Effects of Cinnamon Powder on Glucose Metabolism in Diabetic Mice and the Molecular Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Collection and Preparation
2.3. Animals
2.4. Induction of Experimental Diabetes
2.5. Experimental Design
2.6. Oral Glucose Tolerance Test (OGTT)
2.7. Biochemical Assays in Serum and Liver
2.8. RNA Preparation and Quantitative Real-Time RT-PCR (qPCR)
2.9. Western Blotting
2.10. Data Analysis
3. Results
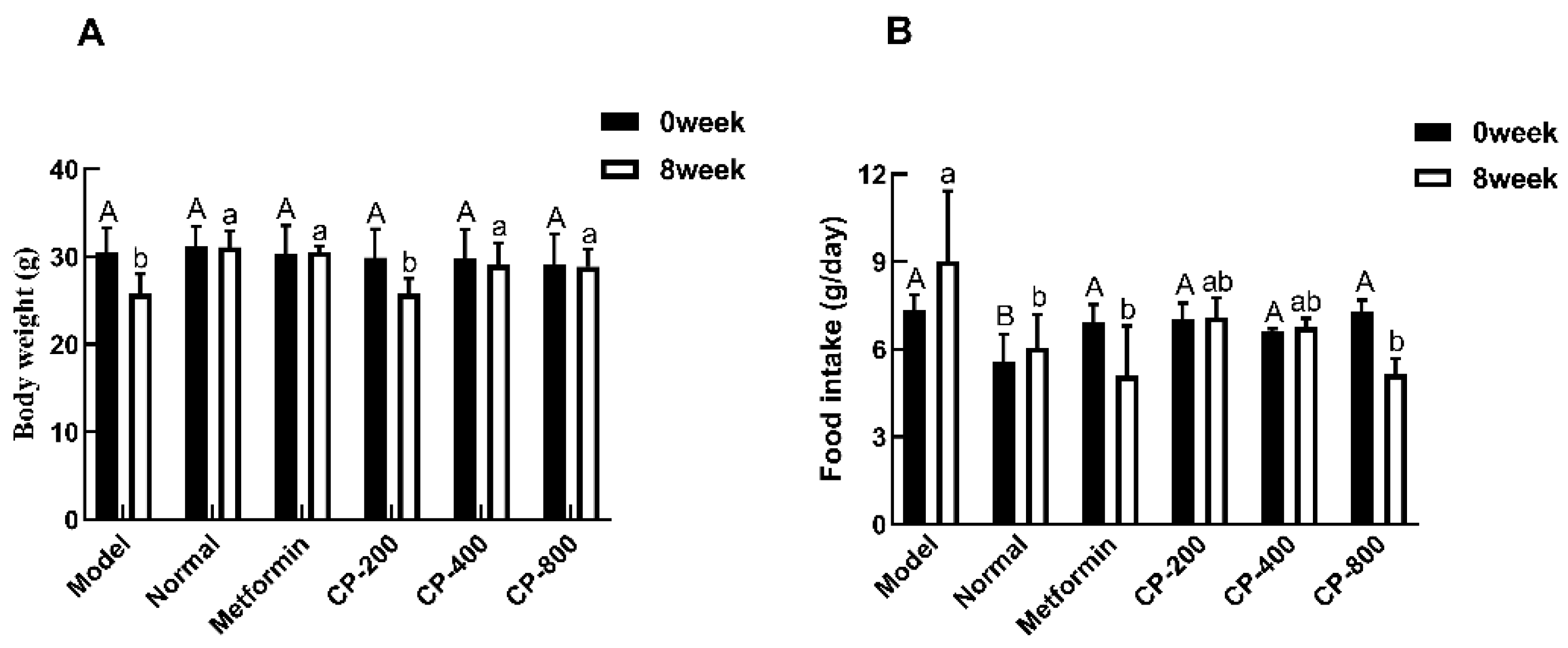
3.1. Effects of CP on Body Weight and Food Intake in Diabetic Mice
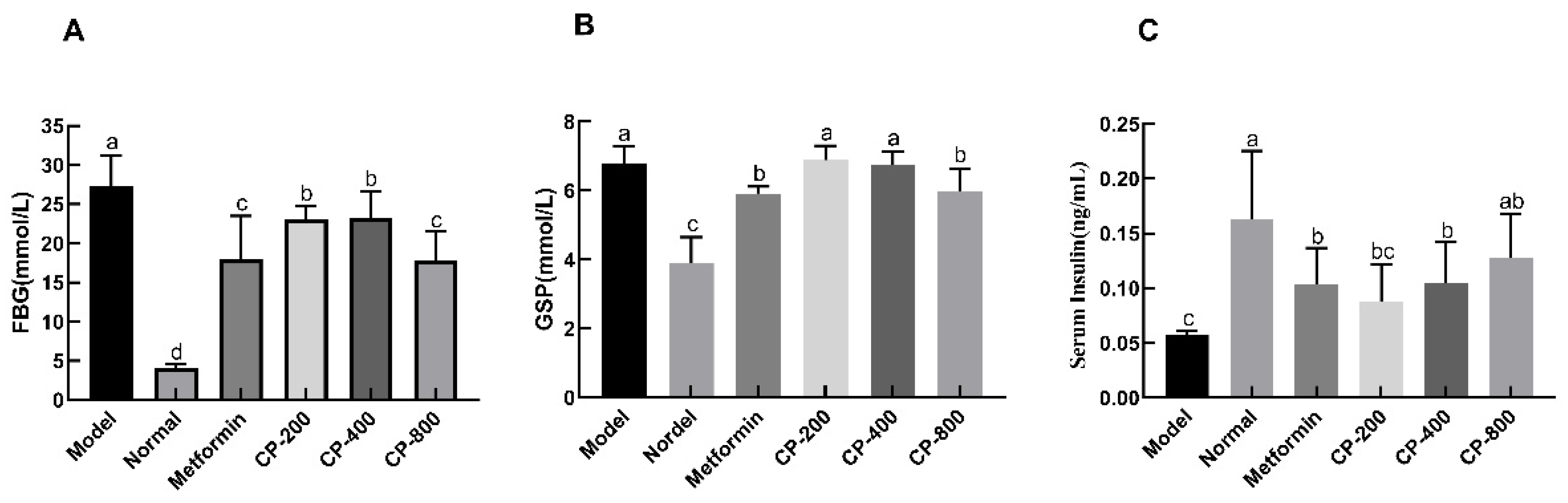
3.2. Effects of CP on FBG, Glycosylated Serum Protein (GSP), and Fasting Serum Insulin (FINS) Levels in Diabetic Mice
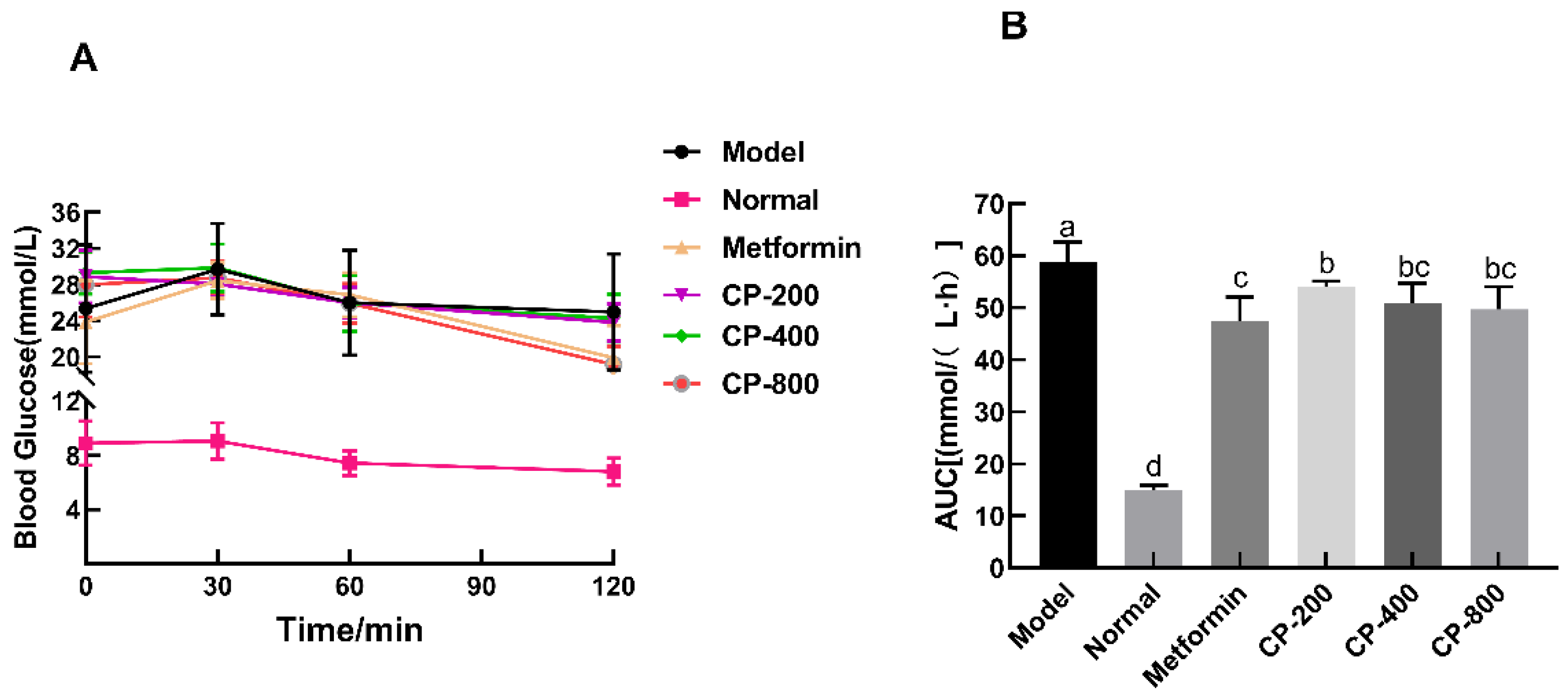
3.3. Effects of CP on OGTT and AUC
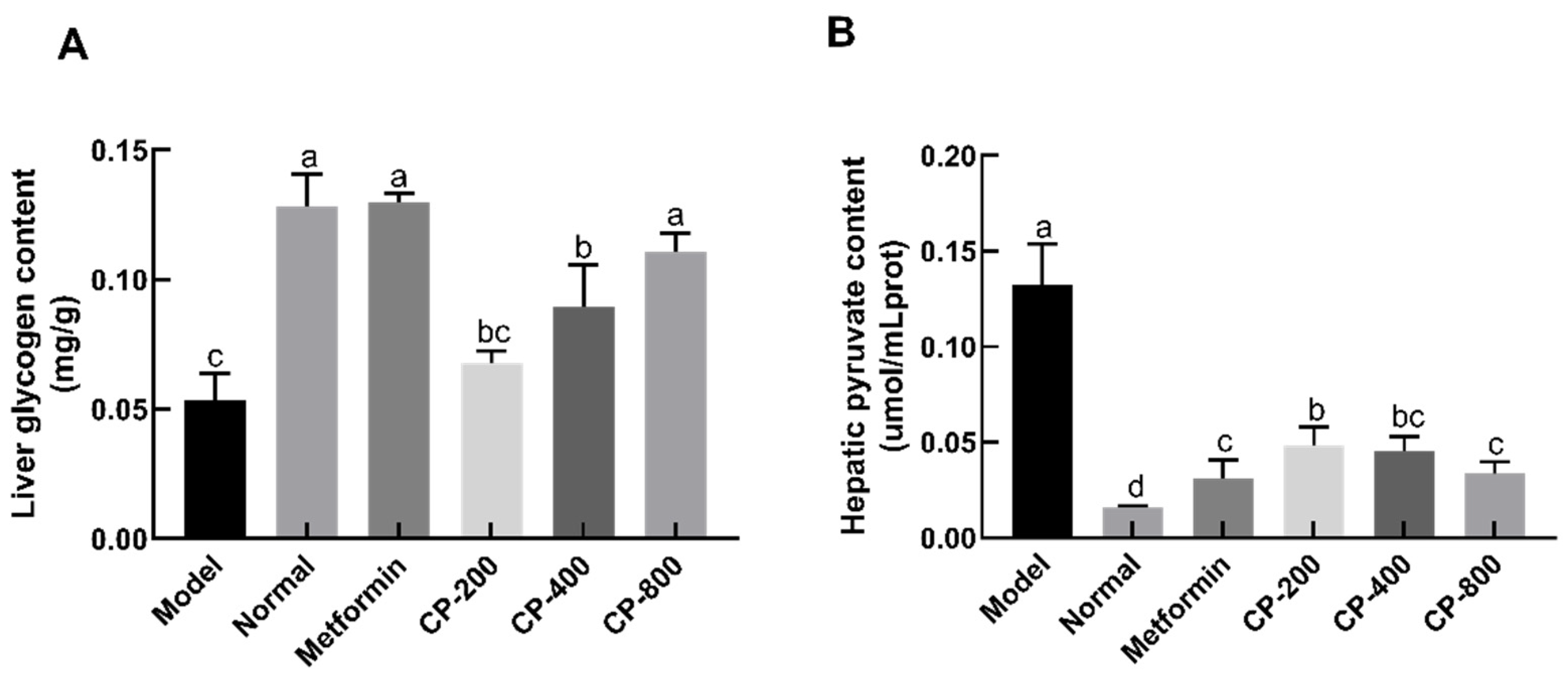
3.4. Effects of CP on the Glycogen and Pyruvate Levels in the Liver of Diabetic Mice
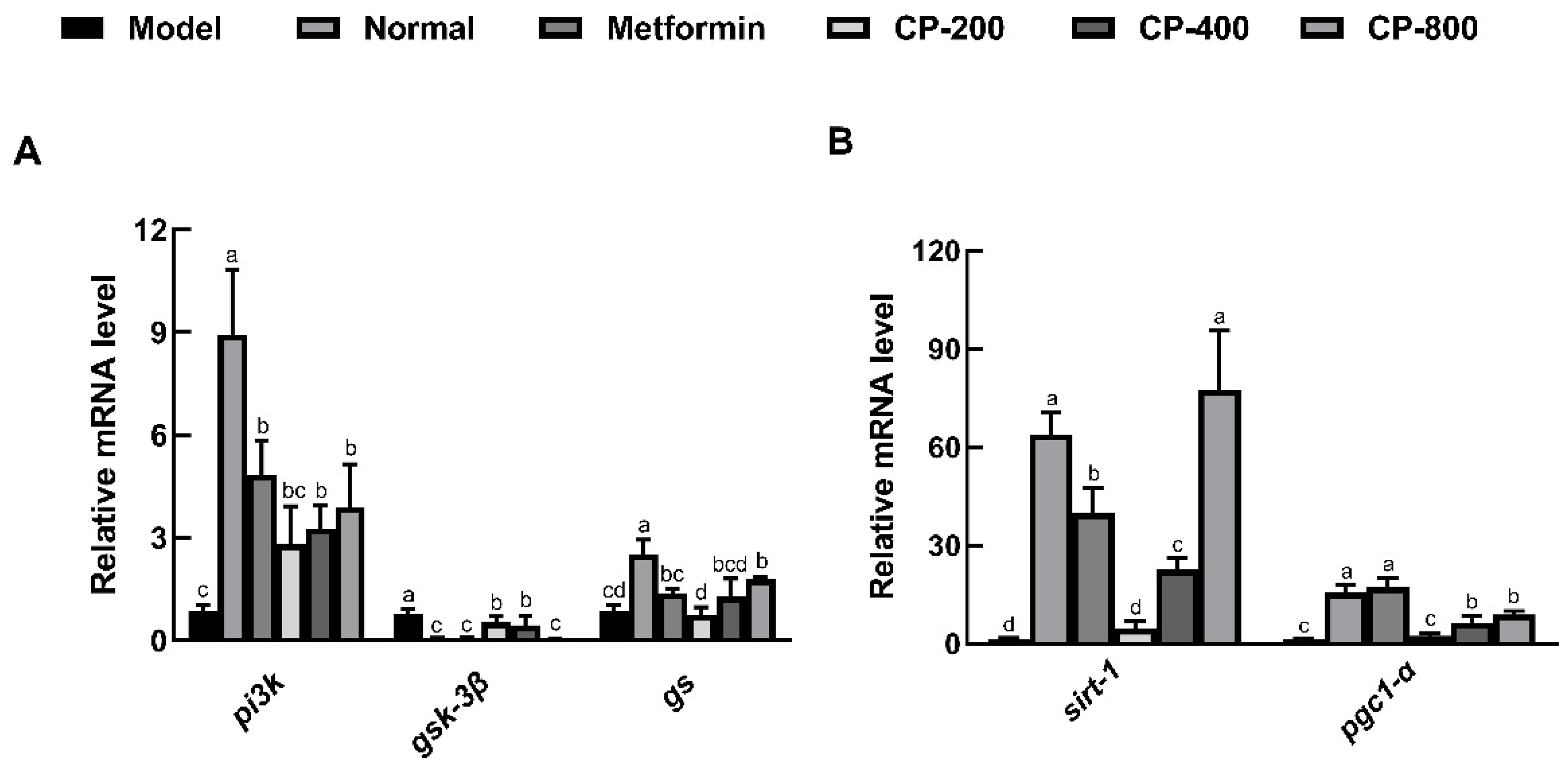
3.5. Effects of CP on Relative Hepatic Expression of Genes Involved in Glycogenesis and Gluconeogenesis
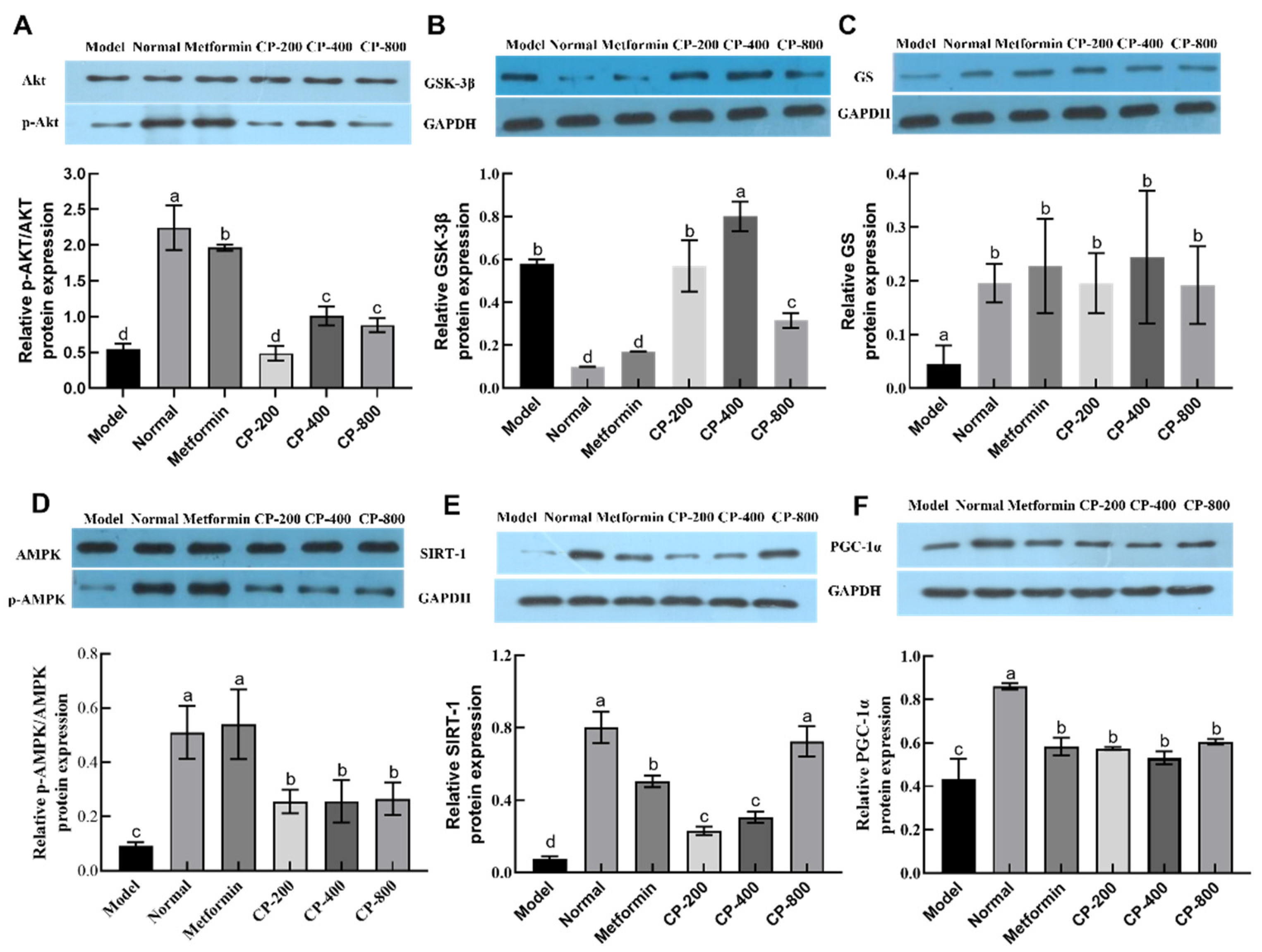
3.6. Effects of CP on Relative Hepatic Expression of Proteins Involved in Glycogenesis and Gluconeogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the glycemic curve |
| AKT | Serine-threonine protein kinase |
| AMPKα | AMP-activated protein kinase alpha |
| CP | Cinnamon powder |
| FBG | Fasting blood glucose |
| FINS | Fasting serum insulin |
| GSK3β | Glycogen synthase kinase 3 beta |
| GSP | Glycosylated serum protein |
| GS | Glycogen synthase |
| HFD | High-fat diet |
| miRNA | microRNA |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PI3K | Phosphatidylinositol 3-kinase |
| p-AMPKα | Phosphorylated-AMPKα |
| p-AKT | Phosphorylated-AKT |
| SIRT1 | Silent information regulator factor 2-related enzyme 1 |
| STZ | Streptozotocin |
References
- Hamidpour, R.; Hamidpour, M.; Hamidpour, S.; Shahlari, M. Cinnamon from the selection of traditional applications to its novel effects on the inhibition of angiogenesis in cancer cells and prevention of Alzheimer’s disease, and a series of functions such as antioxidant, anticholesterol, antidiabetes, antibacterial, antifungal, nematicidal, acaracidal, and repellent activities. J. Tradit. Complement. Med. 2015, 5, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.H.; Graham, L.; Adukwu, E.C. In vitro antifungal activity of Cinnamomum zeylanicum bark and leaf essential oils against Candida albicans and Candida auris. Appl. Microbiol. Biotechnol. 2020, 104, 8911–8924. [Google Scholar] [CrossRef] [PubMed]
- Bento-Bernardes, T.; Toste, F.P.; Pazos-Moura, C.C.; Oliveira, K.J. Maternal cinnamon extract intake during lactation leads to sex-specific endocrine modifications in rat offspring. J. Sci. Food Agric. 2017, 97, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.B.; Dwyer, J.T.; Saldanha, L.; Bailey, R.L.; Merkel, J.; Wambogo, E. Do cinnamon supplements have a role in glycemic control in type 2 diabetes? A narrative review. J. Acad. Nutr. Diet 2016, 116, 1794–1802. [Google Scholar] [CrossRef]
- Hlebowicz, J.; Darwiche, G.; Bjorgell, O.; Almer, L.O. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am. J. Clin. Nutr. 2007, 85, 1552–1556. [Google Scholar] [CrossRef]
- Hayward, N.J.; McDougall, G.J.; Farag, S.; Allwood, J.W.; Austin, C.; Campbell, F.; Horgan, G.; Ranawana, V. Cinnamon shows antidiabetic properties that are species-specific: Effects on enzyme activity inhibition and starch digestion. Plant Foods Hum. Nutr. 2019, 74, 544–552. [Google Scholar] [CrossRef]
- Tian, C.Y.; Zhu, Y.P.; Liu, Y.J.; Hu, H.; Cheng, Q.J.; Yang, F.W.; Pei, L.Q.; Zhou, Y.H.; Li, Y.; Lin, S.D. High albumin level is associated with regression of glucose metabolism disorders upon resolution of acute liver inflammation in hepatitis b-related cirrhosis. Front. Cell Infect. Microbiol. 2022, 12, 721138. [Google Scholar] [CrossRef]
- Huang, X.B.; He, Y.G.; Zheng, L.; Feng, H.; Li, Y.M.; Li, H.Y.; Yang, F.X.; Li, J. Identification of hepatitis B virus and liver cancer bridge molecules based on functional module network. World J. Gastroenterol. 2019, 25, 4921–4932. [Google Scholar] [CrossRef]
- Zhu, X.A.; Ouyang, W.; Lan, Y.Q.; Xiao, H.; Tang, L.; Liu, G.; Feng, K.L.; Zhang, L.Q.; Song, M.Y.; Cao, Y. Anti-hyperglycemic and liver protective effects of flavonoids from Psidium guajava L. (guava) leaf in diabetic mice. Food Biosci. 2020, 35, 100574. [Google Scholar] [CrossRef]
- Luo, Z.H.; Fu, C.F.; Li, T.; Gao, Q.Q.; Miao, D.Y.; Xu, J.; Zhao, Y.Q. Hypoglycemic effects of licochalcone a on the streptozotocin-induced diabetic mice and its mechanism study. J. Agric. Food Chem. 2021, 69, 2444–2456. [Google Scholar] [CrossRef]
- Xiao, Z.Q.; Wang, Y.L.; Gan, S.R.; Chen, J.C. Polysaccharides from liriopes radix ameliorates hyperglycemia via various potential mechanisms in diabetic rats. J. Sci. Food Agric. 2014, 94, 975–982. [Google Scholar] [CrossRef]
- Chen, C.; Tan, S.M.; Ren, T.Y.; Wang, H.; Dai, X.T.; Wang, H. Polyphenol from Rosa roxburghii Tratt fruit ameliorates the symptoms of diabetes by activating the P13K/AKT insulin pathway in db/db mice. Foods 2022, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Zhang, T.T.; Dong, Z.; Shi, H.H.; Xu, J.; Mao, X.Z.; Wang, Y.M.; Xue, C.H. Dietary supplementation with exogenous sea-cucumber-derived ceramides and glucosylceramides alleviates insulin resistance in high-fructose-diet-fed rats by upregulating the IRS/PI3K/Akt signaling pathway. J. Agric. Food Chem. 2021, 69, 9178–9187. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, P.; Wang, T.; Chen, K.X.; Jia, Q.; Wang, H.Y.; Li, Y.M. Diverse mechanisms of antidialbetic effects of the different procyanidin oligomer types of two different cinnamon species on db/db mice. J. Sci. Food Agric. 2012, 60, 9144–9150. [Google Scholar] [CrossRef]
- Cheng, D.M.; Kuhn, P.; Poulev, A.; Rojo, L.E.; Lila, M.A.; Raskin, I. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012, 135, 2994–3002. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Pang, D.R.; Xing, D.X.; Wang, W.F.; Li, Q.; Liao, S.T.; Li, E.N.; Zou, Y.X. Cinnamon free phenolic extract regulates glucose absorption in intestinal cells by inhibiting glucose transporters. Food Biosci. 2023, 52, 102405. [Google Scholar] [CrossRef]
- Jiao, L.H.; Zhang, X.; Huang, L.Q.; Gong, H.; Cheng, B.; Sun, Y.; Li, Y.X.; Liu, Q.; Zheng, L.; Huang, K. Proanthocyanidins are the major anti-diabetic components of cinnamon water extract. Food Chem. Toxicol. 2013, 56, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.L.; Jia, Q.; Wang, R.; Wu, X.M.; Wu, Y.C.; Huang, C.G.; Li, Y.M. Hypoglycemic activities of A- and B-type procyanidin oligomer-rich extracts from different Cinnamon barks. Phytomedicine 2011, 18, 298–302. [Google Scholar] [CrossRef]
- Trinidad, T.P.; Mallillin, A.C.; Valdez, D.H.; Loyola, A.S.; Askali-Mercado, F.C.; Castillo, J.C.; Encabo, R.R.; Masa, D.B.; Maglaya, A.S.; Chua, M.T. Dietary fiber from coconut flour: A functional food. Innov. Food Sci. Emer. 2006, 7, 309–317. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Deng, M.; Zhang, S.; Dong, L.H.; Huang, F.; Jia, X.C.; Su, D.X.; Chi, J.W.; Muhammad, Z.; Ma, Q.; Zhao, D.; et al. Shatianyu (Citrus grandis L. Osbeck) flavonoids and dietary fiber in combination are more effective than individually in alleviating high-fat-diet-induced hyperlipidemia in mice by altering gut microbiota. J. Agric. Food Chem. 2022, 70, 14654–14664. [Google Scholar] [CrossRef]
- Huang, H.R.; Chen, J.J.; Ao, T.X.; Chen, Y.; Xie, J.H.; Hu, X.B.; Yu, Q. Exploration of the role of bound polyphenols on tea residues dietary fiber improving diabetic hepatorenal injury and metabolic disorders. Food Res. Int. 2022, 162, 112062. [Google Scholar] [CrossRef]
- Huang, H.R.; Chen, J.J.; Hu, X.B.; Chen, Y.; Xie, J.H.; Ao, T.X.; Wang, H.; Xie, J.Y.; Yu, Q. Elucidation of the interaction effect between dietary fiber and bound polyphenol components on the anti-hyperglycemic activity of tea residue dietary fiber. Food Funct. 2022, 13, 2710–2728. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.J.; Xiang, L.; Huang, Z.; Zhou, S.; Zhang, S.; Zhang, T.; Jiang, T.P. Overexpression of PIK3R1 promotes hepa-tocellular carcinoma progression. Biol. Res. 2018, 51, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, J.T.; Gomes, P.R.L.; Zanetti, G.; Mezzalira, N.; Lima, O.G.; de Assis, L.V.M.; Guler, A.; Castrucci, A.M.; Moraes, M.N. Lack of trpv1 channel modulates mouse gene expression and liver proteome with glucose metabolism changes. Int. J. Mol. Sci. 2022, 23, 7014. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.X.; Wang, P.; Wang, X.H.; Zhou, X.L.; Hu, X.S.; Chen, F. Dietary platycodon grandiflorus attenuates hepatic insulin resistance and oxidative stress in high-fat-diet induced non-alcoholic fatty liver disease. Nutrients 2020, 12, 480. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Spatz, M.H.; Folk, C.; Chang, A.; Cadenas, E.; Liang, J.; Davies, D.L. Dihydromyricetin improves mitochondrial outcomes in the liver of alcohol-fed mice via the AMPK/Sirt-1/PGC-1α signaling axis. Alcohol 2021, 91, 1–9. [Google Scholar] [CrossRef]
- Liang, X.M.; Yee, S.W.; Chien, H.C.; Chen, E.C.; Luo, Q.; Zou, L.; Piao, M.L.; Mifune, A.; Chen, L.G.; Calvert, M.E.; et al. Organic cation transporter 1 (OCT1) modulates multiple cardiometabolic traits through effects on hepatic thiamine content. PLoS Biol. 2018, 16, e2002907. [Google Scholar] [CrossRef]
- Agbu, P.; Carthew, R.W. MicroRNA-mediated regulation of glucose and lipid metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 425–438. [Google Scholar] [CrossRef]
- Regazzi, R. MicroRNAs as therapeutic targets for the treatment of diabetes mellitus and its complications. Expert Opin. Ther. Targets 2018, 22, 153–160. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Katsiki, N.; Behnam, B.; Iranpanah, H.; Sahebkar, A. MicroRNAs and type 2 diabetes mellitus: Molecular mechanisms and the effect of antidiabetic drug treatment. Metabolism 2018, 87, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Vienberg, S.; Geiger, J.; Madsen, S.; Dalgaard, L.T. MicroRNAs in metabolism. Acta Physiol. 2013, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Lu, Y.G.; Wang, F.H.; Dou, L.; Wang, L.L.; Guo, J.; Li, J. High glucose reduces hepatic glycogenesis by suppression of microRNA-152. Mol. Med. Rep. 2014, 10, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Zhou, F.Y.; Li, M.Z.; Zhang, Y.M.; Li, N.; Shao, L. MiR-34a-5p promotes hepatic gluconeogenesis by suppressing SIRT1 expression. Exp. Cell Res. 2022, 420, 113336. [Google Scholar] [CrossRef]
- Fang, Z.J.; Li, P.; Jia, W.H.; Jiang, T.; Wang, Z.Y.; Xiang, Y. miR-696 plays a role in hepatic gluconeogenesis in ob/ob mice by targeting PGC-1α. Int. J. Mol. Med. 2016, 38, 845–852. [Google Scholar] [CrossRef]
- Mohsin, S.N.; Saleem, F.; Humayun, A.; Tanweer, A.; Muddassir, A. Prospective nutraceutical effects of cinnamon derivatives against insulin resistance in type II diabetes mellitus-evidence from the literature. Dose Response 2023, 21, 15593258231200527. [Google Scholar] [CrossRef]
- Shang, C.; Lin, H.C.; Fang, X.Q.; Wang, Y.L.; Jiang, Z.L.; Qu, Y.; Xiang, M.; Shen, Z.H.; Xin, L.Y.; Lu, Y.D.; et al. Beneficial effects of cinnamon and its extracts in the management of cardiovascular diseases and diabetes. Food Funct. 2021, 12, 12194–12220. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, E.G.; Alfeky, N.H.; Ibrahim, A.O. Upregulation of GLUT4 and PI3K, and downregulation of GSK3 mediate the anti-hyperglycemic effects of proanthocyanidins. Med. Int. 2022, 2, 14–21. [Google Scholar] [CrossRef]
- Kim, W.; Khil, L.Y.; Clark, R.; Bok, S.H.; Kim, E.E.; Lee, S.; Jun, H.S.; Yoon, J.W. Naphthalenemethyl ester derivative of dihydroxyhydrocinnamic acid, a component of cinnamon, increases glucose disposal by enhancing translocation of glucose transporter 4. Diabetologia 2006, 49, 2437–2448. [Google Scholar] [CrossRef]
- Taheri, A.; Lavasani, H.; Kasirzadeh, S.; Sheikholeslami, B.; Ardakani, Y.H.; Rouini, M.R. Changes in CYP2D enzyme activity following induction of type 2 diabetes, and administration of cinnamon and metformin: An experimental animal study. Xenobiotica 2018, 48, 984–989. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| gapdh | GGAGAAACCTGCCAAGTATGATGAC | GAGACAACCTGGTCCTCAGTGTA |
| pi3k | TATGCCTGCTCCGTAGTGGTAGAC | GGTGGCTTGAAGGTGAGGAACTG |
| akt | CACCGTGTGACCATGAACGAGTT | TGGCGACGATGACCTCCTTCTT |
| gsk3β | TAATGCTGGAGACCGTGGACAG | CGTGACCAGTGTTGCTGAGTG |
| gs | GAACAGACGGCCACCCATT | CACTGGGCAGGCATAACCT |
| ampkα | CGTCGCCTACCACCTCATCATAGA | TCGGCAACCAAGAACGGTACTCT |
| sirt-1 | GTGGCAGTAACAGTGACAGTGGC | TCCAGATCCTCCAGCACATTCGG |
| pgc1α | ATGTGTCGCCTTCTTGCTCTTCC | CGGTGTCTGTAGTGGCTTGATTCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, F.; Xing, D.; Wang, W.; Yang, Q.; Liao, S.; Li, E.; Pang, D.; Zou, Y. Effects of Cinnamon Powder on Glucose Metabolism in Diabetic Mice and the Molecular Mechanisms. Foods 2023, 12, 3852. https://doi.org/10.3390/foods12203852
Liu Y, Liu F, Xing D, Wang W, Yang Q, Liao S, Li E, Pang D, Zou Y. Effects of Cinnamon Powder on Glucose Metabolism in Diabetic Mice and the Molecular Mechanisms. Foods. 2023; 12(20):3852. https://doi.org/10.3390/foods12203852
Chicago/Turabian StyleLiu, Yaoyao, Fan Liu, Dongxu Xing, Weifei Wang, Qiong Yang, Sentai Liao, Erna Li, Daorui Pang, and Yuxiao Zou. 2023. "Effects of Cinnamon Powder on Glucose Metabolism in Diabetic Mice and the Molecular Mechanisms" Foods 12, no. 20: 3852. https://doi.org/10.3390/foods12203852
APA StyleLiu, Y., Liu, F., Xing, D., Wang, W., Yang, Q., Liao, S., Li, E., Pang, D., & Zou, Y. (2023). Effects of Cinnamon Powder on Glucose Metabolism in Diabetic Mice and the Molecular Mechanisms. Foods, 12(20), 3852. https://doi.org/10.3390/foods12203852






