix dactylifera L.) Seeds Varieties Grown in the South East Morocco. J. Saudi Soc. Agric. Sci. 2017, 16, 350–357. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Kelly, M.E.; Bielinski, D.F.; Fisher, D.R. Tart Cherry Extracts Reduce Inflammatory and Oxidative Stress Signaling in Microglial Cells. Antioxidants 2016, 5, 33. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.S.; Berná, G.; Otaolaurruchi, E.; Troncoso, A.M.; García-Parrilla, M.C. Changes in Antioxidant Endogenous Enzymes (Activity and Gene Expression Levels) After Repeated Red Wine Intake. J. Agric. Food Chem. 2009, 57, 6578–6583. [Google Scholar] [CrossRef] [PubMed]
- Bianchini Silva, L.S.; Perasoli, F.B.; Caralho, K.V.; Vieira, K.M.; Paz Lopes, M.T.; Bianco de Souza, G.H.; Henrique dos Santos, O.D.; Freitas, K.M. Melaleuca Leucadendron (L.) L. Flower Extract Exhibits Antioxidants and Photoprotective Activities in Human Keratinocytes Exposed to Ultraviolet B Radiation. Free Radic. Biol. Med. 2020, 159, 54–65. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Moldovan, B.; Baldea, I.; Olteanu, D.; Bolfa, P.; Clichici, S.; Filip, G.A. Modulatory Effects of Corus sanguinea L. Mediated Green Synthesized Silver Nanoparticles on Oxidative Stress, COX-2/NOS2 and NKkB/pNFkB Expressions in Experimental Inflammation in Wistar Rats. Mater. Sci. Eng. C. 2020, 110, 110709. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xing, T.; Li, J.; Zhang, L.; Jiang, Y.; Gao, F. Hydrogen Peroxide-Induced Oxidative Stress Impairs Damages Aerobic Metabolism of Breast Muscle in Broilers. Poult. Sci. 2021, 100, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shi, A.; Dong, L.; Lu, X.; Wang, Y.; Zhao, J.; Dai, F.; Guo, X. Chlorogenic Acid Protects Against Liver Fibrosis In Vivo and In Vitro Through Inhibition of Oxidative Stress. Clin. Nutr. 2016, 35, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, J.; Zhao, G.; Lin, M.; Lang, Y.; Zhang, D.; Feng, D.; Tu, C. Apigenin Protects Human Melanocytes Against Oxidative Damage by Activation of the Nrf2 Pathway. Cell Stress Chaperones 2020, 25, 277–285. [Google Scholar] [CrossRef]
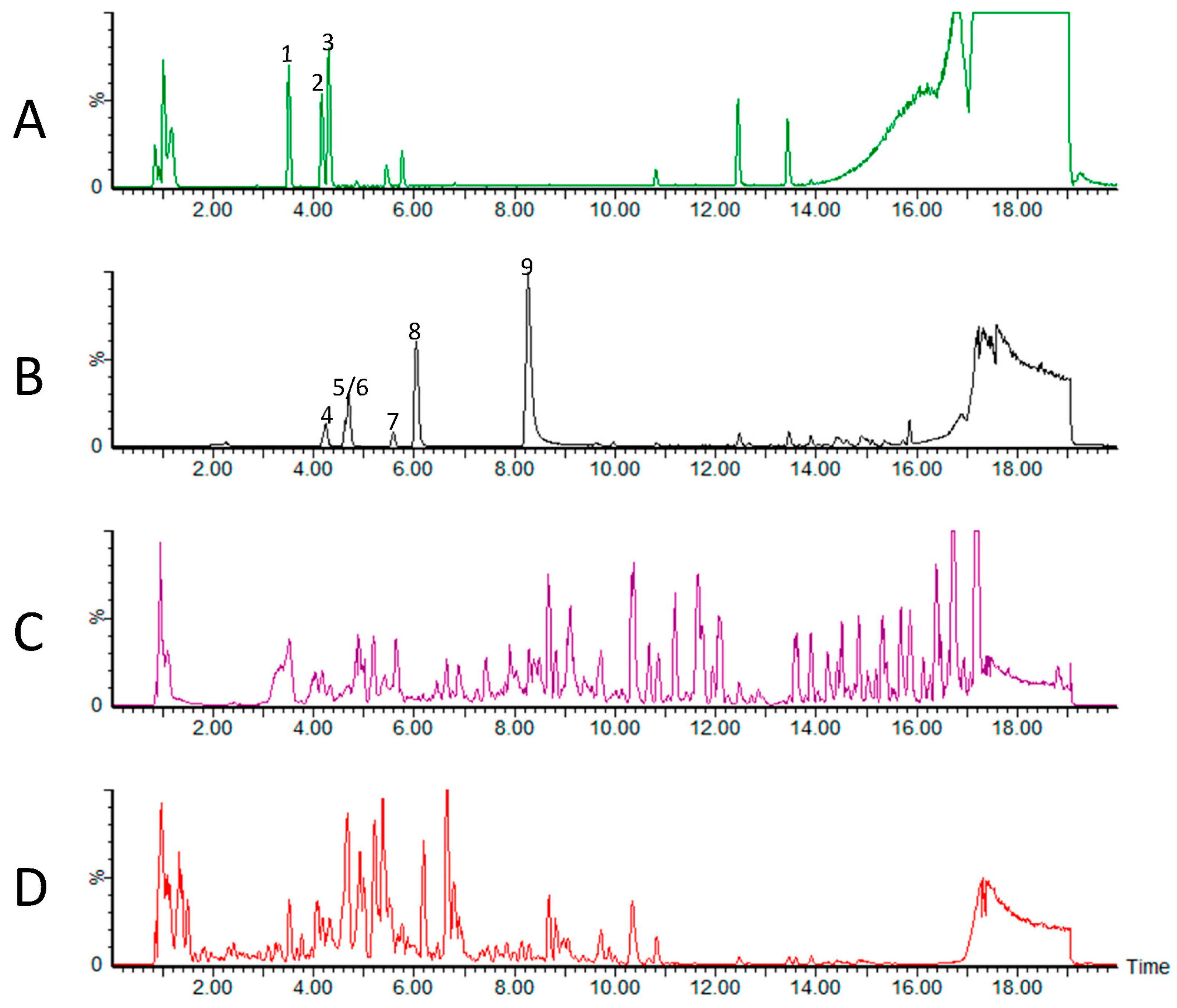



| Assay | CPW | CPE |
|---|---|---|
| TP Yield (mgCaffeic Acid Equivalent/gDried Pit) | 7.20 ± 0.25 | 15.36 ± 1.88 |
| TF Yield (mgQuercetin Equivalent/gDried Pit) | 6.95 ± 0.81 | 7.75 ± 1.11 |
| DPPH (mgDPPH/mLExtract) | 2.80 ± 0.76 | 6.77 ± 1.14 |
| ABTS (mgTrolox Equivalent/mLExtract) | 0.75 ± 0.04 | 1.79 ± 0.00 |
| Compound Name | Concentration in CPW (μg/mL) | Concentration in CPE (μg/mL) | Concentration in Freeze-Dried CPW in DMSO (μg/mL) | Concentration in Freeze-Dried CPE in DMSO (μg/mL) |
|---|---|---|---|---|
| p-Coumarylquinate isomer 2 | 10.42 | 13.84 | 50.08 | 80.38 |
| p-Coumarylquinate isomer 3 | 0.95 | 0.85 | ||
| p-Coumarylquinate isomer 1 | 4.43 | 5.45 | 23.67 | 36.34 |
| Chlorogenic acid | 4.89 | 17.43 | 18.07 | 11.52 |
| Cryptochlorogenic acid | 3.76 | 0.96 | 17.35 | 31.97 |
| Neochlorogenic acid | 2.69 | 0 | ||
| p-Coumaric acid | 0.87 | 2.69 | 1.69 | 6.7 |
| Amygdalin | 0.58 | 4.25 | 1.26 | 7.72 |
| Ferulic acid | 0.44 | 1.35 | 0.17 | 0.56 |
| Caffeic acid | 0.15 | 0.47 | 0.24 | 1.04 |
| Apigenin | 0.01 | 0.69 | 0.27 | 20.27 |
| Naringenin | 0.01 | 0.34 | 0.65 | 31.20 |
| Quercetin | 0 | 0.06 | 0.18 | 0.29 |
| Catechin | 0 | 2.54 | 0 | 2.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decot, H.; Sudhakaran, M.; Boismier, E.; Schilmiller, A.; Claucherty, E.; Doseff, A.I.; Aliakbarian, B. Tart Cherry (Prunus cerasus L.) Pit Extracts Protect Human Skin Cells against Oxidative Stress: Unlocking Sustainable Uses for Food Industry Byproducts. Foods 2023, 12, 3748. https://doi.org/10.3390/foods12203748
Decot H, Sudhakaran M, Boismier E, Schilmiller A, Claucherty E, Doseff AI, Aliakbarian B. Tart Cherry (Prunus cerasus L.) Pit Extracts Protect Human Skin Cells against Oxidative Stress: Unlocking Sustainable Uses for Food Industry Byproducts. Foods. 2023; 12(20):3748. https://doi.org/10.3390/foods12203748
Chicago/Turabian StyleDecot, Hannah, Meenakshi Sudhakaran, Emma Boismier, Anthony Schilmiller, Ethan Claucherty, Andrea I. Doseff, and Bahar Aliakbarian. 2023. "Tart Cherry (Prunus cerasus L.) Pit Extracts Protect Human Skin Cells against Oxidative Stress: Unlocking Sustainable Uses for Food Industry Byproducts" Foods 12, no. 20: 3748. https://doi.org/10.3390/foods12203748
APA StyleDecot, H., Sudhakaran, M., Boismier, E., Schilmiller, A., Claucherty, E., Doseff, A. I., & Aliakbarian, B. (2023). Tart Cherry (Prunus cerasus L.) Pit Extracts Protect Human Skin Cells against Oxidative Stress: Unlocking Sustainable Uses for Food Industry Byproducts. Foods, 12(20), 3748. https://doi.org/10.3390/foods12203748









