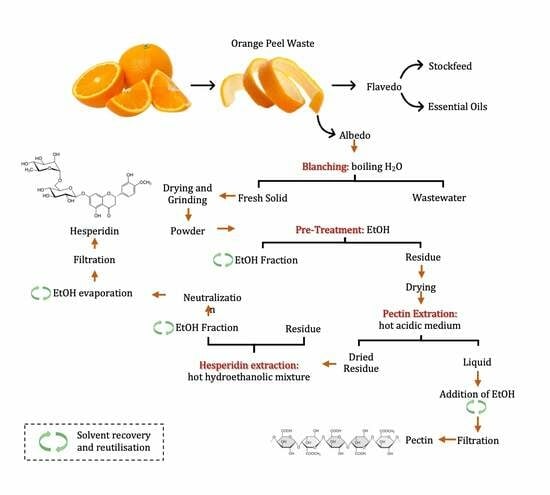
A Two-Step Approach to Orange Peel Waste Valorization: Consecutive Extraction of Pectin and Hesperidin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Reagents
2.2. Pretreatment and Washing
2.3. Consecutive Extractions
- (A)
- Pectin extraction
- (B)
- Hesperidin extraction
2.4. Statistical Analysis
2.5. Characterization
2.5.1. Extractions Yields
2.5.2. Fourier Transform Infrared Spectroscopy
2.5.3. Hesperidin Characterization
Nuclear Magnetic Resonance (NMR)
Melting Point
HPLC-PDA
2.5.4. Pectin Characterization
Equivalent Weight
Methoxyl Content (MeO)
- 31 (g/mol) is the molecular weight of the methoxyl group.
Anhydrouronic Acid Content (AUA)
- 176 (g/mol) is the molecular weight of AUA.
- z is the volume of NaOH (mL) from equivalent weight determination.
- y is the volume of NaOH (mL) from methoxyl content determination.
- W is the mass of pectin (g).
Degree of Esterification (DE)
3. Results and Discussion
3.1. Development of Consecutive Extraction of Pectin and Hesperidin
3.2. Characterization
3.2.1. Pectin Characterization
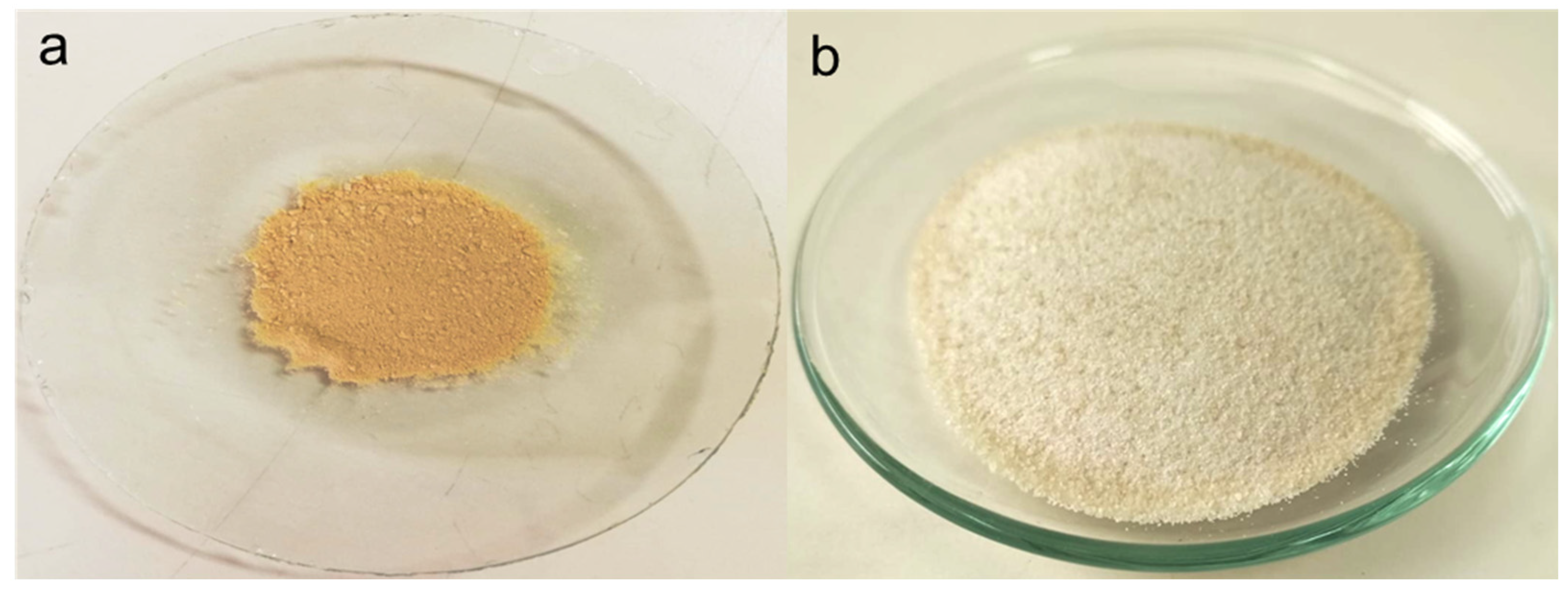
Extraction Yields
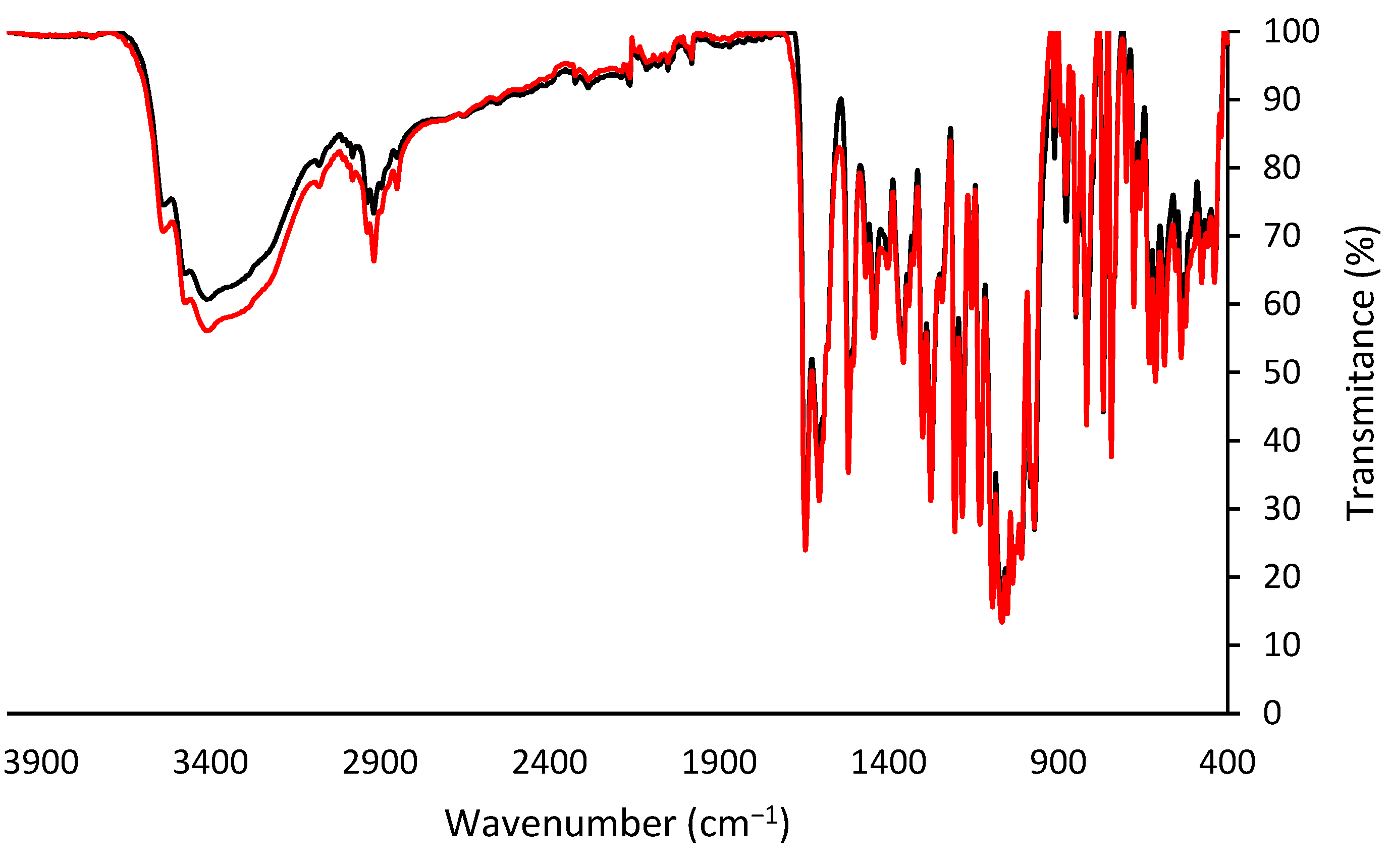
Fourier Transform Infrared Spectroscopy
Equivalent Weight
Methoxyl Content
Anhydrouronic Acid Content
Degree of Esterification
3.2.2. Hesperidin Characterization
Extraction Yields
Fourier Transform Infrared Spectroscopy
Nuclear Magnetic Resonance
Melting Point
HPLC-PDA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.M.; Sameen, A.; Aadil, R.M.; Shahid, M.; Sezen, S.; Zarrabi, A.; Ozdemir, B.; Sevindik, M.; Kaplan, D.N.; Selamoglu, Z.; et al. Citrus Genus and Its Waste Utilization: A Review on Health-Promoting Activities and Industrial Application. Evid. Based Complement. Alternat. Med. 2021, 2021, 2488804. [Google Scholar] [CrossRef] [PubMed]
- Amicarelli, V.; Lagioia, G.; Bux, C. Global Warming Potential of Food Waste through the Life Cycle Assessment: An Analytical Review. Environ. Impact Assess. Rev. 2021, 91, 106677. [Google Scholar] [CrossRef]
- Pandey, A. Food Wastage: Causes, Impacts and Solutions. Sci. Herit. J. 2021, 5, 17–20. [Google Scholar] [CrossRef]
- Singhal, S.; Swami Hulle, N.R. Citrus Pectins: Structural Properties, Extraction Methods, Modifications and Applications in Food Systems—A Review. Appl. Food Res. 2022, 2, 100215. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus Processing Wastes: Environmental Impacts, Recent Advances, and Future Perspectives in Total Valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Chang, Y.; Shi, X.; He, F.; Wu, T.; Jiang, L.; Normakhamatov, N.; Sharipov, A.; Wang, T.; Wen, M.; Aisa, H.A. Valorization of Food Processing Waste to Produce Valuable Polyphenolics. J. Agric. Food Chem. 2022, 70, 8855–8870. [Google Scholar] [CrossRef] [PubMed]
- Victor, M.M.; David, J.M.; Cortez, M.V.M.; Leite, J.L.; da Silva, G.S.B. A High-Yield Process for Extraction of Hesperidin from Orange (Citrus sinensis L. Osbeck) Peels Waste, and Its Transformation to Diosmetin, A Valuable and Bioactive Flavonoid. Waste Biomass Valorization 2021, 12, 313–320. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, J.; Shi, K.; Xu, Y.; Hu, H.; Xu, X.; Hu, T.; Zhang, P.; Yao, J.; Pan, S. Trends in Valorization of Citrus By-Products from the Net-Zero Perspective: Green Processing Innovation Combined with Applications in Emission Reduction. Trends Food Sci. Technol. 2023, 137, 124–141. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sharma, G.D.; Cysneiros, D.; Nayak, S.C.; Thakur, V.K.; Naidu, R.; Pandey, A.; Gupta, V.K. Minimizing Hazardous Impact of Food Waste in a Circular Economy—Advances in Resource Recovery through Green Strategies. J. Hazard. Mater. 2021, 416, 126154. [Google Scholar] [CrossRef]
- Suri, S.; Singh, A.; Nema, P.K. Current Applications of Citrus Fruit Processing Waste: A Scientific Outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Nieto, G.; Fernández-López, J.; Pérez-Álvarez, J.A.; Peñalver, R.; Ros-Berruezo, G.; Viuda-Martos, M. Valorization of Citrus Co-Products: Recovery of Bioactive Compounds and Application in Meat and Meat Products. Plants 2021, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- Lahmer, N.; Belboukhari, N.; Cheriti, A.; Sekkoum, K. Hesperidin and Hesperitin Preparation and Purification from Citrus sinensis Peels. Pharma Chem. 2015, 7, 1–4. [Google Scholar]
- Ding, Z.; Sun, G.; Zhu, Z. Hesperidin Attenuates Influenza a Virus (H1N1) Induced Lung Injury in Rats through Its Anti-Inflammatory Effect. Antivir. Ther. 2018, 23, 611–615. [Google Scholar] [CrossRef]
- Parvez, M.K.; Rehman, M.T.; Alam, P.; Al-Dosari, M.S.; Alqasoumi, S.I.; Alajmi, M.F. Plant-Derived Antiviral Drugs as Novel Hepatitis B Virus Inhibitors: Cell Culture and Molecular Docking Study. Saudi Pharm. J. 2019, 27, 389–400. [Google Scholar] [CrossRef]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New Light on the Healthy Function of Citrus Fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A Therapeutic Agent for Obesity. Drug Des. Devel. Ther. 2019, 2019, 3855–3866. [Google Scholar] [CrossRef]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [PubMed]
- Tamilselvam, K.; Braidy, N.; Manivasagam, T.; Essa, M.M.; Prasad, N.R.; Karthikeyan, S.; Thenmozhi, A.J.; Selvaraju, S.; Guillemin, G.J. Neuroprotective Effects of Hesperidin, a Plant Flavanone, on Rotenone-Induced Oxidative Stress and Apoptosis in a Cellular Model for Parkinson’s Disease. Oxid. Med. Cell. Longev. 2013, 2013, 102741. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, M.; Liu, Z.; Liu, M.; Zhang, S.; Zhang, Y. Hesperidin Inhibits Lung Cancer In Vitro and In Vivo Through PinX1. Front. Pharmacol. 2022, 13, 918665. [Google Scholar] [CrossRef] [PubMed]
- Hewage, S.R.K.M.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Han, X.; Oh, M.C.; Jung, U.; Kim, I.G.; Hyun, J.W. Hesperidin Attenuates Ultraviolet B-Induced Apoptosis by Mitigating Oxidative Stress in Human Keratinocytes. Biomol. Ther. 2016, 24, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.; Liu, L.H.B.; dos Santos, R.V.; Costa, A.F.; Durán, N.; Tasic, L. New Sustainable Process for Hesperidin Isolation and Anti-Ageing Effects of Hesperidin Nanocrystals. Molecules 2020, 25, 4534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Changqing, F.; Zhang, W.; Lei, W.; Wang, D.; Zhou, X. Novel Grasshopper Protein/Soy Protein Isolate/Pullulan Ternary Blend with Hesperidin Derivative for Antimicrobial Edible Film. Arab. J. Chem. 2023, 16, 104563. [Google Scholar] [CrossRef]
- Zhou, J.; Shi, Y.; Fang, J.; Gan, T.; Lu, Y.; Zhu, L.; Chen, X. Efficient Production of α-Monoglucosyl Hesperidin by Cyclodextrin Glucanotransferase from Bacillus Subtilis. Appl. Microbiol. Biotechnol. 2023, 107, 4803–4813. [Google Scholar] [CrossRef] [PubMed]
- MarketWatch. Hesperidin Market 2023: Global Industry Share, Key Dynamics and Forecast to 2028. Available online: https://www.marketwatch.com/press-release/hesperidin-market-2023-global-industry-share-key-dynamics-and-forecast-to-2028-2023-02-15 (accessed on 26 February 2023).
- Mudgil, D. Chapter 3—The Interaction Between Insoluble and Soluble Fiber. In Dietary Fiber for the Prevention of Cardiovascular Disease; Academic Press: Cambridge, MA, USA, 2017; pp. 35–59. ISBN 978-0-12-805130-6. [Google Scholar]
- Benassi, L.; Alessandri, I.; Vassalini, I. Assessing Green Methods for Pectin Extraction from Waste Orange Peels. Molecules 2021, 26, 1766. [Google Scholar] [CrossRef]
- Tsitsagi, M.; Ebralidze, K.; Chkhaidze, M.; Rubashvili, I.; Tsitsishvili, V. Sequential Extraction of Bioactive Compounds from Tangerine (Citrus unshiu) Peel. Ann. Agrar. Sci. 2018, 16, 236–241. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Łopusiewicz, Ł.; Biswas, D.; Chandel, V.; Rhim, J.-W. Recent Progress in Pectin Extraction, Characterization, and Pectin-Based Films for Active Food Packaging Applications: A Review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef]
- Mada, T.; Duraisamy, R.; Guesh, F. Optimization and Characterization of Pectin Extracted from Banana and Papaya Mixed Peels Using Response Surface Methodology. Food Sci. Nutr. 2022, 10, 1222–1238. [Google Scholar] [CrossRef]
- Kozioł, A.; Środa-Pomianek, K.; Górniak, A.; Wikiera, A.; Cyprych, K.; Malik, M. Structural Determination of Pectins by Spectroscopy Methods. Coatings 2022, 12, 546. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Scurria, A.; Ilharco, L.M.; Pagliaro, M. Pectin: New Science and Forthcoming Applications of the Most Valued Hydrocolloid. Food Hydrocoll. 2022, 127, 107483. [Google Scholar] [CrossRef]
- MarketWatch. Pectin Market Scenario 2023 to 2028: New Developments, Economic Situation and Growth Projection. Available online: https://www.marketwatch.com/press-release/pectin-market-scenario-2023-to-2028-new-developments-economic-situation-and-growth-projection-2023-02-09 (accessed on 12 February 2023).
- Kumari, M.; Singh, S.; Chauhan, A.K. A Comparative Study of the Extraction of Pectin from Kinnow (Citrus reticulata) Peel Using Different Techniques. Food Bioprocess Technol. 2023, 16, 2272–2286. [Google Scholar] [CrossRef]
- Padilla de la Rosa, J.; Ruiz-Palomino, P.; Arriola-Guevara, E.; García-Fajardo, J.; Sandoval, G.; Guatemala-Morales, G. A Green Process for the Extraction and Purification of Hesperidin from Mexican Lime Peel (Citrus aurantifolia Swingle) That Is Extendible to the Citrus Genus. Processes 2018, 6, 266. [Google Scholar] [CrossRef]
- Zouambia, Y.; Youcef Ettoumi, K.; Krea, M.; Moulai-Mostefa, N. A New Approach for Pectin Extraction: Electromagnetic Induction Heating. Arab. J. Chem. 2017, 10, 480–487. [Google Scholar] [CrossRef]
- Labrada, A.; Lobarbio, C.F.; Taboada, E. Downstream Purification of Pectin Precipitated from Mango Peel Extract. In Proceedings of the International Conference on Technological and Social Innovations 2018, London, UK, 30 June–2 July 2018; pp. 1–7. [Google Scholar]
- Al-Ashaal, H.A.; El-Sheltawy, S.T. Antioxidant Capacity of Hesperidin from Citrus Peel Using Electron Spin Resonance and Cytotoxic Activity against Human Carcinoma Cell Lines. Pharm. Biol. 2011, 49, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Chand, K.; Shahi, N.C. Effect of Process Parameters on Extraction of Pectin from Sweet Lime Peels. J. Inst. Eng. India Ser. A 2021, 102, 469–478. [Google Scholar] [CrossRef]
- Ranganna, S. HandBook of Analysis and Quality Control for Fruits and Vegetable Products, 2nd ed.; McGraw Hill Publishing Co.: New Delhi, India, 1995; ISBN 978-0-07-451851-9. [Google Scholar]
- Maskey, B.; Dhakal, D.; Pradhananga, M.; Shrestha, N.K. Extraction and Process Optimization of Bael Fruit Pectin. Food Sci. Nutr. 2018, 6, 1927–1932. [Google Scholar] [CrossRef]
- Mohamed, S.; Hasan, Z. Extraction and Characterisation of Pectin from Various Tropical Agrowastes. ASEAN Food J. 1995, 10, 143–150. [Google Scholar]
- Einhorn-Stoll, U.; Kastner, H.; Urbisch, A.; Kroh, L.W.; Drusch, S. Thermal Degradation of Citrus Pectin in Low-Moisture Environment—Influence of Acidic and Alkaline Pre-Treatment. Food Hydrocoll. 2019, 86, 104–115. [Google Scholar] [CrossRef]
- Majumdar, S.; Srirangam, R. Solubility, Stability, Physicochemical Characteristics and In Vitro Ocular Tissue Permeability of Hesperidin: A Natural Bioflavonoid. Pharm. Res. 2009, 26, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Kute, A.B.; Mohapatra, D.; Kotwaliwale, N.; Giri, S.K.; Sawant, B.P. Characterization of Pectin Extracted from Orange Peel Powder Using Microwave-Assisted and Acid Extraction Methods. Agric. Res. 2020, 9, 241–248. [Google Scholar] [CrossRef]
- Kamal, M.M.; Kumar, J.; Mamun, M.A.H.; Ahmed, M.N.U.; Shishir, M.R.I.; Mondal, S.C. Extraction and Characterization of Pectin from Citrus sinensis Peel. J. Biosyst. Eng. 2021, 46, 16–25. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of Pectin Extracted from Banana Peels of Different Varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Fakayode, O.A.; Abobi, K.E. Optimization of Oil and Pectin Extraction from Orange (Citrus sinensis) Peels: A Response Surface Approach. J. Anal. Sci. Technol. 2018, 9, 20. [Google Scholar] [CrossRef]
- Twinomuhwezi, H.; Godswill, C.; Kahunde, D. Extraction and Characterization of Pectin from Orange (Citrus sinensis), Lemon (Citrus limon) and Tangerine (Citrus tangerina). Am. J. Phys. Sci. 2020, 1, 17–30. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lim, S.-B. Extraction of Flavanones from Immature Citrus unshiu Pomace: Process Optimization and Antioxidant Evaluation. Sci. Rep. 2020, 10, 19950. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueira, O.; Pereira, V.; Castilho, P.C. A Two-Step Approach to Orange Peel Waste Valorization: Consecutive Extraction of Pectin and Hesperidin. Foods 2023, 12, 3834. https://doi.org/10.3390/foods12203834
Figueira O, Pereira V, Castilho PC. A Two-Step Approach to Orange Peel Waste Valorization: Consecutive Extraction of Pectin and Hesperidin. Foods. 2023; 12(20):3834. https://doi.org/10.3390/foods12203834
Chicago/Turabian StyleFigueira, Onofre, Verónica Pereira, and Paula C. Castilho. 2023. "A Two-Step Approach to Orange Peel Waste Valorization: Consecutive Extraction of Pectin and Hesperidin" Foods 12, no. 20: 3834. https://doi.org/10.3390/foods12203834
APA StyleFigueira, O., Pereira, V., & Castilho, P. C. (2023). A Two-Step Approach to Orange Peel Waste Valorization: Consecutive Extraction of Pectin and Hesperidin. Foods, 12(20), 3834. https://doi.org/10.3390/foods12203834