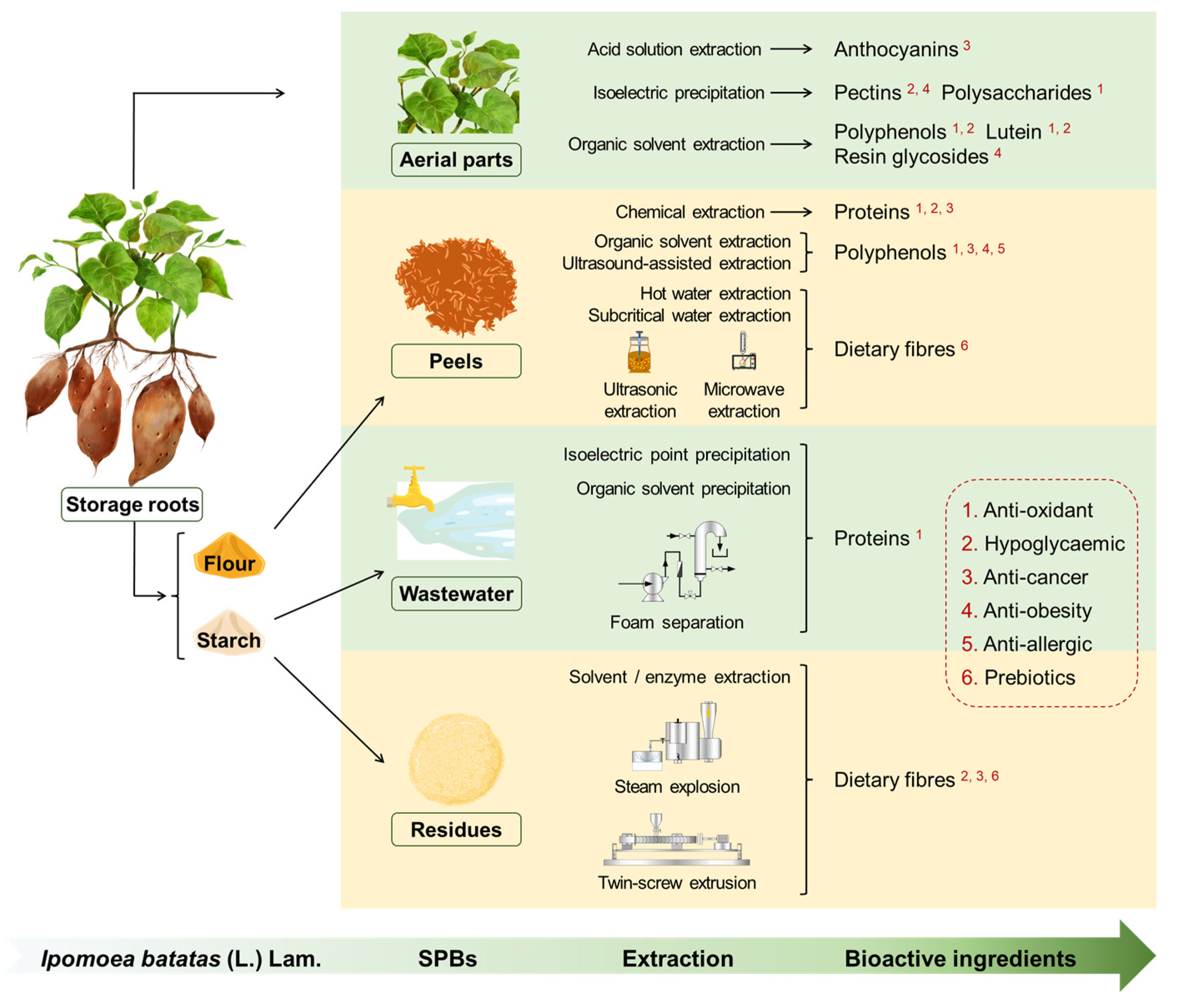
Reclaiming Agriceuticals from Sweetpotato (Ipomoea batatas [L.] Lam.) By-Products
Abstract
1. Introduction
2. SP Aerial Parts
2.1. Polyphenols
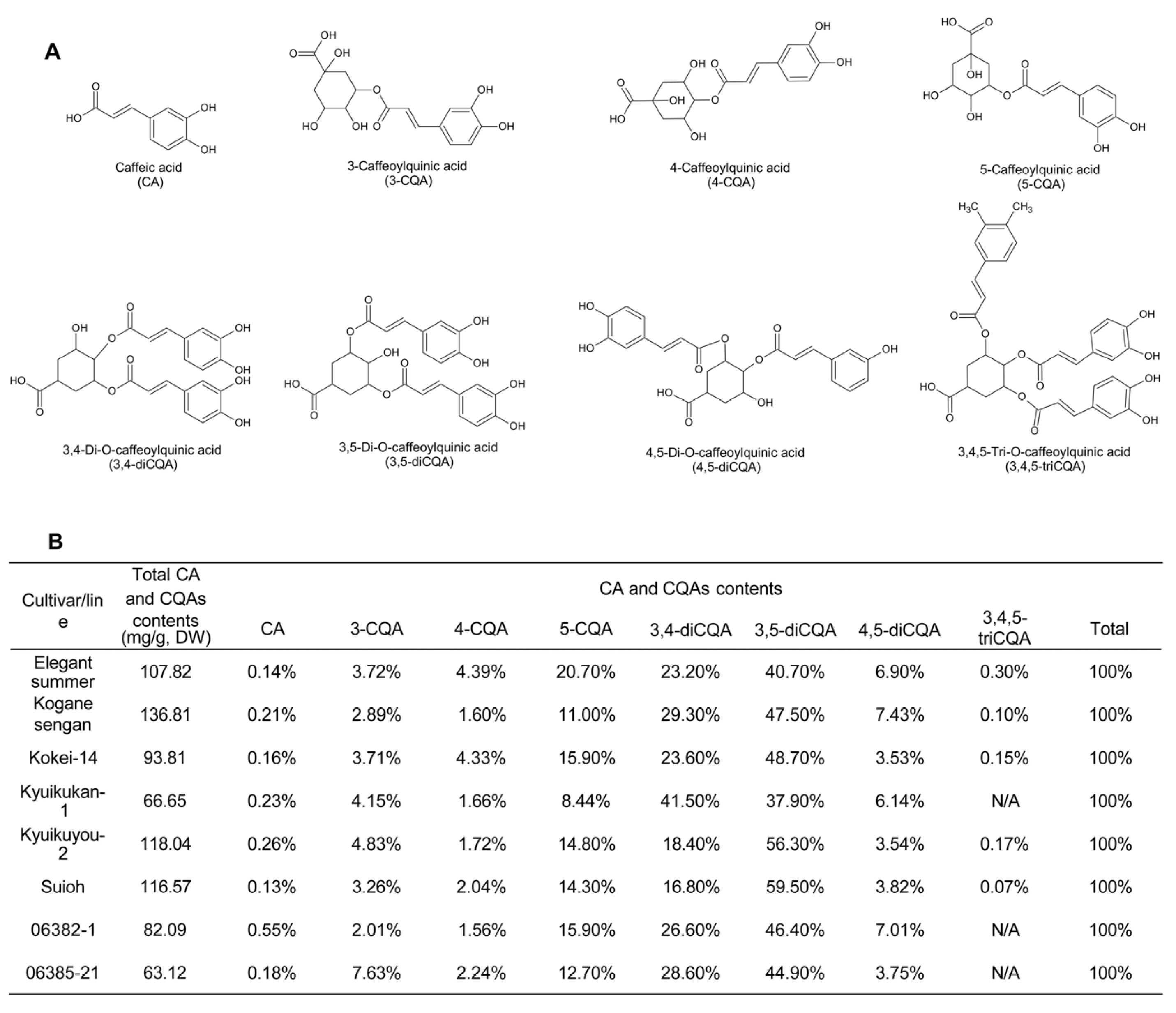
2.1.1. Types and Contents
2.1.2. Bioactivity of SP Leaf Polyphenols
2.1.3. Pretreatment and Extraction Methods
2.2. Functional Carbohydrates and Their Derivatives
2.2.1. Types and Contents
2.2.2. Bioactivities
2.2.3. Extraction Methods
2.3. Lutein
3. SP Storage Root Peels
3.1. Polyphenols
3.2. Dietary Fibres
3.3. Proteins
4. SPBs from Starch Processing
4.1. Proteins
4.1.1. Types and Contents
4.1.2. Functionality as Food Ingredients
4.1.3. Extraction Methods
4.2. Diet Fibres
4.2.1. Types and Contents
4.2.2. Bioactivities
4.2.3. Extraction Methods
5. Applications
6. Challenges and Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Potato Center. Sweet Potato. Available online: https://cipotato.org/sweetpotato/ (accessed on 22 January 2024).
- Food and Agriculture Organization of the United Nations. FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 January 2024).
- Qin, Y.; Naumovski, N.; Ranadheera, C.S.; D’Cunha, N.M. Nutrition-related health outcomes of sweet potato (Ipomoea batatas) consumption: A systematic review. Food Biosci. 2022, 50, 102208. [Google Scholar] [CrossRef]
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Escobar-Puentes, A.A.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Villegas-Ochoa, M.A.; González-Aguilar, G.A.; Olivas-Aguirre, F.J.; Wall-Medrano, A. Sweet potato (Ipomoea batatas L.) phenotypes: From agroindustry to health effects. Foods 2022, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.T.; Trierweiler, L.F.; Trierweiler, J.O. Food waste biorefinery advocating circular economy: Bioethanol and distilled beverage from sweet potato. J. Clean. Prod. 2020, 268, 121788. [Google Scholar] [CrossRef]
- Zhu, L.; Mu, T.; Ma, M.; Sun, H.; Zhao, G. Nutritional composition, antioxidant activity, volatile compounds, and stability properties of sweet potato residues fermented with selected lactic acid bacteria and bifidobacteria. Food Chem. 2022, 374, 131500. [Google Scholar] [CrossRef] [PubMed]
- Dereje, B.; Girma, A.; Mamo, D.; Chalchisa, T. Functional properties of sweet potato flour and its role in product development: A review. Int. J. Food Prop. 2020, 23, 1639–1662. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Li, B.; He, H.-J.; Mahdi, A.A.; Al-Ansi, W.; Saeed, A. An overview of the isolation, modification, physicochemical properties, and applications of sweet potato starch. Food Bioprocess Technol. 2024, 17, 1–32. [Google Scholar] [CrossRef]
- Akoetey, W.; Britain, M.M.; Morawicki, R.O. Potential use of byproducts from cultivation and processing of sweet potatoes. Ciênc. Rural 2017, 47, e20160610. [Google Scholar] [CrossRef]
- Hu, N.; Wu, Z.; Jin, L.; Li, Z.; Liu, W.; Huang, D.; Yang, C. Nanoparticle as a novel foam controller for enhanced protein separation from sweet potato starch wastewater. Sep. Purif. Technol. 2019, 209, 392–400. [Google Scholar] [CrossRef]
- El Sheikha, A.F.; Ray, R.C. Potential impacts of bioprocessing of sweet potato: Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 455–471. [Google Scholar] [CrossRef]
- Wang, S.; Nie, S.; Zhu, F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016, 89, 90–116. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Pace, R.D. Sweet potato leaves: Properties and synergistic interactions that promote health and prevent disease. Nutr. Rev. 2010, 68, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Vithu, P.; Dash, S.K.; Rayaguru, K. Post-harvest processing and utilization of sweet potato: A review. Food Rev. Int. 2019, 35, 726–762. [Google Scholar] [CrossRef]
- Sun, H.; Mu, T.; Xi, L.; Zhang, M.; Chen, J. Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. 2014, 156, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Mu, T.; Sun, H.; Richel, A.; Blecker, C. Valorization of the green waste parts from sweet potato (Impoea batatas L.): Nutritional, phytochemical composition, and bioactivity evaluation. Food Sci. Nutr. 2020, 8, 4086–4097. [Google Scholar] [CrossRef] [PubMed]
- Maloney, K.P.; Truong, V.-D.; Allen, J.C. Susceptibility of sweet potato (Ipomoea batatas) peel proteins to digestive enzymes. Food Sci. Nutr. 2014, 2, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Mu, T.-H.; Han, J.-J. Composition and physicochemical properties of dietary fiber extracted from residues of 10 varieties of sweet potato by a sieving method. J. Agric. Food Chem. 2010, 58, 7305–7310. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.-H.; Sun, H.-N. Chapter 22—Sweet potato leaf polyphenols: Preparation, individual phenolic compound composition and antioxidant activity. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA; pp. 365–380. [CrossRef]
- Truong, V.D.; McFeeters, R.F.; Thompson, R.T.; Dean, L.L.; Shofran, B. Phenolic acid content and composition in leaves and roots of common commercial sweetpotato (Ipomea batatas L.) cultivars in the United States. J. Food Sci. 2007, 72, C343–C349. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, L.; Hu, B.; Sun, Y.; Ye, H.; Ma, D.; Zeng, X. TPC in the leaves of 116 sweet potato (Ipomoea batatas L.) varieties and Pushu 53 leaf extracts. J. Food Compos. Anal. 2010, 23, 599–604. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, L.; Liu, Y.; Zhang, A.; Xiao, S.; Dai, X.; Yuan, R.; Zhou, Z.; Cao, Q. Metabolomic and transcriptomic analyses of the flavonoid biosynthetic pathway for the accumulation of anthocyanins and other flavonoids in sweetpotato root skin and leaf vein base. J. Agric. Food Chem. 2022, 70, 2574–2588. [Google Scholar] [CrossRef]
- Makori, S.I.; Mu, T.-H.; Sun, H.-N. Total polyphenol content, antioxidant activity, and individual phenolic composition of different edible parts of 4 sweet potato cultivars. Nat. Prod. Commun. 2020, 15, 1934578X20936931. [Google Scholar] [CrossRef]
- Luo, D.; Mu, T.; Sun, H. Profiling of phenolic acids and flavonoids in sweet potato (Ipomoea batatas L.) leaves and evaluation of their anti-oxidant and hypoglycemic activities. Food Biosci. 2021, 39, 100801. [Google Scholar] [CrossRef]
- Sasaki, K.; Oki, T.; Kai, Y.; Nishiba, Y.; Okuno, S. Effect of repeated harvesting on the content of caffeic acid and seven species of caffeoylquinic acids in sweet potato leaves. Biosci. Biotechnol. Biochem. 2015, 79, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Du, W.; Zhu, Y.; Du, X.; Song, C.; Chen, X.; Fang, X.; Cao, Q.; Ma, D.; Wang, Y.; et al. Composition and bioactivity of chlorogenic acids in vegetable and conventional sweet potato vine tips. Foods 2023, 12, 3910. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Griffin, J.; Xu, J.; Ouyang, P.; Zhao, Z.; Wang, W. Identification and quantification of anthocyanins in purple-fleshed sweet potato leaves. Heliyon 2019, 5, e01964. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lin, Z.; Zhang, H.; Liu, Z.; Xu, Y.; Xu, G.; Li, H.; Ji, R.; Luo, W.; Qiu, Y.; et al. Anthocyanin accumulation in the leaves of the purple sweet potato (Ipomoea batatas L.) cultivars. Molecules 2019, 24, 3743. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Taira, K.; Ohmine, W.; Nagata, J. Mineral determination and anti-LDL oxidation activity of sweet potato (Ipomoea batatas L.) leaves. J. Food Compos. Anal. 2013, 29, 117–125. [Google Scholar] [CrossRef]
- Jia, R.; Tang, C.; Chen, J.; Zhang, X.; Wang, Z. Total phenolics and anthocyanins contents and antioxidant activity in four different aerial parts of leafy sweet potato (Ipomoea batatas L.). Molecules 2022, 27, 3117. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Song, C.; Liu, J.; Chen, F.; Zhu, Y.; Fang, X.; Cao, Q.; Ma, D.; Wang, Y.; Zhang, C. Chlorogenic acid modulates autophagy by inhibiting the activity of ALKBH5 demethylase, thereby ameliorating hepatic steatosis. J. Agric. Food Chem. 2023, 71, 15073–15086. [Google Scholar] [CrossRef]
- Liao, W.C.; Lai, Y.-C.; Yuan, M.-C.; Hsu, Y.-L.; Chan, C.-F. Antioxidative activity of water extract of sweet potato leaves in Taiwan. Food Chem. 2011, 127, 1224–1228. [Google Scholar] [CrossRef]
- Vishnu, V.R.; Renjith, R.S.; Mukherjee, A.; Anil, S.R.; Sreekumar, J.; Jyothi, A.N. Comparative study on the chemical structure and in vitro antiproliferative activity of anthocyanins in purple root tubers and leaves of sweet potato (Ipomoea batatas). J. Agric. Food Chem. 2019, 67, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Magnuson, B.A.; Lala, G.; Tian, Q.; Schwartz, S.J.; Giusti, M.M. Intact anthocyanins and metabolites in rat urine and plasma after 3 months of anthocyanin supplementation. Nutr. Cancer 2006, 54, 3–12. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar Cipriano, P.; Kim, H.; Fang, C.; Paula Venancio, V.; Mertens-Talcott, S.U.; Talcott, S.T. In vitro digestion, absorption and biological activities of acylated anthocyanins from purple sweet potatoes (Ipomoea batatas). Food Chem. 2022, 374, 131076. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, Z.-f.; Tu, Z.c.; Wang, H.; Zhang, L.; Xie, X.; Liu, G.-x. Influence of in vitro gastrointestinal digestion on the bioavailability and antioxidant activity of polyphenols from Ipomoea batatas leaves. Int. J. Food Sci. Technol. 2017, 52, 1131–1137. [Google Scholar] [CrossRef]
- Jeng, T.L.; Lai, C.C.; Liao, T.C.; Lin, S.Y.; Sung, J.M. Effects of drying on caffeoylquinic acid derivative content and antioxidant capacity of sweet potato leaves. J. Food Drug Anal. 2015, 23, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tu, Z.; Xiao, H.; Li, Z.; Zhang, Q.; Wang, H.; Hu, Y.; Zhang, L. Dynamic high pressure microfluidization-assisted extraction and antioxidant activities of sweet potato (Ipomoea batatas L.) leaves flavonoid. Food Bioprod. Process. 2013, 91, 1–6. [Google Scholar] [CrossRef]
- Fu, Z.-F.; Tu, Z.-C.; Zhang, L.; Wang, H.; Wen, Q.-H.; Huang, T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 2016, 15, 11–18. [Google Scholar] [CrossRef]
- Sun, H.-N.; Mu, T.-H.; Xi, L.-S. Effect of pH, heat, and light treatments on the antioxidant activity of sweet potato leaf polyphenols. Int. J. Food Prop. 2017, 20, 318–332. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Liu, S. (Eds.) Functional Carbohydrates: Development, Characterization, and Biomanufacture, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Food-derived carbohydrates—Structural complexity and functional diversity. Crit. Rev. Biotechnol. 2002, 22, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Mosha, T.C.; Pace, R.D.; Adeyeye, S.; Mtebe, K.; Laswai, H. Proximate composition and mineral content of selected Tanzanian vegetables and the effect of traditional processing on the retention of ascorbic acid, riboflavin and thiamine. Plant Foods Hum. Nutr. 1995, 48, 235–245. [Google Scholar] [CrossRef]
- Ishida, H.; Suzuno, H.; Sugiyama, N.; Innami, S.; Tadokoro, T.; Maekawa, A. Nutritive evaluation on chemical components of leaves, stalks and stems of sweet potatoes (Ipomoea batatas poir). Food Chem. 2000, 68, 359–367. [Google Scholar] [CrossRef]
- Yi Hui Toy, J.; Wei See, J.; Huang, D. Physicochemical and functional characterisation of pectin from margarita sweet potato leaves. Food Chem. 2022, 385, 132684. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Chen, R.; Zhang, Y.; Bai, H.; Tian, L.; Sun, H.; Li, D.; Wu, W. Comparison in structural, physicochemical and functional properties of sweet potato stems and leaves polysaccharide conjugates from different technologies. Int. J. Biol. Macromol. 2023, 247, 125730. [Google Scholar] [CrossRef] [PubMed]
- Toy, J.Y.H.; Song, Z.; Huang, D. Resin glycosides in aerial parts of Ipomoea batatas are potent lipase inhibitors: Potential upcycling of sweet potato by-products to combat obesity. Food Funct. 2022, 13, 5353–5364. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wu, F.; Chen, K.; Pan, B.; Yin, X.; You, Y.; Song, Z.; Li, D.; Huang, D. Sweet potato extract alleviates high-fat-diet-induced obesity in C57BL/6J mice, but not by inhibiting pancreatic lipases. Front. Nutr. 2022, 9, 1016020. [Google Scholar] [CrossRef] [PubMed]
- Hannoufa, A.; Hossain, Z. Regulation of carotenoid accumulation in plants. Biocatal. Agric. Biotechnol. 2012, 1, 198–202. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Young, J.; Zhu, H.; Manolis, T. Separation of geometric isomers of native lutein diesters in marigold (Tagetes erecta L.) by high-performance liquid chromatography-mass spectrometry. J. Chromatogr. A 2004, 1045, 65–70. [Google Scholar] [CrossRef]
- Li, M.; Jang, G.Y.; Lee, S.H.; Kim, M.Y.; Hwang, S.G.; Sin, H.M.; Kim, H.S.; Lee, J.; Jeong, H.S. Comparison of functional components in various sweet potato leaves and stalks. Food Sci. Biotechnol. 2017, 26, 97–103. [Google Scholar] [CrossRef]
- Krishna, T.U.; Pragalyaashree, M.M.; Balamurugan, P. Extraction and quantification of lutein from sweet potato leaves (Ipomoea batatas). Drug Invent. Today 2018, 10, 2618–2621. [Google Scholar]
- Johnson, E.J. A biological role of lutein. Food Rev. Int. 2004, 20, 1–16. [Google Scholar] [CrossRef]
- Becerra, M.; Contreras, L.; Lo, M.; Mateos-Díaz, J.; Herrera, G. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Safiyyu’d-din Bin Hisamuddin, A.; Naomi, R.; Manan, K.A.B.; Bahari, H.; Othman, F.; Embong, H.; Ismail, A.; Ahmed, Q.U.; Jumidil, S.H.; Hussain, M.K.; et al. The role of lutein-rich purple sweet potato leaf extract on the amelioration of diabetic retinopathy in streptozotocin-induced Sprague–Dawley rats. Front. Pharmacol. 2023, 14, 1175907. [Google Scholar] [CrossRef] [PubMed]
- Safiyyu’d-din Bin Hisamuddin, A.; Naomi, R.; Bin Manan, K.A.; Bahari, H.; Yazid, M.D.; Othman, F.; Embong, H.; Hadizah Jumidil, S.; Hussain, M.K.; Zakaria, Z.A. Phytochemical component and toxicological evaluation of purple sweet potato leaf extract in male Sprague–Dawley rats. Front. Pharmacol. 2023, 14, 1132087. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Carotenoids of biotechnological importance. Adv. Biochem. Eng. Biotechnol. 2015, 148, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Sandhu, K.S. Sweet potato flour and starch. In Tropical Roots and Tubers; Wiley: Hoboken, NJ, USA, 2016; pp. 479–506. [Google Scholar] [CrossRef]
- Shaari, N.; Shamsudin, R.; Nor, M.Z.M.; Hashim, N. Quality attributes of Malaysia purple-fleshed sweet potato at different peel condition. Agronomy 2021, 11, 872. [Google Scholar] [CrossRef]
- Šeregelj, V.; Ćetković, G.; Čanadanović-Brunet, J.; Tumbas Šaponjac, V.; Vulić, J.; Stajčić, S. Encapsulation and degradation kinetics of bioactive compounds from sweet potato peel during storage. Food Technol. Biotechnol. 2020, 58, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Anastácio, A.; Carvalho, I.S. Phenolics extraction from sweet potato peels: Key factors screening through a Placket–Burman design. Ind. Crops Prod. 2013, 43, 99–105. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L.A. Bioactive and functional properties of purple sweetpotato (Ipomoea batatas (L.) Lam). Acta Hortic. 2002, 583, 195–203. [Google Scholar] [CrossRef]
- Althwab, S.A.; Mousa, H.M.; El-Zaha, K.M.; Zaher, A.A. Protective effect of sweet potato peel against oxidative stress in hyperlipidemic albino rats. Food Nutr. Sci. 2019, 10, 503–516. [Google Scholar] [CrossRef]
- Anastácio, A.; Silva, R.; Carvalho, I.S. Phenolics extraction from sweet potato peels: Modelling and optimization by response surface modelling and artificial neural network. J. Food Sci. Technol. 2016, 53, 4117–4125. [Google Scholar] [CrossRef]
- Phomkaivon, N.; Amilia, N.; Tanintaratan, W.; Yonekura, L.; Tamura, H. Polyphenols in Naruto Kintoki sweet potato enhanced antiallergic activity after baking and microwave cooking. Food Sci. Technol. Res. 2022, 28, 275–283. [Google Scholar] [CrossRef]
- Oluyori, A.P.; Shaw, A.K.; Olatunji, G.A.; Rastogi, P.; Meena, S.; Datta, D.; Arora, A.; Reddy, S.; Puli, S. Sweet potato peels and cancer prevention. Nutr. Cancer 2016, 68, 1330–1337. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Sousa, V.M.; Rodrigues, S.; de Brito, E.S.; Fernandes, F.A.N. Green ultrasound-assisted extraction of chlorogenic acids from sweet potato peels and sonochemical hydrolysis of caffeoylquinic acids derivatives. Ultrason. Sonochemistry 2020, 63, 104911. [Google Scholar] [CrossRef] [PubMed]
- Bovell-Benjamin, A.C.; Hathorn, C.; Gichuhi, P. Preparation and Characterization of Sweetpotato Peels for Use as Dietary Fiber Enhancement in Space Foods; SAE Technical Paper 2007-01-3053; SAE International: Warrendale, PA, USA, 2007. [Google Scholar]
- Cao, Y.; Tian, B.; Zhang, Z.; Yang, K.; Cai, M.; Hu, W.; Guo, Y.; Xia, Q.; Wu, W. Positive effects of dietary fiber from sweet potato [Ipomoea batatas (L.) Lam.] peels by different extraction methods on human fecal microbiota in vitro fermentation. Front. Nutr. 2022, 9, 986667. [Google Scholar] [CrossRef]
- Maloney, K.P.; Truong, V.-D.; Allen, J.C. Chemical optimization of protein extraction from sweet potato (Ipomoea batatas) peel. J. Food Sci. 2012, 77, E307–E312. [Google Scholar] [CrossRef]
- Zhu, F.; Yang, X.; Cai, Y.-Z.; Bertoft, E.; Corke, H. Physicochemical properties of sweetpotato starch. Starch—Stärke 2011, 63, 249–259. [Google Scholar] [CrossRef]
- Cervantes-Flores, J.C.; Sosinski, B.; Pecota, K.V.; Mwanga, R.O.M.; Catignani, G.L.; Truong, V.D.; Watkins, R.H.; Ulmer, M.R.; Yencho, G.C. Identification of quantitative trait loci for dry-matter, starch, and β-carotene content in sweetpotato. Mol. Breed. 2011, 28, 201–216. [Google Scholar] [CrossRef]
- Vithu, P.; Dash, S.K.; Rayaguru, K.; Panda, M.K.; Nedunchezhiyan, M. Optimization of starch isolation process for sweet potato and characterization of the prepared starch. J. Food Meas. Charact. 2020, 14, 1520–1532. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, H.; Li, H.; Li, Y. Analysis on the nutrition composition and antioxidant activity of different types of sweet potato cultivars. Food Nutr. Sci. 2015, 6, 161–167. [Google Scholar] [CrossRef]
- Mu, T.-H.; Tan, S.-S.; Xue, Y.-L. The amino acid composition, solubility and emulsifying properties of sweet potato protein. Food Chem. 2009, 112, 1002–1005. [Google Scholar] [CrossRef]
- Sun, M.; Mu, T.; Zhang, M.; Arogundade, L.A. Nutritional assessment and effects of heat processing on digestibility of Chinese sweet potato protein. J. Food Compos. Anal. 2012, 26, 104–110. [Google Scholar] [CrossRef]
- Yang, S.; Liu, H.; Liao, X.; Kong, X.; Xu, Z. Extraction and profiling of proteins in yellow powder from sweet potato starch wastewater using response surface methodology and proteomic approach. J. Food Sci. 2022, 87, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhang, Y.-F.; Zeng, Z.-Q.; Lin, J.; Zhang, Y.-W.; Ni, H.; Li, H.-H. Screening, separating, and completely recovering polyphenol oxidases and other biochemicals from sweet potato wastewater in starch production. Appl. Microbiol. Biotechnol. 2015, 99, 1745–1753. [Google Scholar] [CrossRef]
- Li, Q.-R.; Luo, J.-L.; Zhou, Z.-H.; Wang, G.-Y.; Chen, R.; Cheng, S.; Wu, M.; Li, H.; Ni, H.; Li, H.-H. Simplified recovery of enzymes and nutrients in sweet potato wastewater and preparing health black tea and theaflavins with scrap tea. Food Chem. 2018, 245, 854–862. [Google Scholar] [CrossRef]
- Bovell-Benjamin, A.C. Sweet potato: A review of its past, present, and future role in human nutrition. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2007; Volume 52, pp. 1–59. [Google Scholar]
- Hou, W.-C.; Lin, Y.-H. Dehydroascorbate reductase and monodehydroascorbate reductase activities of trypsin inhibitors, the major sweet potato (Ipomoea batatas [L.] Lam) root storage protein. Plant Sci. 1997, 128, 151–158. [Google Scholar] [CrossRef]
- Huang, G.J.; Chen, H.-J.; Chang, Y.-S.; Sheu, M.-J.; Lin, Y.-H. Recombinant sporamin and its synthesized peptides with antioxidant activities in vitro. Bot. Stud. 2007, 48, 133–140. [Google Scholar]
- Lu, G.; Gao, Q. Chapter 37—Use of sweet potato in bread and flour fortification. In Flour and Breads and Their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 407–416. [Google Scholar] [CrossRef]
- Chen, H.-J.; Liang, S.-H.; Huang, G.-J.; Lin, Y.-H. Sweet potato cysteine proteases SPAE and SPCP2 participate in sporamin degradation during storage root sprouting. J. Plant Physiol. 2015, 186–187, 39–49. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, T.-S.; Mu, T.-H. Production and in vitro gastrointestinal digestion of antioxidant peptides from enzymatic hydrolysates of sweet potato protein affected by pretreatment. Plant Foods Hum. Nutr. 2019, 74, 225–231. [Google Scholar] [CrossRef]
- Dziedzoave, N.T.; Graffham, A.J.; Westby, A.; Otoo, J.; Komlaga, G. Influence of variety and growth environment on β-amylase activity of flour from sweet potato (Ipomea batatas). Food Control 2010, 21, 162–165. [Google Scholar] [CrossRef]
- Mu, T.-H.; Liu, Y.; Zhang, M.; Sun, H.-N. Protein recovery from sweet potato starch wastewater by foam separation. Sep. Sci. Technol. 2014, 49, 2255–2260. [Google Scholar] [CrossRef]
- Jin Yanling, Z.H.Z.F. Value-added utilization of sweet potato starch residue: A review. J. Agric. 2021, 11, 98–103. [Google Scholar]
- Liu, M.; Li, X.; Zhou, S.; Wang, T.T.Y.; Zhou, S.; Yang, K.; Li, Y.; Tian, J.; Wang, J. Dietary fiber isolated from sweet potato residues promotes a healthy gut microbiome profile. Food Funct. 2020, 11, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhou, S.; Li, Y.; Tian, J.; Zhang, C. Structure, physicochemical properties and effects on nutrients digestion of modified soluble dietary fiber extracted from sweet potato residue. Food Res. Int. 2021, 150, 110761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, W.; Duan, L.; Zhu, H.; Wang, H.; Wang, J.; Sun, J.; Niu, F. Protective effect of dietary fiber from sweet potato (Ipomoea batatas L.) against lead-induced renal injury by inhibiting oxidative stress via AMPK/SIRT1/PGC1α signaling pathways. J. Food Biochem. 2018, 42, e12513. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Mu, T.-H.; Zhang, M. Optimisation of acid extraction of pectin from sweet potato residues by response surface methodology and its antiproliferation effect on cancer cells. Int. J. Food Sci. Technol. 2013, 48, 778–785. [Google Scholar] [CrossRef]
- Wang, T.; Liang, X.; Ran, J.; Sun, J.; Jiao, Z.; Mo, H. Response surface methodology for optimisation of soluble dietary fibre extraction from sweet potato residue modified by steam explosion. Int. J. Food Sci. Technol. 2017, 52, 741–747. [Google Scholar] [CrossRef]
- Lu, H.; Gui, Y.; Zheng, L.; Liu, X. Morphological, crystalline, thermal and physicochemical properties of cellulose nanocrystals obtained from sweet potato residue. Food Res. Int. 2013, 50, 121–128. [Google Scholar] [CrossRef]
- Zhang, C.; Mu, T. Optimisation of pectin extraction from sweet potato (Ipomoea batatas, Convolvulaceae) residues with disodium phosphate solution by response surface method. Int. J. Food Sci. Technol. 2011, 46, 2274–2280. [Google Scholar] [CrossRef]
- Qiao, H.; Shao, H.; Zheng, X.; Liu, J.; Liu, J.; Huang, J.; Zhang, C.; Liu, Z.; Wang, J.; Guan, W. Modification of sweet potato (Ipomoea batatas Lam.) residues soluble dietary fiber following twin-screw extrusion. Food Chem. 2021, 335, 127522. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Gawlik-Dziki, U. Soymilk enriched with green coffee phenolics—Antioxidant and nutritional properties in the light of phenolics-food matrix interactions. Food Chem. 2017, 223, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.J.L.; Yu, J.; Zhou, W.; Liu, M.H. Effects of anthocyanins on bread microstructure, and their combined impact on starch digestibility. Food Chem. 2022, 374, 131744. [Google Scholar] [CrossRef] [PubMed]
- Bodjrenou, D.M.; Li, X.; Lu, X.; Lei, S.; Zheng, B.; Zeng, H. Resistant starch from sweet potatoes: Recent advancements and applications in the food sector. Int. J. Biol. Macromol. 2023, 225, 13–26. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Castro, L.M.G.; Moreira, S.A.; Pintado, M.; Saraiva, J.A. Comparison of emerging technologies to extract high-added value compounds from fruit residues: Pressure- and electro-based technologies. Food Eng. Rev. 2017, 9, 190–212. [Google Scholar] [CrossRef]
- Angoy, A.; Ginies, C.; Goupy, P.; Bornard, I.; Ginisty, P.; Sommier, A.; Valat, M.; Chemat, F. Development of a green innovative semi-industrial scale pilot combined microwave heating and centrifugal force to extract essential oils and phenolic compounds from orange peels. Innov. Food Sci. Emerg. Technol. 2020, 61, 102338. [Google Scholar] [CrossRef]
- García-Pérez, J.S.; Cuéllar-Bermúdez, S.P.; Cruz-Quiroz, R.d.l.; Arévalo-Gallegos, A.; Esquivel-Hernandez, D.A.; Rodríguez-Rodríguez, J.; García-García, R.; Iqbal, H.M.N.; Parra-Saldivar, R. Supercritical CO2-based tailor made valorization of Origanum vulgare L extracts: A green approach to extract high-value compounds with applied perspectives. J. Environ. Manag. 2019, 232, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, É.R.; Silva, R.F.; Santos, P.R.; Queiroz, F. Potential of alternative solvents to extract biologically active compounds from green coffee beans and its residue from the oil industry. Food Bioprod. Process. 2019, 115, 47–58. [Google Scholar] [CrossRef]
- Lu, X.-F.; Zhou, Y.; Ren, Y.-P.; Zhang, J. Improved sample treatment for the determination of flavonoids and polyphenols in sweet potato leaves by ultra performance convergence chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 169, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, C.; Zhou, C.; Wen, Z.; Dong, X. An improved microwave-assisted extraction of anthocyanins from purple sweet potato in favor of subsequent comprehensive utilization of pomace. Food Bioprod. Process. 2019, 115, 1–9. [Google Scholar] [CrossRef]
- Rodríguez-Mena, A.; Ochoa-Martínez, L.A.; González-Herrera, S.M.; Rutiaga-Quiñones, O.M.; González-Laredo, R.F.; Olmedilla-Alonso, B.; Vega-Maturino, S. Coloring potential of anthocyanins from purple sweet potato paste: Ultrasound-assisted extraction, enzymatic activity, color and its application in ice pops. Food Chem. Adv. 2023, 3, 100358. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Qin, D.; Xi, J. Flash extraction: An ultra-rapid technique for acquiring bioactive compounds from plant materials. Trends Food Sci. Technol. 2021, 112, 581–591. [Google Scholar] [CrossRef]
- Cheng, Z.; Song, H.; Yang, Y.; Zhou, H.; Liu, Y.; Liu, Z. Smashing tissue extraction of five lignans from the fruit of Schisandra chinensis. J. Chromatogr. Sci. 2016, 54, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Boyadzhiev, L.; Dimitrov, K.; Metcheva, D. Integration of solvent extraction and liquid membrane separation: An efficient tool for recovery of bio-active substances from botanicals. Chem. Eng. Sci. 2006, 61, 4126–4128. [Google Scholar] [CrossRef]

| Recyclable Part | Cultivar | Moisture | Crude Fibre | Crude Protein | Crude Fat | Ash | Dietary Fibre | Carbohydrate | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Aerial part | Ximeng No. 1 | 88.70 ± 1.81 | 12.76 ± 0.05 | 25.66 ± 0.63 | 3.06 ± 0.15 | - | - | - | [16] |
| Jinyu No. 1 | 88.10 ± 2.03 | 11.28 ± 0.02 | 27.53 ± 0.33 | 3.43 ± 0.06 | - | - | - | ||
| Jishu | 87.60 ± 0.23 | 11.26 ± 0.06 | 29.27 ± 0.02 | 3.99 ± 0.11 | - | - | - | ||
| Shi No. 5 | 87.95 ± 1.85 | 11.06 ± 0.07 | 31.08 ± 0.09 | 5.13 ± 0.09 | - | - | - | ||
| Xushu No. 55-2 | 87.85 ± 0.12 | 10.62 ± 0.05 | 29.08 ± 0.35 | 4.88 ± 0.12 | - | - | - | ||
| Jishu No. 22 | 87.57 ± 0.58 | 12.98 ± 0.07 | 27.15 ± 0.13 | 4.90 ± 0.04 | - | - | - | ||
| Yanshu No. 25 | 87.33 ± 0.93 | 11.26 ± 0.05 | 23.46 ± 0.21 | 4.08 ± 0.06 | - | - | - | ||
| Xushu No. 23 | 84.54 ± 0.66 | 11.36 ± 0.00 | 30.53 ± 0.32 | 4.95 ± 0.06 | - | - | - | ||
| Sushu No. 14 | 87.63 ± 0.16 | 11.03 ± 0.10 | 26.75 ± 0.16 | 4.47 ± 0.15 | - | - | - | ||
| Wanshu No. 5 | 86.79 ± 0.19 | 12.45 ± 0.17 | 27.20 ± 0.12 | 5.23 ± 0.18 | - | - | - | ||
| Longshu No. 9 | 86.25 ± 0.69 | 13.00 ± 0.02 | 25.71 ± 0.04 | 4.90 ± 0.12 | - | - | - | ||
| Hongxinwang | 87.52 ± 0.31 | 10.55 ± 0.54 | 24.72 ± 0.17 | 3.71 ± 0.08 | - | - | - | ||
| Xushu No. 053601 | 88.92 ± 0.34 | 10.04 ± 0.50 | 23.43 ± 0.11 | 3.75 ± 0.01 | - | - | - | ||
| Nongda No. 6-2 | 88.84 ± 1.02 | 9.86 ± 0.35 | 24.21 ± 0.17 | 3.84 ± 0.16 | - | - | - | ||
| Miyuan No. 6 | 88.59 ± 0.53 | 9.25 ± 0.38 | 23.49 ± 0.43 | 3.97 ± 0.04 | - | - | - | ||
| Yuzi No. 7 | 87.52 ± 0.20 | 10.68 ± 1.15 | 21.12 ± 0.25 | 2.24 ± 0.08 | - | - | - | ||
| Beijing No. 553 | 86.75 ± 0.87 | 9.71 ± 1.50 | 22.03 ± 0.01 | 5.17 ± 0.10 | - | - | - | ||
| Xinong No.1 | 87.78 ± 0.62 | 10.19 ± 0.85 | 18.35 ± 0.01 | 5.28 ± 0.15 | - | - | - | ||
| Jishu No.04150 | 87.82 ± 1.16 | 10.24 ± 0.69 | 23.18 ± 0.13 | 4.22 ± 0.04 | - | - | - | ||
| Pushu No.53 | 88.28 ± 1.02 | 11.33 ± 0.46 | 24.04 ± 0.11 | 4.39 ± 0.16 | - | - | - | ||
| Xushu No. 22-1 | 86.81 ± 0.22 | 11.88 ± 0.93 | 22.96 ± 0.25 | 2.08 ± 0.06 | - | - | - | ||
| Shangshu No. 19 (spring) | 88.56 ± 0.14 | 10.01 ± 0.75 | 16.69 ± 0.09 | 2.94 ± 0.10 | - | - | - | ||
| Shangshu No. 19 (summer) | 87.85 ± 0.65 | 9.15 ± 0.49 | 17.92 ± 0.11 | 2.85 ± 0.16 | - | - | - | ||
| Sushu No. 16 | 84.09 ± 0.81 | 12.70 ± 0.35 | 27.55 ± 0.35 | 2.37 ± 0.08 | - | - | - | ||
| Chuanshu No. 294 | 87.76 ± 0.14 | 12.32 ± 0.74 | 28.57 ± 0.04 | 2.53 ± 0.01 | - | - | - | ||
| Xinxiang No. 1 | 86.33 ± 0.90 | 13.11 ± 0.72 | 28.62 ± 0.08 | 2.42 ± 0.03 | - | - | - | ||
| Xushu No. 038008 | 86.75 ± 3.31 | 11.54 ± 0.68 | 25.94 ± 0.06 | 3.17 ± 0.04 | - | - | - | ||
| Yanzi No. 337 | 88.65 ± 2.56 | 10.33 ± 0.79 | 23.77 ± 0.19 | 3.57 ± 0.12 | - | - | - | ||
| Shanchuanzi | 88.76 ± 1.44 | 11.26 ± 1.19 | 21.46 ± 0.13 | 3.25 ± 0.06 | - | - | - | ||
| Pushu No. 17 | 88.89 ± 1.69 | 14.26 ± 0.38 | 18.62 ± 0.11 | 3.16 ± 0.01 | - | - | - | ||
| Jinong No. 2694 | 86.20 ± 1.44 | 10.82 ± 1.28 | 25.26 ± 0.26 | 3.31 ± 0.08 | - | - | - | ||
| Fushu No. 2 | 88.53 ± 2.36 | 12.10 ± 1.02 | 24.59 ± 0.33 | 3.81 ± 0.08 | - | - | - | ||
| Ningzi No. 23-1 | 88.45 ± 2.19 | 13.00 ± 1.02 | 22.76 ± 0.35 | 3.54 ± 0.01 | - | - | - | ||
| Langshu No. 7-12 | 88.42 ± 1.90 | 12.40 ± 0.58 | 22.25 ± 0.01 | 3.89 ± 0.02 | - | - | - | ||
| Jingshu No. 6 | 87.24 ± 2.64 | 12.70 ± 0.49 | 23.76 ± 0.07 | 3.27 ± 0.06 | - | - | - | ||
| Ningzi No. 1 | 87.53 ± 2.55 | 13.59 ± 1.00 | 22.45 ± 0.26 | 3.37 ± 0.07 | - | - | - | ||
| Yuzi No. 263 | 87.93 ± 0.37 | 13.13 ± 0.67 | 22.76 ± 0.01 | 3.22 ± 0.02 | - | - | - | ||
| Xushu No. 26 | 88.15 ± 2.14 | 12.20 ± 1.80 | 22.63 ± 0.07 | 2.93 ± 0.16 | - | - | - | ||
| Jishu No. 65 | 87.58 ± 1.53 | 11.81 ± 1.29 | 21.80 ± 0.56 | 3.30 ± 0.00 | - | - | - | ||
| Xushu No. 22 (spring) | 87.68 ± 1.39 | 12.62 ± 0.23 | 17.53 ± 0.29 | 3.04 ± 0.01 | - | - | - | ||
| Guang2 | 89.67 ± 0.87 | 10.92 ± 0.07 | 33.64 ± 0.83 | 3.87 ± 0.64 | 15.62 ± 0.05 | 37.28 ± 0.1 | 36.31 ± 0.49 | [17] | |
| Guang5 | 88.67 ± 1.34 | 9.26 ± 0.03 | 31.41 ± 0.69 | 2.75 ± 0.41 | 14.86 ± 0.05 | 38.87 ± 0.33 | 41.98 ± 0.55 | ||
| Ecai1 | 87.92 ± 0.43 | 9.82 ± 0.08 | 35.66 ± 0.2 | 4.28 ± 0.92 | 13.43 ± 0.15 | 40.32 ± 0.1 | 36.79 ± 1.24 | ||
| Ecai10 | 89.95 ± 0.16 | 10.63 ± 0.01 | 38.52 ± 0.33 | 4.25 ± 0.33 | 16.61 ± 0.12 | 38.71 ± 0.01 | 30.13 ± 0.74 | ||
| Zhecai1 | 89.89 ± 0.36 | 9.74 ± 0.12 | 35.45 ± 0.31 | 2.78 ± 0.23 | 15.51 ± 0.03 | 38.48 ± 0.42 | 36.75 ± 0.88 | ||
| Zhe726 | 90.01 ± 1.2 | 9.91 ± 0.09 | 33.65 ± 0.34 | 2.74 ± 0.22 | 14.61 ± 0.18 | 39.06 ± 0.3 | 38.15 ± 0.3 | ||
| Fu18 | 88.41 ± 0.98 | 10.19 ± 0.02 | 36.44 ± 0.25 | 2.78 ± 0.23 | 16.48 ± 0.03 | 38.91 ± 0.04 | 34.01 ± 0.19 | ||
| Fu22 | 87.37 ± 0.82 | 10.11 ± 0.02 | 28.01 ± 0.19 | 2.74 ± 0.22 | 16.47 ± 0.01 | 41.45 ± 0.11 | 42.64 ± 0.12 | ||
| Fu23 | 87.96 ± 2.03 | 11.4 ± 0.06 | 36.16 ± 0 | 2.75 ± 0.06 | 15.45 ± 0.07 | 40.35 ± 0.14 | 34.22 ± 0.19 | ||
| Taninong71 | 88.24 ± 0.13 | 10.2 ± 0.07 | 35.49 ± 0.07 | 3.3 ± 0.21 | 15.93 ± 0.07 | 40.06 ± 0.13 | 35 ± 0.34 | ||
| Shulv1 | 90.27 ± 0.17 | 9.77 ± 0.06 | 36.04 ± 0.14 | 3.03 ± 0.75 | 16.99 ± 0.1 | 39.58 ± 0.14 | 33.64 ± 0.03 | ||
| Pushu53 | 88.61 ± 0.02 | 9.39 ± 0 | 31.36 ± 0.2 | 3.25 ± 0.08 | 13.74 ± 0.14 | 38.48 ± 0.13 | 42.19 ± 0.19 | ||
| Ningcai | 88.14 ± 0.4 | 10.66 ± 0.05 | 31.14 ± 0.08 | 2.49 ± 0.56 | 14.88 ± 0.02 | 38.42 ± 0.14 | 41.11 ± 0.45 | ||
| Peel ** | Primary | 22.4–22.3 | 4.74–4.76 | 6.40–6.49 | 2.33–2.65 | 9.47–9.70 | 55.2–56.1 | 76.4–77.0 | [18] |
| Blanched | 14.2–14.0 | 3.65–4.01 | 8.11–8.20 | 1.28–1.35 | 6.45–6.60 | 29.6–30.1 | 79.9–80.4 | ||
| Residue of tuber after starch extraction | Beijing553 | - | - | 5.97 ± 0.43 | 0.45 ± 0.05 | 2.14 ± 0.01 | 23.81 ± 0.14 | 42.44 ± 0.04 | [19] |
| Jishu21 | - | - | 4.23 ± 0.05 | 0.52 ± 0.03 | 2.65 ± 0.08 | 17.15 ± 0.05 | 60.89 ± 0.11 | ||
| Jishu71 | - | - | 4.05 ± 0.10 | 0.37 ± 0.06 | 1.59 ± 0.02 | 17.83 ± 0.08 | 59.41 ± 0.12 | ||
| Jishu82 | - | - | 5.06 ± 0.05 | 0.38 ± 0.04 | 2.67 ± 0.03 | 24.49 ± 0.07 | 49.73 ± 0.05 | ||
| Jishu98 | - | - | 4.37 ± 0.15 | 0.25 ± 0.03 | 2.09 ± 0.05 | 20.05 ± 0.06 | 53.76 ± 0.25 | ||
| Jishu99 | - | - | 4.20 ± 0.49 | 0.21 ± 0.03 | 1.88 ± 0.04 | 16.33 ± 0.11 | 59.10 ± 0.06 | ||
| Lvya18 | - | - | 3.38 ± 0.42 | 0.59 ± 0.02 | 1.99 ± 0.01 | 18.75 ± 0.04 | 53.53 ± 0.06 | ||
| Weiduoli | - | - | 6.11 ± 0.42 | 0.33 ± 0.02 | 3.02 ± 0.03 | 26.55 ± 0.04 | 43.45 ± 0.09 | ||
| Xinong431 | - | - | 4.12 ± 0.16 | 0.37 ± 0.01 | 2.63 ± 0.15 | 23.35 ± 0.13 | 45.13 ± 0.11 | ||
| Xu55-2 | - | - | 3.97 ± 0.01 | 0.48 ± 0.05 | 1.95 ± 0.04 | 25.82 ± 0.20 | 52.32 ± 0.06 |
| Variety | Pectin | Hemicellulose | Lignin | Cellulose | Monosaccharide | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhamnose | Arabinose | Galactose | Glucose | Xylose | Mannose | Uronic Acid | |||||
| Beijing553 | 10.56 ± 0.18 g | 12.09 ± 0.11 c | 16.57 ± 0.06 f | 27.66 ± 0.08 i | 1.86 ± 0.05 f | 4.29 ± 0.03 a | 13.86 ± 0.28 a | 57.29 ± 0.78 cd | 2.63 ± 0.07 e | 1.21 ± 0.06 de | 18.86 ± 0.32 d |
| Jishu21 | 15.36 ± 0.11 ef | 9.13 ± 0.04 g | 22.61 ± 0.03 a | 33.07 ± 0.30 b | 2.39 ± 0.02 b | 3.89 ± 0.09 bc | 7.50 ± 0.09 f | 58.95 ± 0.66 c | 3.11 ± 0.04 cd | 1.51 ± 0.05 c | 22.65 ± 0.16 c |
| Jishu71 | 9.31 ± 0.61 h | 12.01 ± 0.03 c | 14.83 ± 0.05 i | 36.54 ± 0.03 a | 1.38 ± 0.01 g | 3.96 ± 0.07 b | 9.47 ± 0.22 d | 65.73 ± 0.52 a | 2.49 ± 0.06 e | 2.14 ± 0.08 a | 14.83 ± 0.23 e |
| Jishu82 | 21.02 ± 0.06 b | 10.94 ± 0.10 e | 8.96 ± 0.11 k | 30.12 ± 0.13 f | 2.24 ± 0.06 bc | 3.79 ± 0.06 cd | 8.52 ± 0.13 e | 51.44 ± 0.41 f | 2.92 ± 0.06 d | 1.10 ± 0.08 e | 26.35 ± 0.30 b |
| Jishu98 | 15.67 ± 0.30 e | 9.09 ± 0.02 g | 21.12 ± 0.11 c | 27.90 ± 0.05 h | 2.21 ± 0.05 cd | 3.64 ± 0.05 e | 8.75 ± 0.11 de | 53.60 ± 0.37 ef | 3.32 ± 0.08 c | 2.08 ± 0.15 a | 26.38 ± 0.11 b |
| Jishu99 | 9.01 ± 0.11 h | 12.13 ± 0.05 c | 17.41 ± 0.21 d | 29.89 ± 0.11 g | 1.51 ± 0.07 g | 3.61 ± 0.05 e | 12.11 ± 0.14 b | 63.56 ± 0.43 b | 3.17 ± 0.05 c | 0.82 ± 0.03 f | 15.22 ± 0.15 e |
| Lvya18 | 19.25 ± 0.17 c | 13.32 ± 0.30 b | 15.20 ± 0.06 h | 31.93 ± 0.08 d | 2.13 ± 0.04 de | 3.88 ± 0.07 bc | 10.91 ± 0.17 c | 52.61 ± 0.29 f | 3.68 ± 0.11 b | 1.88 ± 0.07 b | 25.91 ± 0.26 b |
| Weiduoli | 18.29 ± 0.16 d | 10.45 ± 0.05 f | 22.01 ± 0.02 b | 32.49 ± 0.04 c | 2.30 ± 0.06 bc | 2.90 ± 0.10 g | 10.62 ± 0.18 c | 54.47 ± 0.33 def | 3.85 ± 0.09 ab | 0.56 ± 0.04 g | 25.31 ± 0.31 b |
| Xinong431 | 22.93 ± 0.16 a | 8.70 ± 0.02 h | 15.80 ± 0.07 g | 25.92 ± 0.06 j | 2.51 ± 0.08 a | 3.19 ± 0.04 f | 7.99 ± 0.17 ef | 46.63 ± 0.51 g | 4.07 ± 0.03 a | 0.92 ± 0.05 f | 34.69 ± 0.27 a |
| Xu55-2 | 15.13 ± 0.07 f | 15.98 ± 0.07 a | 13.94 ± 0.07 j | 36.34 ± 0.21 a | 2.01 ± 0.08 e | 3.69 ± 0.11 de | 14.21 ± 0.34 a | 56.24 ± 0.62 cde | 4.06 ± 0.08 a | 0.47 ± 0.02 g | 19.32 ± 0.19 d |
| Average | 15.65 ± 0.06 e | 11.38 ± 0.05 d | 16.85 ± 0.13 e | 31.19 ± 0.08 e | 2.05 ± 0.05 e | 3.68 ± 0.06 de | 10.39 ± 0.27 c | 56.05 ± 0.49 cde | 3.33 ± 0.10 c | 1.27 ± 0.09 d | 22.95 ± 0.28 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Xie, Q.; Zhang, M.; Gu, J.; Huang, D.; Cao, Q. Reclaiming Agriceuticals from Sweetpotato (Ipomoea batatas [L.] Lam.) By-Products. Foods 2024, 13, 1180. https://doi.org/10.3390/foods13081180
Liu T, Xie Q, Zhang M, Gu J, Huang D, Cao Q. Reclaiming Agriceuticals from Sweetpotato (Ipomoea batatas [L.] Lam.) By-Products. Foods. 2024; 13(8):1180. https://doi.org/10.3390/foods13081180
Chicago/Turabian StyleLiu, Tiange, Qingtong Xie, Min Zhang, Jia Gu, Dejian Huang, and Qinghe Cao. 2024. "Reclaiming Agriceuticals from Sweetpotato (Ipomoea batatas [L.] Lam.) By-Products" Foods 13, no. 8: 1180. https://doi.org/10.3390/foods13081180
APA StyleLiu, T., Xie, Q., Zhang, M., Gu, J., Huang, D., & Cao, Q. (2024). Reclaiming Agriceuticals from Sweetpotato (Ipomoea batatas [L.] Lam.) By-Products. Foods, 13(8), 1180. https://doi.org/10.3390/foods13081180








