The Impact of Photosynthetic Characteristics and Metabolomics on the Fatty Acid Biosynthesis in Tea Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions and Materials
2.2. Determination of Biological Characteristics, Oil Content, Individual Plant Yield, and Fatty Acid Composition of Mature Tea Fruits
2.3. Determination of Chlorophyll Content during the Peak Period of Tea Tree Oil Transformation
2.4. Determination of Photosynthesis during the Peak Period of Tea Tree Oil Transformation
2.5. Determination of Chlorophyll Fluorescence during the Peak Period of Tea Tree Oil Transformation
2.6. Targeted Metabolomics Determination of Tea Fruit during the Peak Period of 1.5 Oil Conversion
2.7. Data Statistics and Analysis
3. Results
3.1. The Biological Characteristics of Matured Fruits in Jincha 2 and Wuniuzao
3.2. Analysis of Fatty Acid Composition of Jincha 2 and Wuniuzao
3.3. Examination of Photosynthetic Characteristics of Jincha 2 and Wuniuzao during the Peak Period of Oil Transformation
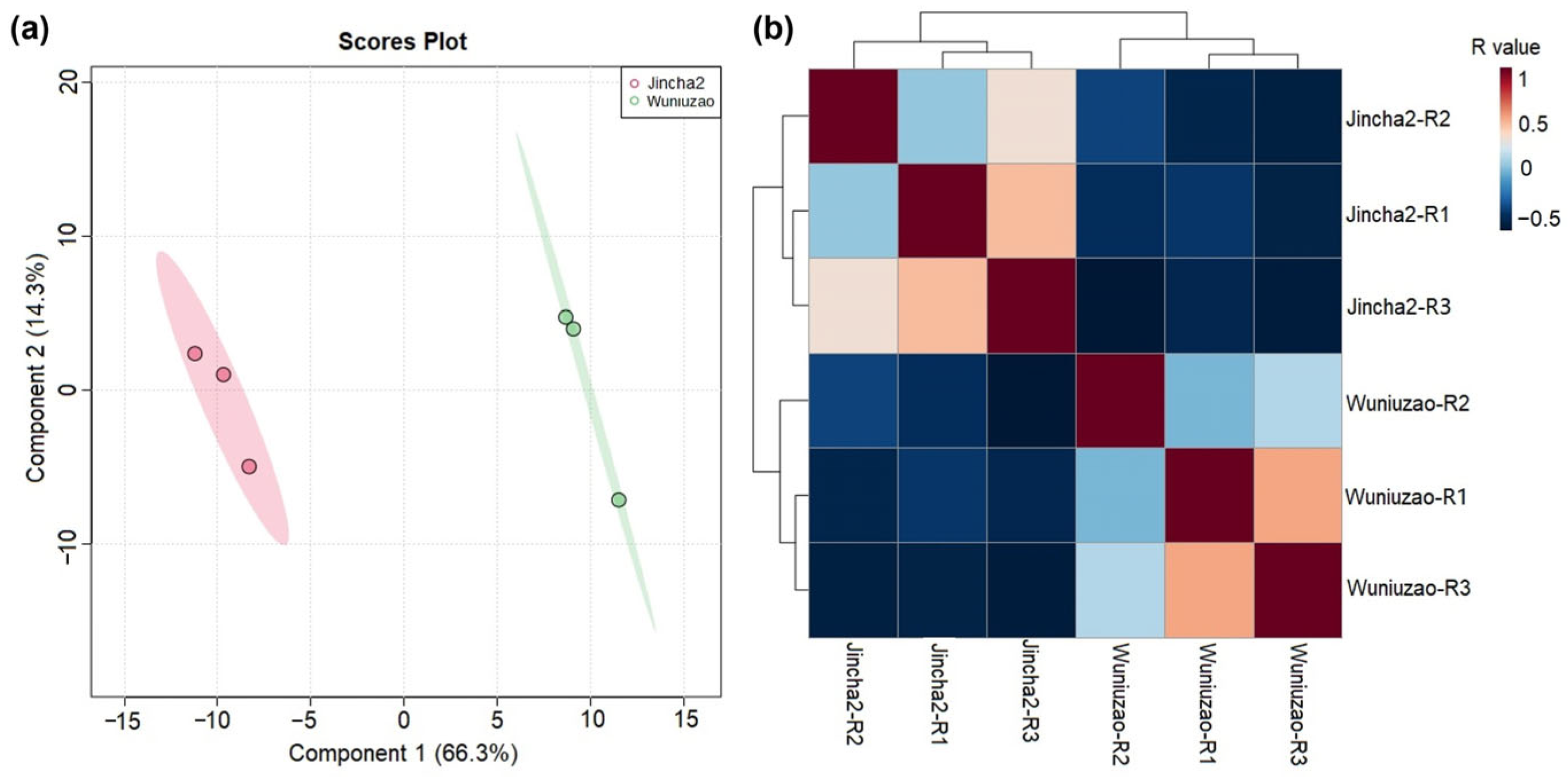
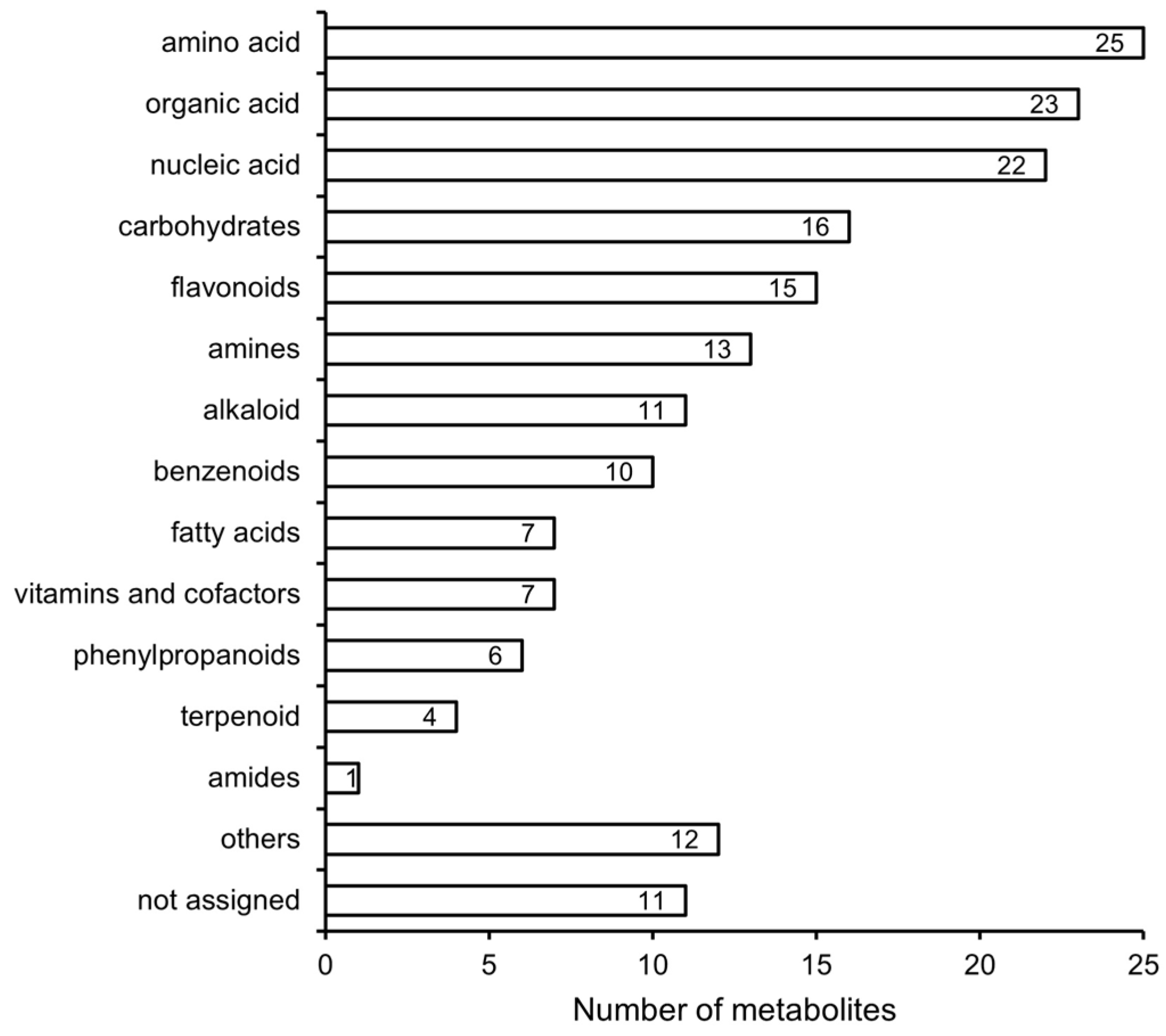
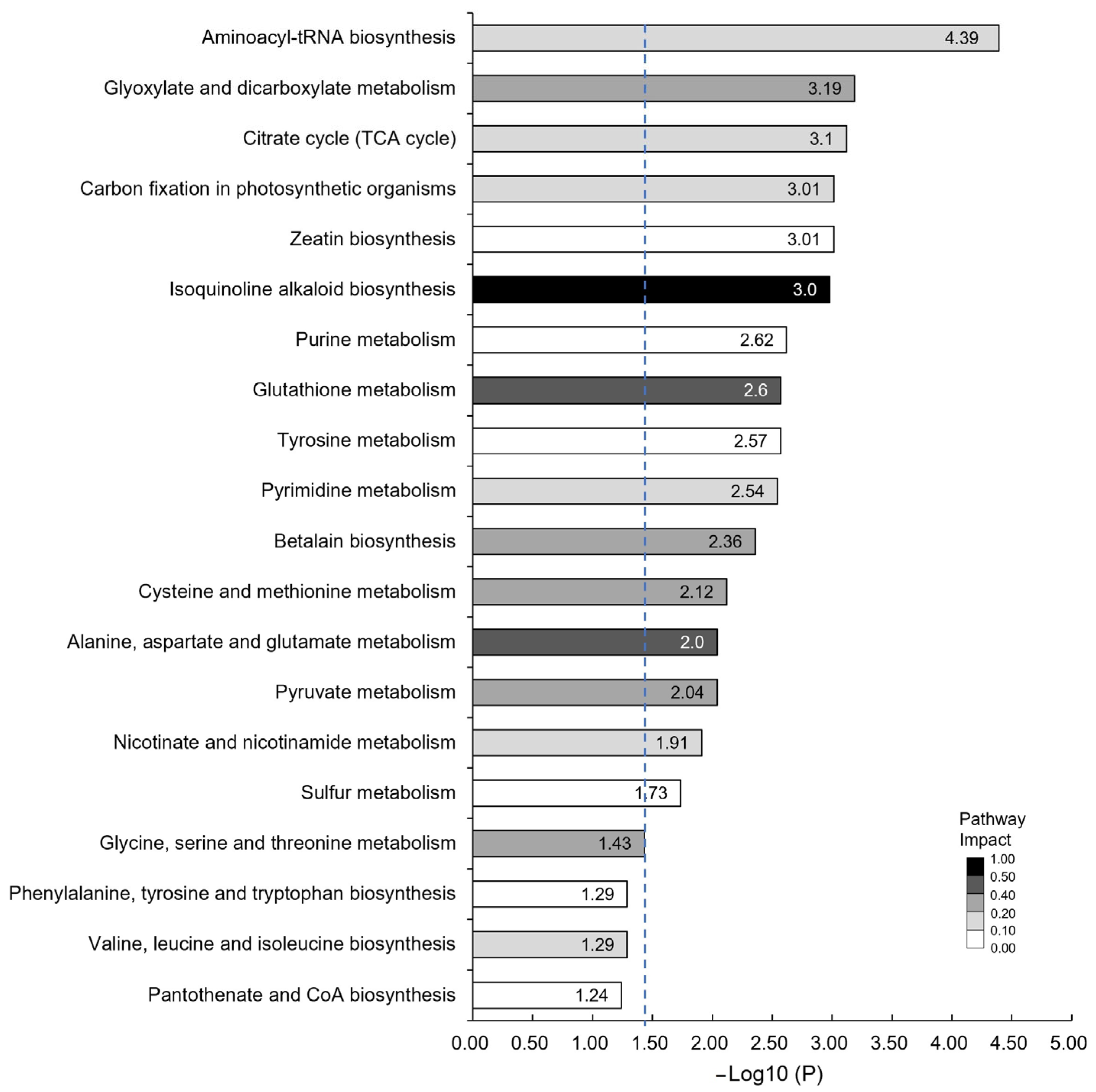
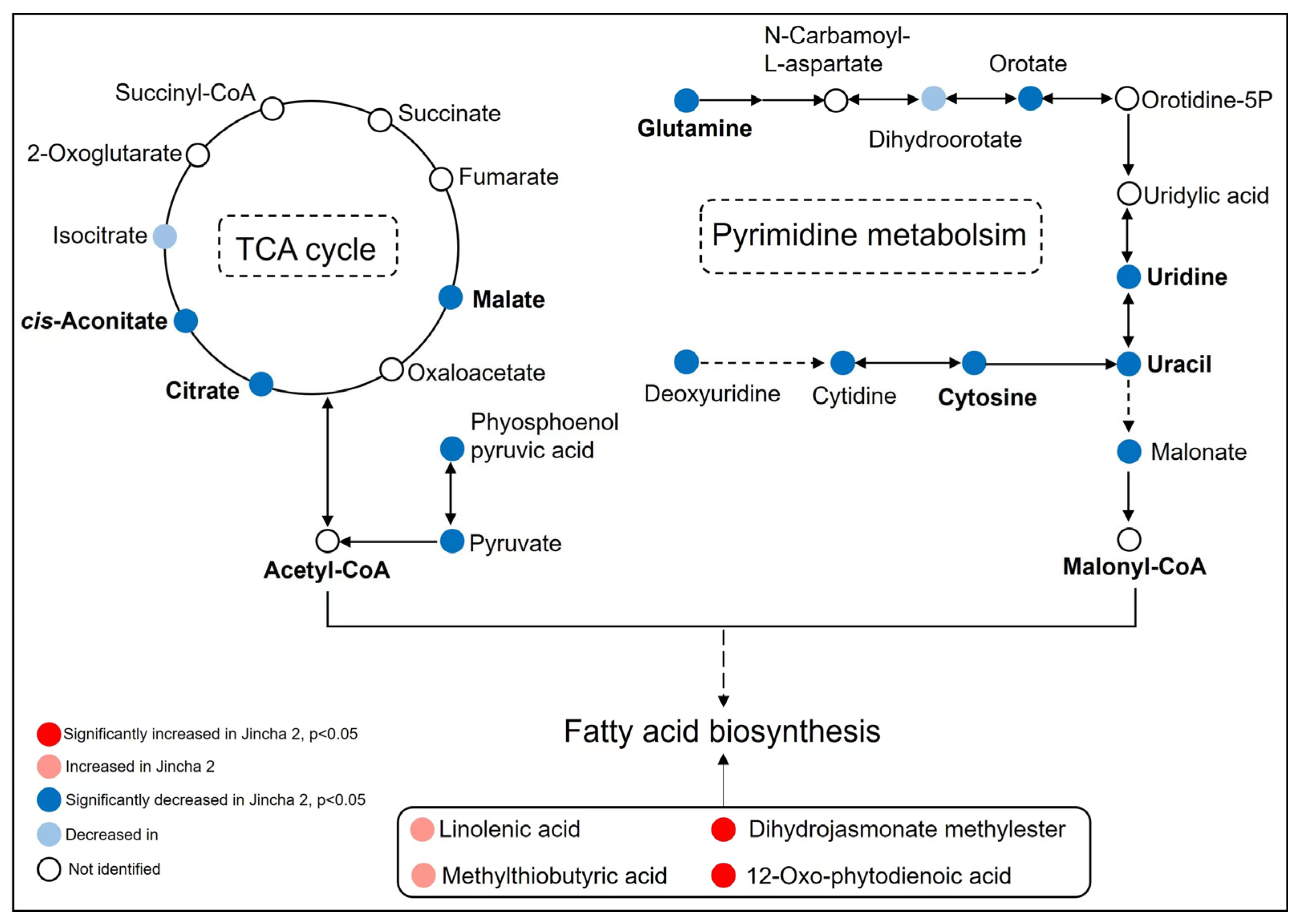
3.4. Differences in Metabolites during the Peak Period of Oil Transformation between Jincha 2 and Wuniuzao
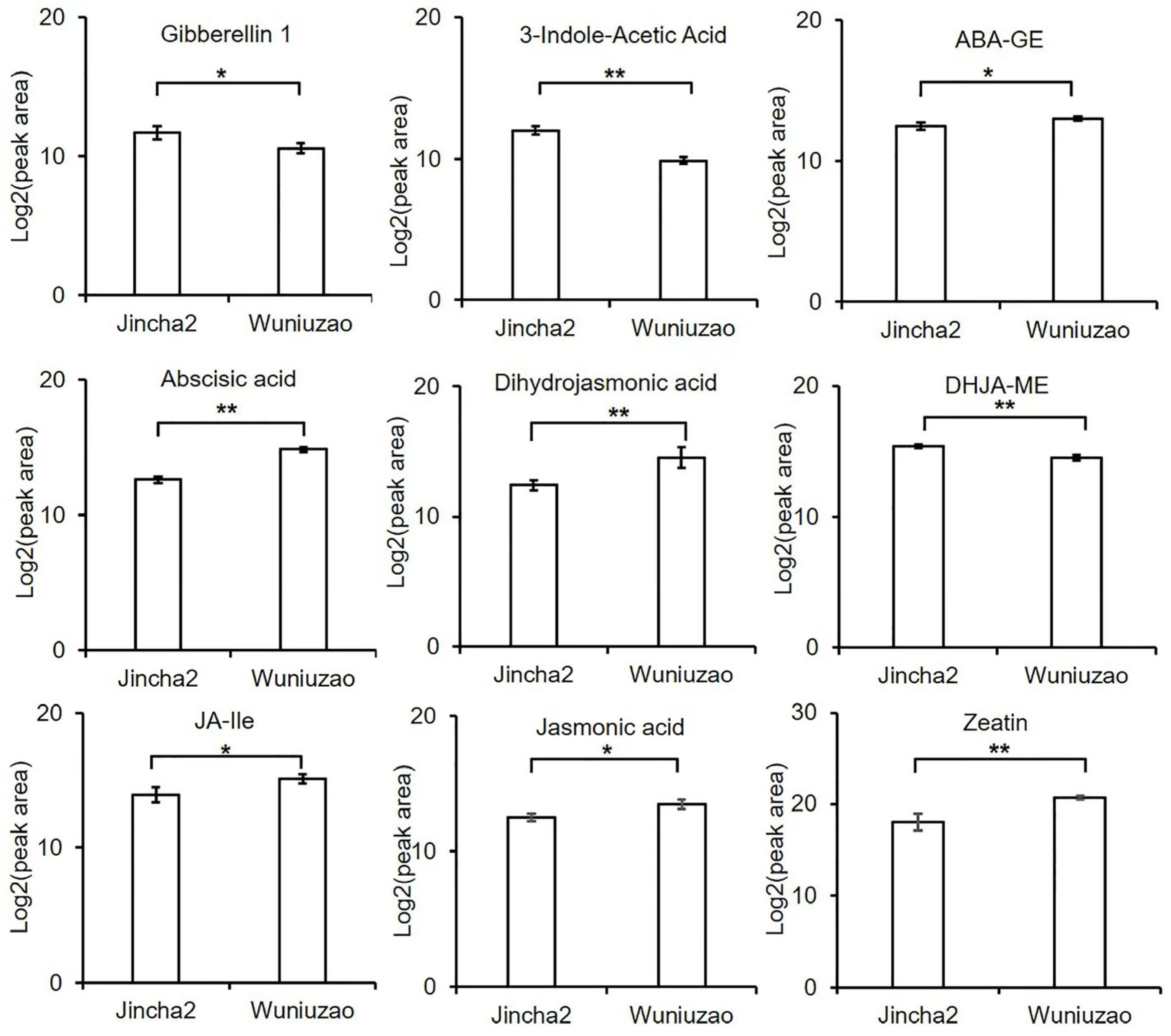
3.5. Differential Analysis of Hormones during the Peak Period of Oil Conversion between Jincha 2 and Wuniuzao
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Chang, H.; Na, Y.J.; Kang, Y.G.; Kim, B.G.; Park, J.S. Extract of enzyme-hydrolyzed green tea seed as potent melanin synthesis inhibitor. Bull. Korean Chem. Soc. 2013, 34, 2199–2202. [Google Scholar] [CrossRef]
- Kumar, M.; Kannan, A.; Bhar, R.; Gulati, A.; Gaurav, A.; Sharma, V.K. Nutrient intake, digestibility and performance of Gaddi kids supplemented with tea seed or tea seed saponin extract. Asian-Australas. J. Anim. Sci. 2017, 30, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Park, S.K.; Kang, J.Y.; Park, S.B.; Yoo, S.K.; Han, H.J.; Cho, K.H.; Kim, J.C.; Heo, H.J. Green tea seed oil suppressed Aβ 1-42 induced behavioral and cognitive deficit via the Aβ-related Akt pathway. Int. J. Mol. Sci. 2019, 20, 1865. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Hsu, Y.J.; Chien, Y.W. Tea seed oil prevents obesity, reduces physical fatigue and improves exercise performance in high-fat-diet-induced obese ovariectomized mice. Molecules 2019, 24, 980. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Du, X.; Zhu, Y.; Wang, Z.; Hua, S.J.; Li, Z.L.; Guo, W.L.; Zhang, G.P.; Peng, J.R.; Jiang, L.X. Seed fatty acid reducer acts downstream of gibberellin signalling pathway to lower seed fatty acid storage in Arabidopsis. Plant Cell Environ. 2012, 35, 2155–2169. [Google Scholar] [CrossRef]
- Ji, H.G.; Lee, Y.R.; Lee, M.S. Metabolic phenotyping of various tea (Camellia sinensis L.) cultivars and understanding of their intrinsic metabolism. Food Chem. 2017, 233, 321–330. [Google Scholar] [CrossRef]
- Myers, R.A.; Fuller, E.; Yang, W. Identification of native catechin fatty acid esters in green tea (Camellia sinensis). J. Agric. Food Chem. 2013, 61, 11484–11493. [Google Scholar] [CrossRef]
- Jiang, C.; Ma, J.; Apostolides, Z.; Chen, L. Metabolomics for a millenniums-old crop: Tea plant (Camellia sinensis). J. Agrc. Food Chem. 2019, 67, 6445–6457. [Google Scholar] [CrossRef]
- Samanta, T.; Kotamreddy, J.N.R.; Ghosh, B.C.; Mitra, A. Changes in targeted metabolites, enzyme activities and transcripts at different developmental stages of tea leaves: A study for understanding the biochemical basis of tea shoot plucking. Acta Physiol. Plant. 2017, 39, 91–96. [Google Scholar] [CrossRef]
- Antolin, E.M.; Delange, D.M.; Canavaciolo, V.G. Evaluation of five methods for derivatization and GC determination of a mixture of very long chain fatty acids (C24:0–C36:0). J. Pharm. Biomed. Anal. 2008, 46, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.F.; Lv, S.D.; Yang, X.F.; Li, Y.; Zhang, J.G. Study of characteristic indexes and fatty acid composition in big-leaf tea plant (Camellia sinensis var. assamica) seed oils from Yunnan province. J. Tea Sci. 2021, 41, 463–470. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, S.; Wang, X.D.; Shi, X.B.; Wang, Z.Q.; Liu, F.Z. Effect of different light qualities on the photosynthetic properties and ultrastructure of chloroplast in senescing grape leaves. Acta Hortic. Sin. 2019, 46, 205–214. [Google Scholar]
- Dai, Y.; Shen, Z.; Liu, Y.; Wang, L.L.; Hannaway, D.; Lu, H.F. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ. Exp. Bot. 2009, 65, 177–182. [Google Scholar] [CrossRef]
- Geng, S.; Misra, B.B.; de Armas, E.; Huhman, D.V.; Alborn, H.T.; Sumner, L.W.; Chen, S. Jasmonate-mediated stomatal closure under elevated CO2 revealed by time-resolved metabolomics. Plant J. 2016, 88, 947–962. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Han, H.; Liu, A.; Guan, Q.J.; Kang, J.N.; David, L.; Dufresne, C.; Chen, S.X.; Tian, J.K. Combined ultraviolet and darkness regulation of medicinal metabolites in Mahonia bealei revealed by proteomics and metabolomics. J. Proteom. 2021, 233, 104081. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Ferguson, J.N.; Jithesh, T.; Lawson, T.; Kromdijk, J. Leaf excision introduces limited and species-specific effects on photosynthetic parameters across crop functional types. J. Exp. Bot. 2023, erad319. [Google Scholar] [CrossRef]
- Zhang, Y.; Fernie, A.R. On the role of the tricarboxylic acid cycle in plant productivity: The role of TCA in the plant productivity. J. Integr. Plant Biol. 2018, 60, 1199–1216. [Google Scholar] [CrossRef]
- Rocha, M.; Licausi, F.; Araújo, W.L.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; van Dongen, J.T. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef]
- Bergman, J.M.; Wrande, M.; Hughes, D. Acetate availability and utilization supports the growth of mutant sub-populations on aging bacterial colonies. PLoS ONE 2014, 9, e109255. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.J.; Behal, R.H.; Back, S.L.; Nikolau, B.J.; Wurtele, E.S.; Oliver, D.J. The role of pyruvate dehydrogenase and acetyl-coenzyme a synthetase in fatty acid synthesis in developing Arabidopsis seeds1. Plant Physiol. 2000, 123, 497–508. [Google Scholar] [CrossRef]
- Zrenner, R.; Stitt, M.; Sonnewald, U.; Boldt, R. Pyrimidine and purine biosynthesis and degradation in plants. Annu. Rev. Plant Biol. 2006, 57, 805–836. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Savka, M.A.; Hudson, A.O. The synthesis and role of β-alanine in plants. Front. Plant Sci. 2019, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, N.; Soon, C.L.; Shimizu, S.; Ogawa, J. Oxidative pyrimidine metabolism in Rhodococcus erythropolis useful for valuable nucleoside synthesis: Discovery of a novel amidohydorolase, ureidomalonase. Biocatal. Agri. Biotechnol. 2012, 1, 264–266. [Google Scholar] [CrossRef]
- Guan, X.; Nikolau, B.J. AAE13 encodes a dual-localized malonyl-CoA synthetase that is crucial for mitochondrial fatty acid biosynthesis. Plant J. 2016, 85, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Ohlrogge, J.; Browse, J. Lipid biosynthesis. Plant Cell 1995, 7, 957–970. [Google Scholar]
- West, A.K.; Bailey, C.B. Crosstalk between primary and secondary metabolism: Interconnected fatty acid and polyketide biosynthesis in prokaryotes. Bioorg. Med. Chem. Lett. 2023, 91, 129377. [Google Scholar] [CrossRef]
- Crane, J.C. Growth substances in fruit setting and development. Annu. Rev. Plant Physiol. 1964, 15, 303–326. [Google Scholar] [CrossRef]
- Davies, C.; Boss, P.K.; Robinson, S.P. Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol. 1997, 115, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Keawmanee, N.; Ma, G.; Zhang, L.C.; Yahata, M.; Murakami, K.; Yamamoto, M.; Kojima, N.; Kato, M. Exogenous gibberellin induced regreening through the regulation of chlorophyll and carotenoid metabolism in Valencia oranges. Plant Physiol. Biochem. 2022, 173, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liao, X.; He, R.; Zhong, M.; Feng, P.; Li, X.; Tang, D.; Liu, X.; Zhao, X. Ectopic expression of GA 2-oxidase 6 from rapeseed (Brassica napus L.) causes dwarfism, late flowering and enhanced chlorophyll accumulation in Arabidopsis thaliana. Plant Physiol. Biochem. 2017, 111, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Guanter, L.; Zhang, Y.; Jung, M.; Joiner, J.; Voigt, M.; Berry, J.A.; Frankenberg, C.; Huete, A.R.; Zarco-Tejada, P.; Lee, J.E.; et al. Global and time-resolved monitoring of crop photosynthesis with chlorophyll fluorescence. Proc. Natl. Acad. Sci. USA 2014, 111, E1327–E1333. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Incharoensakdi, A. Plant hormone induced enrichment of Chlorella sp. Omega-3 fatty acids. Biotechnol. Biofuels 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Vlad, F.; Sirichandra, C.; Redko, Y.; Jammes, F.; Valon, C.; dit Frey, N.F.; Leung, J. An update on abscisic acid signaling in plants and more. Mol. Plant 2008, 1, 198–217. [Google Scholar] [CrossRef] [PubMed]
- Mandal, N.K.; Kumari, K.; Kundu, A.; Arora, A.; Bhowmick, P.K.; Iquebal, M.A.; Jaiswal, S.; Behera, T.K.; Munshi, A.D.; Dey, S.S. Cross-talk between the cytokinin, auxin, and gibberellin regulatory networks in determining parthenocarpy in cucumber. Front. Genet. 2022, 13, 957360. [Google Scholar] [CrossRef]
- Du, P.X.; Deng, Q.Q.; Wang, W.Y.; Garg, V.; Lu, Q.; Huang, L.; Wang, R.F.; Li, H.F.; Huai, D.X.; Chen, X.P.; et al. scRNA-seq reveals the mechanism of fatty acid desaturase 2 mutation to repress leaf growth in Peanut (Arachis hypogaea L.). Cells 2023, 12, 2305. [Google Scholar] [CrossRef]


| Cultivars | One Compartment (Spherical) | Two Compartments (Renal) | Three Compartments (Triangular) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Percentage (%) | Height of Fruit (cm) | Diameter of Fruit (cm) | Percentage (%) | Height of Fruit (cm) | Diameter of Fruit (cm) | Percentage (%) | Height of Fruit (cm) | Diameter of Fruit (cm) | |
| Jincha 2 | 72.18 ± 1.11% | 1.81 ± 0.05 | 1.84 ± 0.08 | 18.79 ± 1.12% * | 1.65 ± 0.17 | 2.79 ± 0.09 | 9.03 ± 0.2% * | 1.76 ± 0.05 | 2.66 ± 0.07 |
| Wuniuzao | 84.05 ± 2.44% * | 2.07 ± 0.06 * | 2.22 ± 0.16 * | 11.44 ± 0.9% | 1.95 ± 0.16 * | 3.20 ± 0.11 * | 4.51 ± 1.5% | 1.94 ± 0.1 * | 2.89 ± 0.21 * |
| Cultivars | One Compartment | Two Compartments | Three Compartments | Water Content (%) | Seed Oil Yield (%) | Single Plant Seed Yield (kg) | Single Plant Oil Yield (kg) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Fresh Weight (g) | Dry Weight (g) | Fresh Weight (g) | Dry Weight (g) | Fresh Weight (g) | Dry Weight (g) | |||||
| Jincha 2 | 3.91 ± 0.03 | 1.23 ± 0.08 | 6.15 ± 0.01 | 2.62 ± 0.05 | 7.73 ± 0.07 | 3.44 ± 0.17 | 65.27% | 35.18 ± 4.37% ** | 4.04 ± 0.27 ** | 1.42 ± 0.18 ** |
| Wuniuzao | 4.44 ± 0.12 * | 1.34 ± 0.14 * | 7.93 ± 0.11 ** | 2.83 ± 0.05 * | 9.95 ± 0.11 * | 4.20 ± 0.14 * | 66.14% * | 19.94 ± 1.78% | 0.53 ± 0.06 | 0.11 ± 0.08 |
| No. | Compound Name | Formula | Contents in Jincha 2 (g∙kg−1 DW) | Percentage in Jincha 2 (%) | Contents in Wuniuzao (g∙kg−1 DW) | Percentage in Wuniuzao (%) |
|---|---|---|---|---|---|---|
| Unsaturated fatty acid | ||||||
| 1 | Palmitoleic acid a | C16H30O2 | 0.13 ± 0.004 | 0.08 | 0.14 ± 0.008 | 0.07 |
| 2 | 10-Heptadecenoic acid a | C17H32O2 | 0.05 ± 0.001 * | 0.03 | 0.03 ± 0.002 | 0.04 |
| 3 | Oleic acid a | C18H34O2 | 80.73 ± 1.61 * | 52.32 | 32.48 ± 0.81 | 38.53 |
| 4 | Linoleic acid b | C18H32O2 | 43.2 ± 0.86 * | 28 | 20.81 ± 0.5 | 24.69 |
| 5 | Gadoleic acid a | C21H40O2 | 1.17 ± 0.02 * | 0.76 | 0.30 ± 0.01 | 0.36 |
| 6 | α-Linolenic acid b | C18H30O2 | 0.47 ± 0.01 | 0.3 | 0.53 ± 0.01 * | 0.63 |
| 7 | Eicosadienoic acid b | C20H36O2 | 0.03 ± 0.002 * | 0.02 | 0.02 ± 0.003 | 0.02 |
| 8 | Arachidonic acid b | C20H32O2 | 0.03 ± 0.004 | 0.02 | 0.03 ± 0.008 | 0.04 |
| 9 | Nervonic acid b | C24H46O2 | 0.02 ± 0.0001 * | 0.01 | n.d. | n.d. |
| Saturated fatty acid | ||||||
| 10 | Myristic acid | C14H28O2 | 0.15 ± 0.002 | 0.1 | 0.27 ± 0.01 * | 0.32 |
| 11 | Pentadecylic acid | C15H30O2 | 0.022 ± 0.01 | 0.01 | 0.03 ± 0.005 * | 0.04 |
| 12 | Palmitic acid | C16H32O2 | 22.94 ± 0.41 | 14.87 | 26.44 ± 0.75 * | 31.37 |
| 13 | Erucic acid | C22H42O2 | 0.09 ± 0.002 | 0.06 | 0.08 ± 0.002 | 0.09 |
| 14 | Lignoceric acid | C24H48O2 | 0.13 ± 0.003 * | 0.08 | 0.11 ± 0.01 | 0.13 |
| 15 | Stearic acid | C18H36O2 | 4.98 ± 0.1 * | 3.23 | 2.97 ± 0.07 | 3.52 |
| 16 | Arachidic acid | C20H40O2 | 0.15 ± 0.001 * | 0.1 | 0.13 ± 0.04 | 0.15 |
| Cultivars | Pn (umol∙m−2∙s−1) | Gs (mol∙m−2∙s−1) | Ci (umol∙m−2∙s−1) | Tr (m−2∙s−1) |
|---|---|---|---|---|
| Jincha 2 | 9.6 ± 0.3 * | 0.37 ± 0.01 * | 252.23 ± 1.48 | 12.39 ± 0.18 * |
| Wuniuzao | 7.93 ± 0.43 | 0.3 ± 0 | 256.18 ± 2.4 | 11.15 ± 0.09 |
| Cultivars | Chl a (mg∙g−1) | Chl b (mg∙g−1) | Chl a + Chl b (mg∙g−1) | Chl a/Chl b |
|---|---|---|---|---|
| Jincha 2 | 3.79 ± 0.73 | 1.68 ± 0.12 | 5.47 ± 0.62 | 2.25 ± 0.57 |
| Wuniuzao | 3.09 ± 0.52 | 1.28 ± 0.19 | 4.38 ± 0.71 | 2.42 ± 0.06 |
| Chl a | Chl b | Pn | Gs | Ci | Tr | Yield | ETR | GA-1 | IAA | ABA-GE | ABA | DHJA-ME | JA-Ile | JA | ZR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single plant Seed yield | 0.607 | 0.821 * | 0.954 ** | 0.992 ** | −0.896 * | 0.976 ** | 0.863 * | 0.863 * | 0.762 | 0.974 ** | −0.809 | −0.976 ** | 0.931 ** | −0.818 * | −0.833 * | −0.974 ** |
| Single plant oil yield | 0.518 | 0.825 * | 0.924 ** | 0.968 ** | −0.897 * | 0.937 ** | 0.911 * | 0.911 * | 0.789 | 0.931 ** | −0.808 | −0.978 ** | 0.907 * | −0.864 * | −0.817 * | −0.966 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Liu, S.; Hu, X.; Li, D.; Chen, L.; Weng, X.; Zheng, Z.; Chen, X.; Zhuang, J.; Li, X.; et al. The Impact of Photosynthetic Characteristics and Metabolomics on the Fatty Acid Biosynthesis in Tea Seeds. Foods 2023, 12, 3821. https://doi.org/10.3390/foods12203821
Jiang L, Liu S, Hu X, Li D, Chen L, Weng X, Zheng Z, Chen X, Zhuang J, Li X, et al. The Impact of Photosynthetic Characteristics and Metabolomics on the Fatty Acid Biosynthesis in Tea Seeds. Foods. 2023; 12(20):3821. https://doi.org/10.3390/foods12203821
Chicago/Turabian StyleJiang, Li, Shujing Liu, Xinrong Hu, Duojiao Li, Le Chen, Xiaoxing Weng, Zhaisheng Zheng, Xuan Chen, Jing Zhuang, Xinghui Li, and et al. 2023. "The Impact of Photosynthetic Characteristics and Metabolomics on the Fatty Acid Biosynthesis in Tea Seeds" Foods 12, no. 20: 3821. https://doi.org/10.3390/foods12203821
APA StyleJiang, L., Liu, S., Hu, X., Li, D., Chen, L., Weng, X., Zheng, Z., Chen, X., Zhuang, J., Li, X., Chen, Z., & Yuan, M. (2023). The Impact of Photosynthetic Characteristics and Metabolomics on the Fatty Acid Biosynthesis in Tea Seeds. Foods, 12(20), 3821. https://doi.org/10.3390/foods12203821






