Abstract
A one-year survey was undertaken of the microbiological quality of carcases and the derived primal cuts, manufacturing meat and offals at twelve Australian export establishments (six beef, three sheep/lamb and three pork). A total of 27,157 microbiological results for aerobic plate count (APC) and generic Escherichia coli were gathered, 15,155 from beef, 8405 from sheep and 3597 from pig establishments. The mean log10 APCs on beef, sheep and pig carcases were 0.84, 1.60 and 1.30 log10 cfu/cm2, respectively. For primals, the mean log10 APC was higher for beef but was similar for sheep and pork primals, with ‘outside’ cuts having higher counts. For manufacturing meat, the concentration was 2–3 log10 cfu/g, irrespective of species. The prevalence (%) of generic E. coli from beef, sheep and pork was 2.3, 28.4 and 5.4 on carcases; 7.0, 20.6 and 3.2 on primals; and 5.8, 33.6 and 6.1 on manufacturing meat, respectively. The mean log10 APCs of beef, sheep and pork offal were 3.23, 3.18 and 3.37 log10 cfu/g, with tripes and tongues having APCs 1–2 log10 units higher than organ offals. The results reflect improvements in total bacterial loadings compared with previous national baseline surveys.
1. Introduction
Following several food poisoning incidents associated with the consumption of hamburgers, the Food Safety and Inspection Service in the United States introduced the Pathogen reduction: hazard analysis and critical control point (HACCP) systems; final rule, also known as the ‘Mega Reg’ [1]. As a major exporter to the USA of manufacturing meat for grinding, in 1998, Australia mandated a government-supervised monitoring program for carcases, the E. coli and Salmonella Monitoring (ESAM) program. The ESAM program is performed by all export establishments, which are required to respond to results considered unacceptable based on a three-class sampling plan and a moving window [2], the original criteria having been set using 2001 data [3]. The results are stored in a national database which is “active”, with each export establishment being able to generate reports and summaries of their data and the national microbiological profile.
In the 25 years since the inception of mandatory monitoring, the Australian industry has undergone significant improvements in infrastructure and in process control. These changes were documented by a series of national baseline studies of beef and sheep carcases and cuts with a trend towards improved microbiological profiles of both categories [4,5,6,7,8,9,10,11]. Typically, few samples, particularly beef, had E. coli counts above the limit of detection, prompting establishments to question the utility of E. coli testing of carcases as it provided no meaningful relationship with end-product verification testing or port-of-entry testing.
This thinking, together with a parallel trend over the same period of a decrease in marketing of carcases per se and of increased processing of meat cuts and offals, led to a review of the microbiological monitoring of Australian meat [12]. The review, undertaken with representatives from industry and the controlling authority (the Department of Agriculture, Fisheries and Forestry, DAFF), canvassed the microbiological monitoring regimes of other meat exporting countries, analysed the ESAM database and recommended an industry trial be undertaken to provide baseline data on carcases, primals (individually packed), manufacturing or bulk-packed meat and offals. Accordingly, the trial was undertaken at six beef, three sheep and three pig establishments and generated more than 20,000 data points for carcases, primals, bulk meat and offal [13]. The resulting database provides a unique linkage between the carcase and products derived from it, bulk meat, primals and offals, and is described in the present paper. In addition, it was the intention to use these data to develop alternative microbiological criteria by which to assess the performance of the Australian meat industry and to submit them for review by Australia’s major trading partners.
2. Materials and Methods
2.1. Selection of Establishments
Twelve establishments (six beef, three sheep and three pig) were selected from the Australian states of Queensland, New South Wales, Victoria, South Australia, Western Australia and Tasmania. An additional selection criterion was based on the size of the establishment and, hence, slaughter volume; other process characteristics of each establishment are presented in Table 1, Table 2 and Table 3.

Table 1.
Process characteristics of participating beef establishments.

Table 2.
Process characteristics of participating sheep establishments.

Table 3.
Process characteristics of participating pork establishments.
As indicated in Table 1, the slaughter volume/hour varied considerably across the six participating beef establishments, with two of the larger establishments, C and D, having separate hide-on and hide-off areas and a hot water intervention followed by spray chilling. A two-knife cleaning system, where one knife resides in an 82 °C water bath while the other is in use, was employed by beef establishments exporting to the European Union.
Sealing of the bung with a plastic bag and elastic band was standard practice in all beef and sheep establishments, except for establishment I (Table 2) and all three pig processors (Table 3). All three sheep establishments used steam/vacuum devices to remove macro contamination on cutting lines and sheep establishments G and I operated a two-knife system.
As indicated in Table 3, pig slaughter volumes/hour varied considerably and one processor (K) used steaming to loosen the bristles, an operation considered superior to water scalding where build-up of organic material occurs in the scald tank [14].
2.2. Sampling Regime
All samples were collected over a 13-month period from October 2017 to October 2018. Carcases and bulk meat were sampled after overnight chilling according to the Microbiological Manual for Sampling and Testing of Export Meat and Meat Products [2] at a frequency of 1 per 300 beef carcases and 1 per 1000 sheep or pig carcases and at the corresponding carcase equivalent rate for bulk meat. Bulk meat comprised mainly manufacturing meat (trim) destined for grinding, packed in cartons. Carcase and bulk meat samples are routinely collected under the national monitoring program. Primals comprised cuts individually vacuum packed and chilled. Offals comprised so-called ‘red’ offals such as hearts and livers and ‘green’ offals such as tripes, which were scalded. There is currently no regulatory requirement for the sampling and testing of primal cuts under the national monitoring program. So, for this study, establishments sampled primals and offal at a carcase equivalent rate of 1 per 1000 for beef and 1 per 3000 for sheep and pigs. Primal and offal samples were taken immediately before packing and chilling or freezing, with the exception of one pig establishment which sampled offals after chilling.
Samples were taken by quality assurance personnel at each establishment under the authorisation of the on-plant government inspection service and processed at a laboratory accredited to the ISO/IEC 17025-2005 standard [15] by the National Association of Testing Authorities, Australia. Where the laboratory was located on-site, samples were refrigerated until same-day processing. Samples transported to an off-site laboratory were refrigerated to arrive at 4 °C or cooler and processed the next day.
Bacteria were removed from the carcase by back-and-forth strokes with a single Whirlpak sponge resuscitated in Butterfields solution over an area of 100 cm2 at three sites on beef and pig carcases (limit of detection, LOD 0.08 cfu/cm2) and 25 cm2 at three sites on sheep carcases (LOD 0.33 cfu/cm2) [2]. A similar methodology as used for carcases was employed for primals, sponging an area of 100 cm2 at a single site on the surface (LOD 0.25 cfu/cm2). Excision sampling was used for bulk meat and offal samples, with approximately 25 g, including some outer surface being taken (LOD 10 cfu/g).
2.3. Microbiological Analysis
Testing of samples was as per the DAFF-approved methods for the microbiological testing of meat and meat products [16]. For example, bacteria were removed from the sponge either by massaging sponges in a stomacher or by “squishing” sponges by hand in the sample bags for 30 s and, from the moisture expressed, preparing serial dilutions in 0.1% buffered peptone water blanks (9 mL) using 1 mL aliquots. Excision samples were homogenised in a stomacher with 0.1% buffered peptone to give a 10-fold dilution. Aliquots (1 mL) from each dilution were spread on E. coli Petrifilm (3M, Sydney, Australia) and Aerobic Plate Count (APC) Petrifilm (3M) and incubated at 30 °C/48 h. Colonies were identified and counted as per the manufacturer’s instructions.
2.4. Statistical Analysis
Establishment data were sent to the South Australian Research and Development Institute either daily or weekly for entry into a database. Counts/g or cm2 were converted to log10 cfu/g or cm2 and the statistical analysis (means, analysis of variance and Tukey HSD) was carried out using the statistical software R (version 4.2.2) [17] at a significance level of 0.05.
3. Results and Discussion
A total of 27,157 microbiological results were gathered as part of the trial: 15,155 from beef, 8405 from sheep and 3597 from pig establishments comprising 11,512 carcase, 9872 bulk meat, 2169 primal and 3604 offal samples.
Box plots for APC from beef carcases, primals and bulk meat at individual beef establishments are presented in Figure 1, together with the whole industry combined, based on ESAM data, indicating that the trial establishments were broadly representative of the industry. The mean from carcases from all six establishments was 0.84 log10 cfu/cm2 with establishment means ranging from 0.39 log10 cfu/cm2 (establishment F) to 1.65 log10 cfu/cm2 (establishment A). Two establishments (A and B) had mean APC counts more than 0.5 log10 higher than other establishments, which may reflect the fact that these establishments produced carcases slaughtered from long-haired European breeds of B. taurus. Industry information indicates that these cattle present challenges during rain events due to build-up of “tag”, a mixture of soil and faeces on hide incision lines, the problem being magnified particularly on feedlot cattle. However, as seen from Table 1, establishment E slaughtered similar stock at a similar line speed in the same geographical region as establishments A and B and produced carcases with much lower APCs. At three northern establishments (C, D and F), the livestock mix contained a substantial proportion of both B. indicus and grain-fed cattle, which were slaughtered at line speeds of 100–300 head/hour. The low mean log10 APCs on carcases from these establishments are probably linked to slaughter floor interventions: establishments C and D passed carcase sides through a hot water cabinet and at establishment F lactic acid was sprayed on the tail/bung area immediately after stunning. In addition, industry information suggests that short-haired B. indicus cattle, particularly those grain-fed for 100 days, are more easily processed on the slaughter floor because the fat layer beneath the hide facilities its removal. However, during the northern raining season, feedlot cattle enter abattoirs with a considerable amount of soil and faecal contamination of the hide, as do European breeds in the southern states. Establishments C and D also differed from other beef establishments by using spray chilling to offset the weight loss, which accompanies air chilling. After overnight chilling, passage of beef carcases through the boning room resulted in higher mean APCs of 0.4–1.3 log10 cfu/cm2 for primals; bulk meat APCs were also higher, although their comparison with carcases and primals is not possible because counts were obtained by excision sampling (log10 cfu/g).
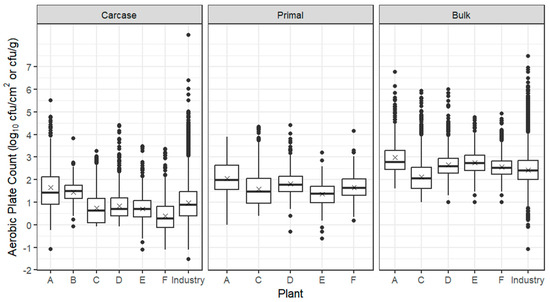
Figure 1.
Box plots of the APC of beef carcases (log10 cfu/cm2), primals (log10 cfu/cm2) and bulk meat (log10 cfu/g) from establishments A–F. The box encompasses data between the 25th and 75th percentiles, with the mean indicated by ‘X’ and median by a solid line. Box plots of the whole industry (ESAM data including trial establishments) are also given for carcases and bulk meat; industry data are currently not collected for primals.
While the prevalence of E. coli on beef carcases was generally low, there were more frequent detections at each establishment after fabrication to bulk meat and primals (Table 4). While concentrations remained low on primal meat, higher concentrations were detected from bulk product, possibly because bulk meat has a higher proportion of trim from external carcase surfaces.

Table 4.
Prevalence (%) and (mean log10 concentration *) of E. coli on carcases (log10 cfu/cm2), primals (log10 cfu/cm2) and bulk meat (log10 cfu/g) at beef establishments A–F. Mean values with the same letter within the same column are not significantly different.
With respect to primal cuts, the mean log10 APC of primals from the five beef establishments was 1.65 log10 cfu/cm2 with means for specific primals ranging from 1.41 and 1.42 log10 cfu/cm2 on internal cuts, such as tenderloins and cube rolls, to 1.80 to 1.99 log10 cfu/cm2 on cuts with external surfaces such as outside, brisket and blade; not unexpectedly, the prevalence of E. coli was also higher on external cuts (Table 5).

Table 5.
APC of beef primals (log10 cfu/cm2) and E. coli prevalence (%) at participating beef establishments. Mean values with the same letter are not significantly different.
Box plots for APC from carcases, primals and bulk meat at individual sheep establishments are presented in Figure 2, together with the whole industry combined for carcases and bulk meat, based on ESAM data, indicating that the trial establishments were broadly representative of the industry. The mean log10 APC from carcases across all three sheep establishments was 1.56 log10 cfu/cm2, with establishment H having a mean around 1 log10 units higher than establishments G and I. All three sheep establishments used inverted dressing, but line speed differed from 8/minute (establishment G) to 8.5 (establishment H) and 10 (establishment I), as did the use of a two-knife system and legging paper to prevent roll-back of the pelt and consequent contamination of the forequarters (Table 2).
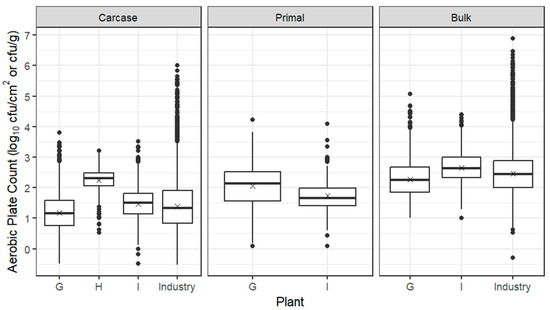
Figure 2.
Box plots of the APC of sheep carcases (log10 cfu/cm2), primals (log10 cfu/cm2) and bulk meat (log10 cfu/g) from Establishments G–I. The box encompasses data between the 25th and 75th percentiles, with the mean indicated by ‘X’ and median by a solid line. Box plots of the whole industry (ESAM data including trial establishments) are also given for carcases and bulk meat; industry data are currently not collected for primals.
After overnight chilling and passage of sheep carcases through the boning room, the mean log10 APC of primals was around 1 log10 cfu/cm2 higher at establishment G and less than 0.5 log10 cfu/cm2 higher at establishment I; bulk meat mean log10 APCs at establishments G and I were 2.27 and 2.66 log10 cfu/g, respectively. All carcases from establishment H were shipped off-site for fabrication at independent boning rooms, hence the absence of data for primals and bulk meat.
Following boning, the detection of E. coli was higher on sheep primals, compared with carcases, with the average concentration of E. coli from positive samples of carcases and primals ≤ 0.1 log10 cfu/cm2 (2 cfu/cm2). On excised samples of bulk meat, the prevalence of E. coli ranged from 15 to 24%, with the average concentrations ≥ 1.3 log10 cfu/g (20 cfu/g) at sheep establishments G and I (Table 6).

Table 6.
Prevalence (%) and (mean log10 concentration *) of E. coli on carcases (log10 cfu/cm2), primals (log10 cfu/cm2) and bulk meat (log10 cfu/g) at sheep establishments G–I. Mean values with the same letter within the same column are not significantly different.
The mean log10 APC of primals from the two sheep establishments was 1.89 log10 cfu/cm2 with means for individual primals close to the overall mean. The prevalence of E. coli was much higher than on beef primals, especially on legs and shoulders (Table 7).

Table 7.
APC of sheep primals (log10 cfu/cm2) and E. coli prevalence (%) at establishments G and I. Mean values with the same letter are not significantly different.
As for beef and sheep, box plots for APC from carcases, primals and bulk meat at individual pork establishments are presented in Figure 3, together with the whole industry combined, based on ESAM data, indicating that the trial establishments were broadly representative of the industry. The mean log10 APC from carcases from all three pork establishments was 1.35 log10 cfu/cm2, with the average being 1 log10 cfu/cm2 higher at pork establishment J compared with establishments K and L, possibly related to its faster line speed. Steam scalding at establishment K may also be linked with its lower APC. After overnight chilling and passage of pig carcases through the boning room, the mean log10 APCs of primals were higher at establishments K and L but lower at establishment J; bulk meat mean APCs ranged between 2.5 and 3.0 log10 cfu/g.
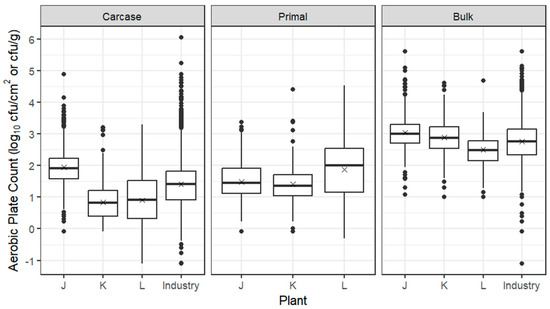
Figure 3.
Box plots of the APC of pork carcases (log10 cfu/cm2), primals (log10 cfu/cm2) and bulk meat (log10 cfu/g) from establishments J–L. The box encompasses data between the 25th and 75th percentiles, with the mean indicated by ‘X’ and median by a solid line. Box plots of the whole industry (ESAM data including trial establishments) are also given for carcases and bulk meat; industry data are currently not collected for primals.
Following boning, the prevalence of E. coli was lower on pork primals compared with carcases. On excised samples of bulk meat, the prevalence of E. coli ranged from 1.4 to 3.9%, with the average concentrations approximately 1.2 log10 cfu/g (16 cfu/g) (Table 8).

Table 8.
Prevalence (%) and (mean log10 concentration *) of E. coli on carcases (log10 cfu/cm2), primals (log10 cfu/cm2) and bulk meat (log10 cfu/g) at pig establishments J–L. Mean values with the same letter within the same column are not significantly different.
The mean log10 APC of primals from the three pork establishments was 1.57 log10 cfu/cm2, with means for individual primal cuts similar to the overall mean, with the exception of trotters, which were considerably higher than other primals (Table 9).

Table 9.
APC of pork primals (log10 cfu/cm2) at establishments J–L. Mean values with the same letter are not significantly different.
Establishments reported APCs for more than 40 offal types, of which the most commonly collected (n > 25) are presented in Table 10. All establishments collected ‘red’ offals (hearts, kidneys, livers, etc.) while ‘green’ offals (stomach parts processed by scalding) were collected predominately from beef and sheep. Some offals were specific for only sheep (brains) or pigs (chitterlings, ears, snouts and trotters).

Table 10.
Mean log10 APC (log10 cfu/g) of beef, sheep and pig offals. Mean values with the same letter within the same column are not significantly different.
While microbiological quality varied between offal type, there was comparably little variability between the same offals taken from beef, sheep or pig carcases. Offal from organs (heart, liver and kidney) were generally 2 log10 cfu/g while tripes were 3 log10 cfu/g and tongues were 4 log10 cfu/g. It might be expected that organ offals could be removed without significantly increasing their bacterial load, and so would pick up contamination whilst passing down chutes and from handling in the offal room. In contrast, offals derived from the gastrointestinal tract would have a high bacterial loading prior to washing, scalding and cooling, a proportion of which would be retained on the finished product. Tongues and meats derived from the head might also be expected to have a higher bacterial loading, stemming from contamination with saliva. The mean APCs in Table 10 are very similar to those obtained in a contemporaneous survey of chilled and frozen offals from 17 Australian export establishments which stated “the average APC on beef, sheep and lamb offal was 3.25, 3.38 and 3.70 log10 cfu/g, respectively” [18].
Previous surveys [4,5,6,7,8,9,10,11] monitored carcases and cuts at establishments which represented approximately 80% of industry output. By contrast, the present 13-month survey monitored establishments representing approximately 26% (beef), 15% (sheep) and 41% (pork) of national output on a daily basis. In addition, the present survey sampled carcases plus products derived from them: primal cuts, bulk meat and offals; for carcases and the derived end products, there was little evidence of seasonal effects on APCs [13].
In Table 11 are presented summary data of surveys of Australian beef and sheep carcases, which all used the same methodology. As a result, it may be construed that there has been a meaningful reduction in total bacterial loadings over the period 1998–2018, reflecting significant improvements in livestock handling, establishment infrastructure, operator training and the uptake of HACCP systems throughout the industry.

Table 11.
Beef and sheep carcase contamination in Australia from 1998 to 2018.
Currently, the performance of individual establishments is assessed against criteria set by the Australian regulator, the Department of Agriculture, Fisheries and Forestry (current name) in the Microbiological Manual for Sampling and Testing of Export Meat and Meat Products [2], using limits for APC and generic E. coli and three-class sampling plans that are assessed on a moving window of consecutive samples (n = 15), as described by FAO/WHO [19]. A window failure occurs when the number of marginal results (> m but ≤ M) exceeds c, or a single result exceeds the unacceptable level (M); there are different values for c, m and M according to livestock category [2]. In the present survey, there were 19 failed windows in five establishments over the 13-month survey period—13 for beef, 5 for sheep and 1 for pig carcases (Table 12). No other establishment had a moving window failure.

Table 12.
Failed windows for APC and E. coli on beef, sheep and pig carcases; in the ‘m’ column are listed the number of failures due to exceeding ‘m’ too many times in the moving window, while in the ‘M’ column are listed the number of failures due to exceeding ‘M’.
As set out by DAFF [2], Australia’s current performance monitoring system sets different sampling and evaluation criteria for carcases of bovines, ovines, porcines, caprines, cervines, equines, Camelidae, ratites, macropods and wild boars, and for various categories within them (steer/heifers versus cow/bulls). For the three most processed species (bovines, ovines and porcines), the criteria for n, c, m and M were formulated based on performance data from 2001 to 2002 [3]. However, as indicated in Table 11, the hygienic condition of carcases has improved greatly over the ensuing period and the export of carcase parts, particularly primals and offals, has also increased substantially, e.g., offal exports now exceed 200,000 t/annum [20].
The results of this trial have enabled representatives from industry, the regulator and research establishments to develop criteria which better reflect the performance of the current meat industry. Major proposed changes include setting identical criteria for n and c and an m-limit for products (carcases, primals, bulk meat and offals) from all species (beef, sheep, pork, etc.); removing Salmonella testing; and reducing frequency of carcase monitoring balanced by monitoring primals, bulk meat and offals. The window system is retained, and failure to meet any criterion for any product triggers an Alert requiring the establishment to review the process to identify any factors that may have caused the Alert and take any corrective and preventative action to control those factors in discussion with the on-plant veterinarian.
The resultant alternative monitoring system is currently being reviewed by Australia’s major trading partners.
Author Contributions
Conceptualization, J.J., A.K. and J.S.; methodology, J.J., A.K. and J.S.; software, J.J.; validation, J.J., A.K. and J.S.; formal analysis, J.J., A.K. and J.S.; investigation, J.J., A.K. and J.S.; resources, J.J.; data curation, J.J. and A.K.; writing—original draft preparation, J.J. and J.S.; writing—review and editing, J.J., A.K. and J.S.; visualization, J.J., A.K. and J.S.; supervision, J.J., A.K. and J.S.; project administration, J.J.; funding acquisition, J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Australian Meat Processor Corporation (Project 2018.1070) and Meat and Livestock Australia (Project V.MFS.0004) with matching funds from the Australian Government and with contributions from participating establishments.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy obligations to the participating processing establishments.
Acknowledgments
The authors wish to thank the staff of processing establishments for collection of the required samples and submission of microbiological data.
Conflicts of Interest
Author Andreas Kiermeier was employed by the company Statistical Process Improvement Consulting and Training Pty Ltd. Author John Sumner was employed by the company M&S Food Consultants Pty Ltd.
References
- Food Safety and Inspection Service. Pathogen Reduction: Hazard Analysis and Critical Control Point (HACCP) Systems; Final Rule, Federal Register; Department of Agriculture, Food Safety and Inspection Service: Washington, DC, USA, 1996; Volume 61, pp. 38806–38989.
- Department of Agriculture, Water and the Environment. Microbiological Manual for Sampling and Testing of Export Meat and Meat Products; version 1.05; Department of Agriculture, Water and the Environment: Canberra, Australia, 2021.
- Vanderlinde, P.; Jenson, I.; Sumner, J. Using national microbiological data to set meaningful performance criteria for slaughter and dressing of animals at Australian export abattoirs. Int. J. Food Microbiol. 2005, 104, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Vanderlinde, P.; Shay, B.; Murray, J. Microbiological quality of Australian beef carcass meat and frozen bulk packed beef. J. Food Protect. 1999, 61, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Vanderlinde, P.; Shay, B.; Murray, J. Microbiological status of Australian sheep meat. J. Food Protect. 1999, 62, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.; Sumner, J.; Alexander, J.; Dutton, K. Microbiological quality of Australian beef. J. Food Protect. 2001, 64, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.; Sumner, J.; Alexander, J.; Dutton, K. Microbiological quality of Australian sheep meat. J. Food Protect. 2001, 64, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.; Jordan, D.; Morris, S.; Jenson, I.; Sumner, J. A national survey of the microbiological quality of beef carcasses and frozen boneless beef in Australia. J. Food Protect. 2006, 69, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.; Jordan, D.; Morris, S.; Jenson, I.; Sumner, J. Microbiological quality of Australian sheep meat in 2004. Meat Sci. 2006, 74, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.; Bridger, K.; Jenson, I.; Sumner, J. An Australian national survey of the microbiological quality of frozen boneless beef and beef primal cuts. J. Food Protect. 2012, 75, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.; Bridger, K.; Jenson, I.; Sumner, J. Microbiological quality of Australian sheep meat in 2011. Food Control 2012, 31, 291–294. [Google Scholar] [CrossRef]
- Jolley, J.; Sumner, J.; Kiermeier, A. Process Control Monitoring—Is There a Better Way? AMPC Report 2017-1068. 2018. Available online: https://www.ampc.com.au/research-development/product-process-integrity/process-control-monitoring-is-there-a-better-way (accessed on 13 July 2023).
- Jolley, J.; Kiermeier, A.; Sumner, J. Process Monitoring for the Australian Meat Industry—An Industry Trial. AMPC Project 2018-1070. 2019. Available online: https://www.ampc.com.au/research-development/product-process-integrity/process-monitoring-for-the-australian-meat-industry-a-comparative-industry-trial (accessed on 13 July 2023).
- Delhalle, L.; De Sadeleer, L.; Bollaerts, K.; Farnir, F.; Saegerman, C.; Korsak, N.; Dewulf, J.; De Zutter, L.; Daube, G. Risk factors for Salmonella and hygiene indicators in the 10 largest Belgian pig slaughterhouses. J. Food Protect. 2008, 71, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- ISO/IEC 17025:2005; General Requirements for the Competence of Testing and Calibration Laboratories. International Standards Organization: Geneva, Switzerland, 2005.
- Department of Agriculture, Fisheries and Forestry. Approved Methods for Microbiological Testing of Meat and Meat Products; Department of Agriculture, Water and the Environment: Canberra, Australia, 2022. Available online: https://www.agriculture.gov.au/biosecurity-trade/export/controlled-goods/meat/elmer-3/approved-methods-manual#daff-page-main (accessed on 13 July 2023).
- R Core Team. R 4.2.2: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Vanderlinde, P.; Horchner, P.; Huynh, L.; Jenson, I. Microbiological quality of red meat offal produced at Australian export establishments. Foods 2022, 11, 3007. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization; World Health Organization. Statistical Aspects of Microbiological Criteria Related to Foods: A Risk Managers Guide; Microbiological Risk Assessment Series 24; FAO: Rome, Italy; WHO: Rome, Italy, 2016; Available online: https://www.who.int/foodsafety/publications/mra_24/en/ (accessed on 13 July 2023).
- Department of Agriculture, Water and the Environment. Red Meat Export Statistics. 2020. Available online: https://www.agriculture.gov.au/biosecurity-trade/export/controlled-goods/meat/statistics (accessed on 23 April 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).