Microscopic Imaging to Visualize the Distribution of Dietary Nucleic Acids in Food Products of Various Origins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Biochemicals
2.2. Food Samples
2.3. Preparation of Paraffin-Embedded Sections of Meat and Cheese Samples
2.4. Preparation of Paraffin-Embedded Sections of Plant Samples
2.5. Preparation of Cryosections of Food Samples
2.6. Basic Tissue Staining
2.7. Fluorescent Staining of Nucleic Acids
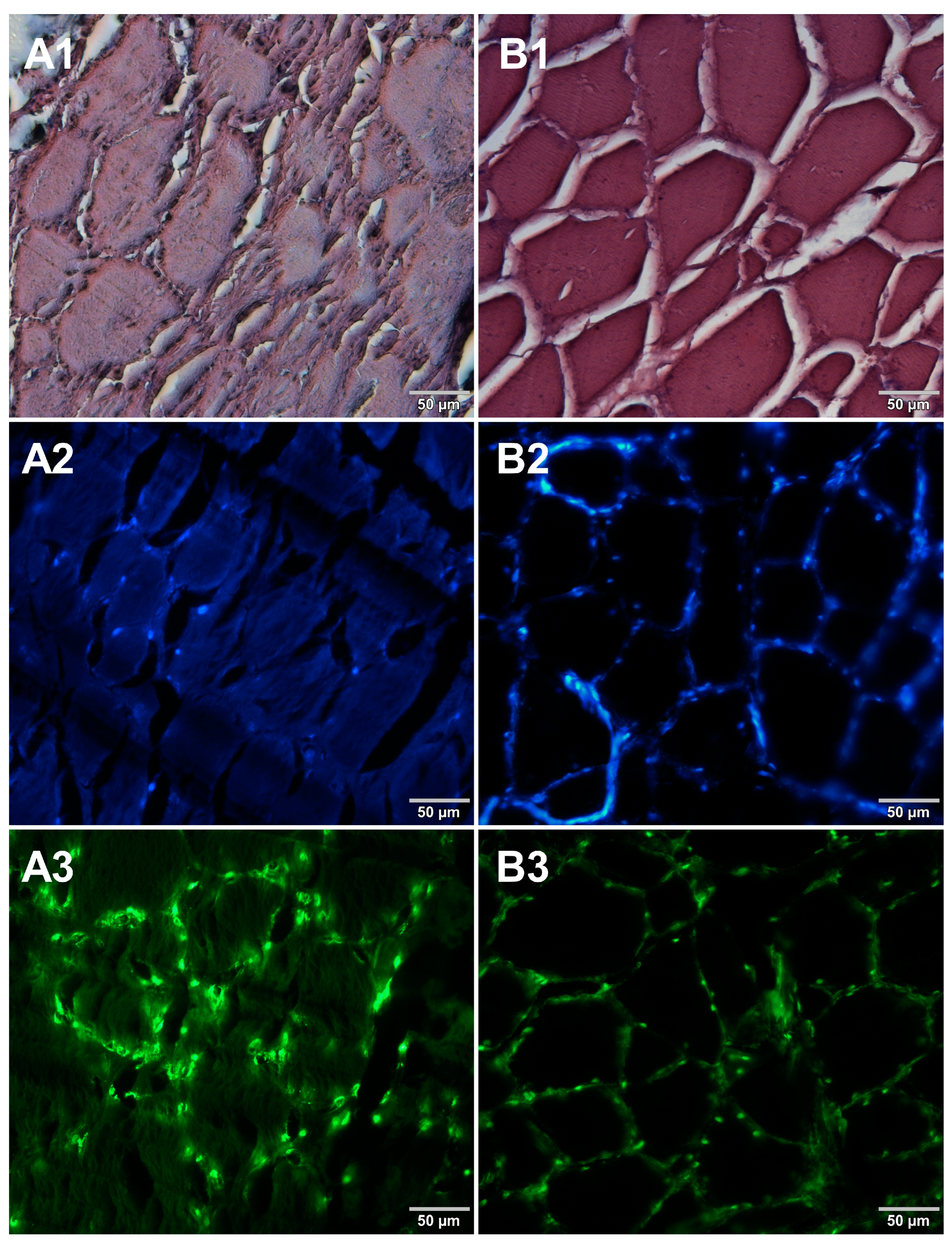
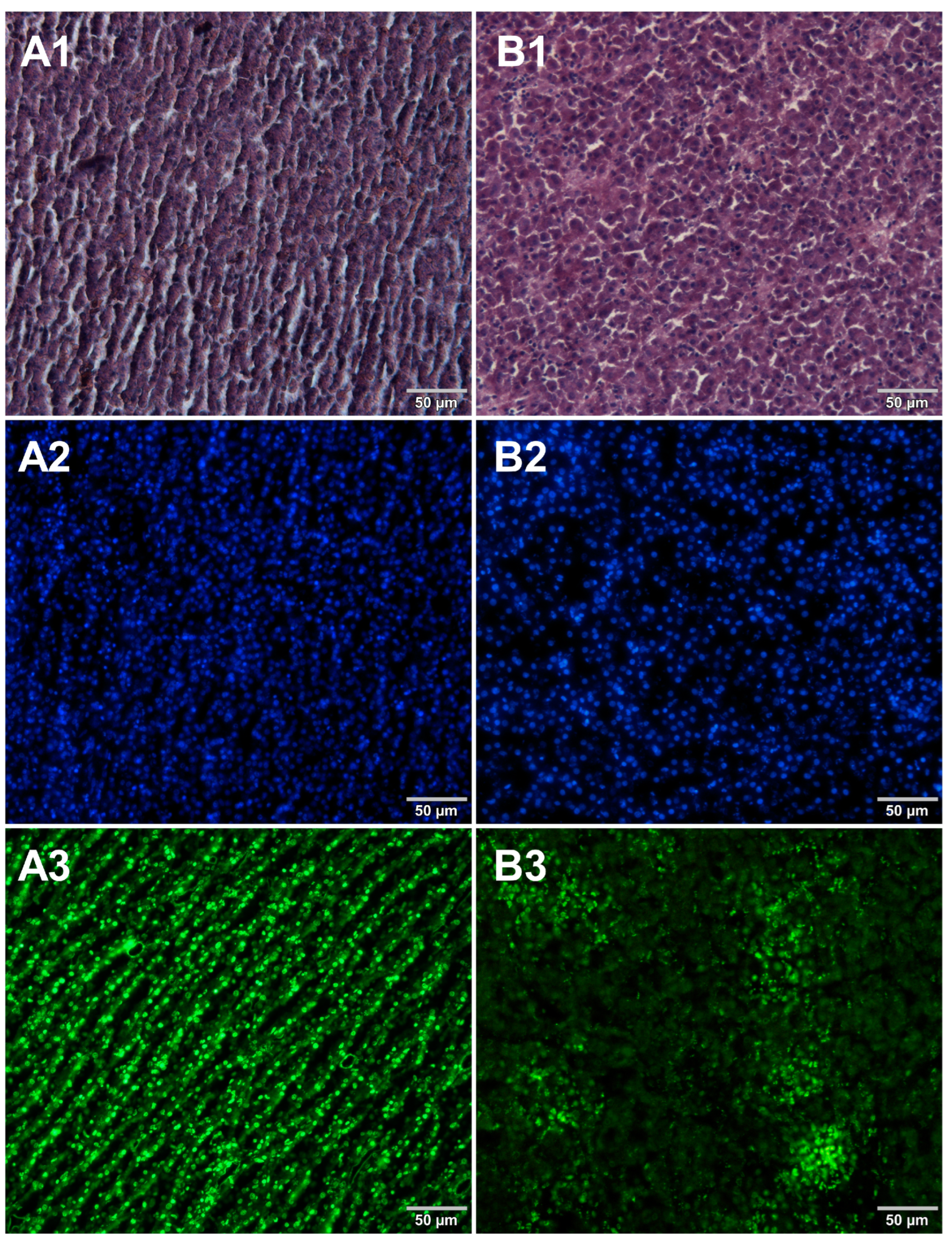
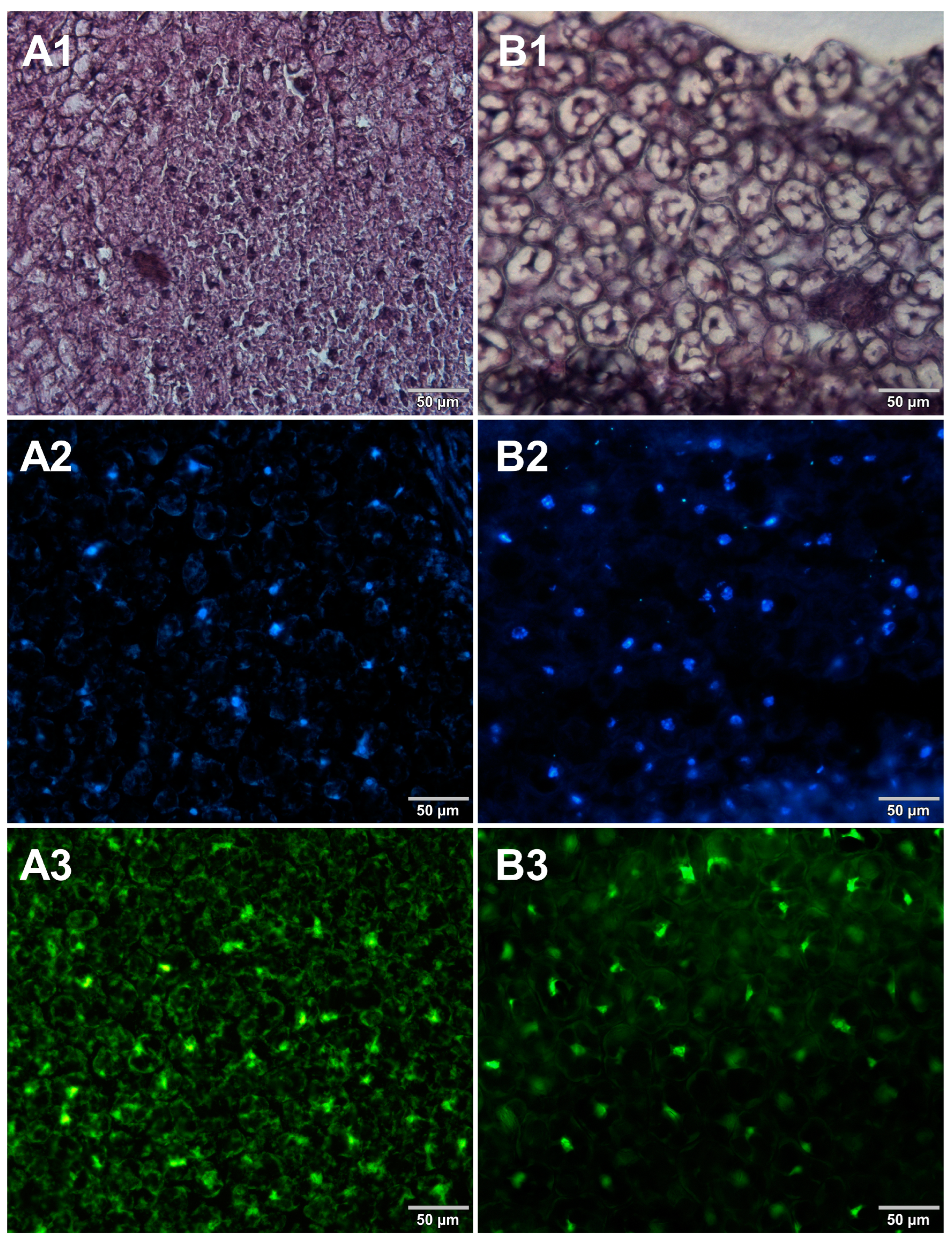
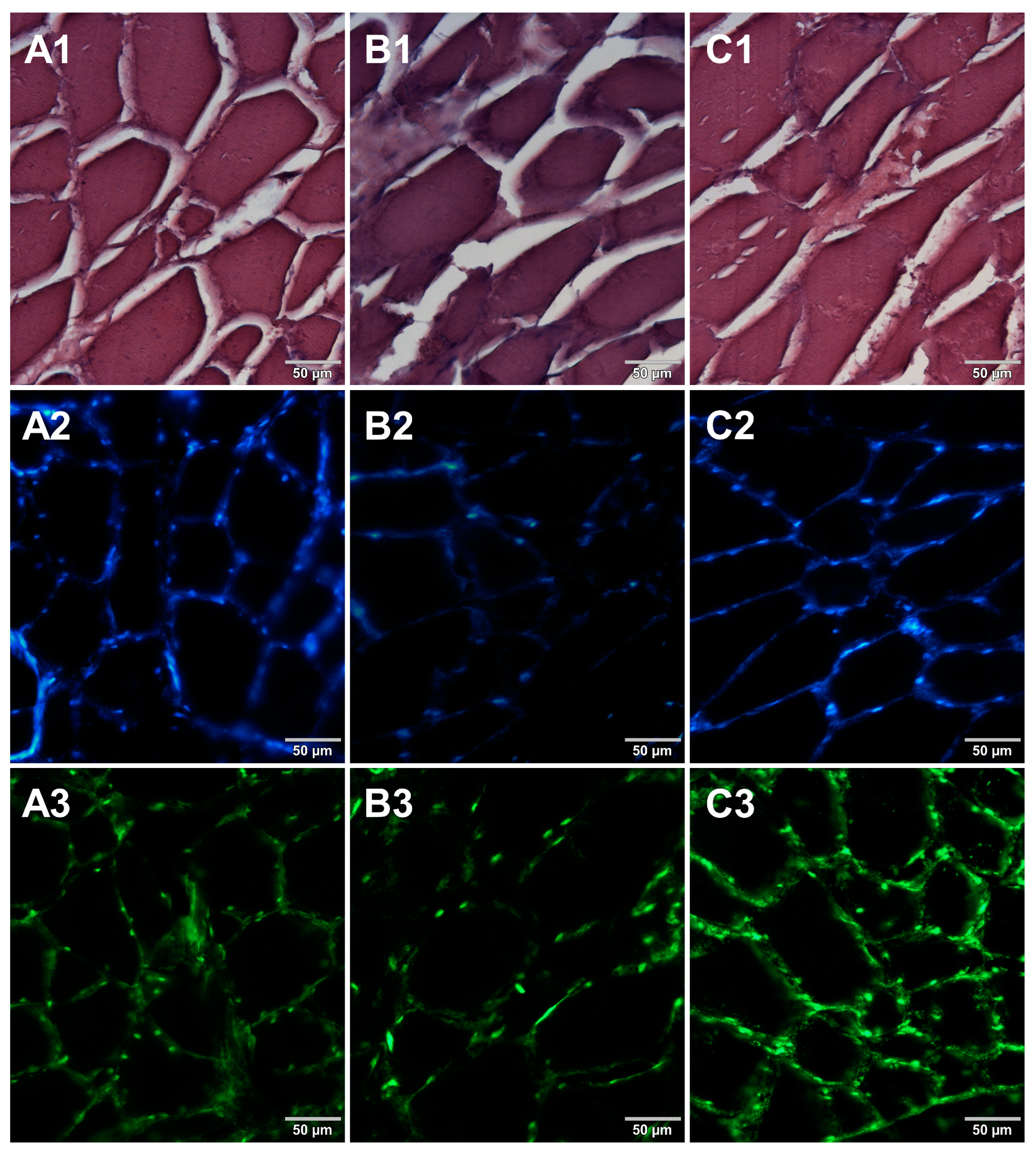
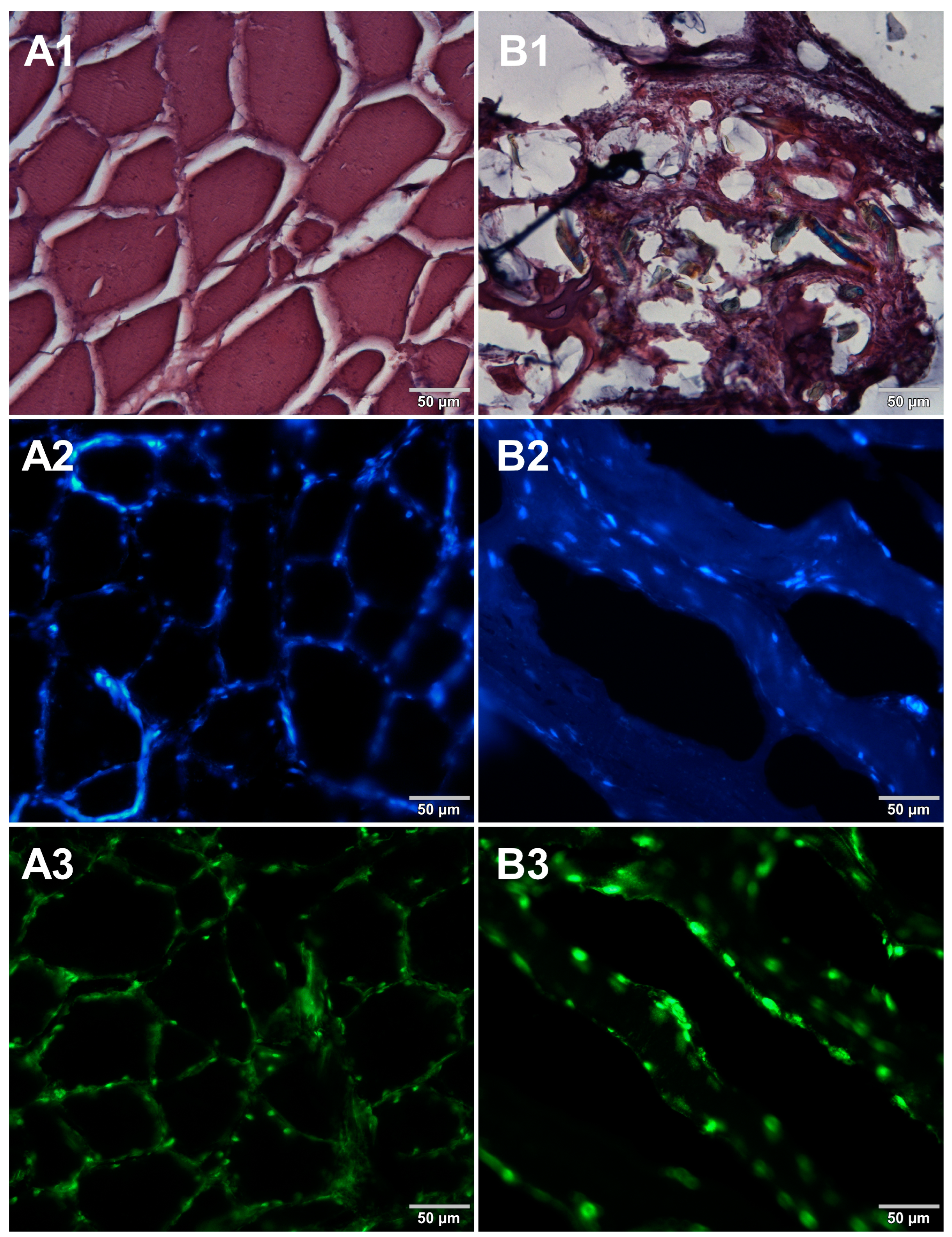
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verkerk, R. An ‘essential’ nutrient? New Food Mag. 2020, 4, 118427. [Google Scholar]
- Ding, T.; Song, G.; Liu, X.; Xu, M.; Li, Y. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Cieślewicz, J.; Koziara, Z.; Ćwiklińska, W.; Bartoszek, A. The toolbox of methods for multidirectional characterization of dietary nucleic acids; verification for raw and processed food products. Food Anal. Methods 2021, 14, 1482–1497. [Google Scholar] [CrossRef]
- Yeung, J.W.Y.; Leung, K.M.Y. Effects of animal size and nutritional status on the RNA/DNA ratio in different tissues of the green-lipped mussel Perna viridis. J. Mar. Biol. Assoc. 2013, 93, 217–225. [Google Scholar] [CrossRef]
- Bhat, H.; Ahmad, I.; Asmi, O.; Shah, F.; Bhat, B.; Hussain, T.; Hafeez, M.; Rashid, S.; Razak, N.; Hussain, R.A. RNA: DNA ratio as an indicator of growth, nutritional status and condition of fish: A review. J. Entomol. Zool. Stud. 2020, 8, 654–658. [Google Scholar]
- Grayson, K. An improved method for staining mammalian oocytes. Stain Technol. 1978, 53, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Scouten, C.W.; Cunningham, M. Freezing biological samples. Microsc. Today 2006, 14, 48. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Cryosectioning tissues. Cold Spring Harb. Protoc. 2008, 3, pdb.prot4991. [Google Scholar] [CrossRef] [PubMed]
- Sadeghipour, A.; Babaheidarian, P. Making formalin-fixed, paraffin embedded blocks. In Biobanking. Methods in Molecular Biology; Yong, W., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1897, pp. 253–268. [Google Scholar] [CrossRef]
- Farragher, S.M.; Tanney, A.; Kennedy, R.D.; Paul Harkin, D. RNA expression analysis from formalin fixed paraffin embedded tissues. Histochem. Cell Biol. 2008, 130, 435–445. [Google Scholar] [CrossRef]
- Gancarz, M.; Konstankiewicz, K.; Pawlak, K.; Zdunek, A. Analysis of plant tissue images obtained by confocal tandem scanning reflected light microscope. Int. Agrophysics 2007, 21, 49–53. [Google Scholar]
- Peighambardoust, S.H.; Dadpour, M.R.; Dokouhaki, M. Application of epifluorescence light microscopy (EFLM) to study the microstructure of wheat dough: A comparison with confocal scanning laser microscopy (CSLM) technique. J. Cereal Sci. 2010, 51, 21–27. [Google Scholar] [CrossRef]
- Ong, L.; Dagastine, R.R.; Kentish, S.E.; Gras, S.L. Microstructure of milk gel and cheese curd observed using cryo scanning electron microscopy and confocal microscopy. LWT—Food Sci. Technol. 2011, 44, 1291–1302. [Google Scholar] [CrossRef]
- Błaszczak, W.; Lewandowicz, G. Light microscopy as a tool to evaluate the functionality of starch in food. Foods 2020, 9, 670. [Google Scholar] [CrossRef]
- Meistro, S.; Pezzolato, M.; Muscolino, D.; Giarratana, F.; Baioni, E.; Panebianco, A.; Bozzetta, E. Histology as a valid tool to differentiate fresh from frozen-thawed marinated fish. J. Food Prot. 2016, 79, 1457–1459. [Google Scholar] [CrossRef]
- Flint, F.O. Light microscopy preparation techniques for starch and lipid containing snack foods. Food Struct. 1982, 1, 5. [Google Scholar]
- Dodd, C.E.R.; Waites, W.M. The use of toluidine blue for in situ detection of micro-organisms in foods. Lett. Appl. Microbiol. 1991, 13, 220–223. [Google Scholar] [CrossRef]
- Slot, J.W.; Geuze, H.J. Cryosectioning and immunolabeling. Nat. Protoc. 2007, 2, 2480–2491. [Google Scholar] [CrossRef]
- Shi, S.R.; Liu, C.; Pootrakul, L.; Tang, L.; Young, A.; Chen, R.; Cote, R.J.; Taylor, C.R. Evaluation of the value of frozen tissue section used as "gold standard" for immunohistochemistry. Am. J. Clin. Pathol. 2008, 129, 358–366. [Google Scholar] [CrossRef]
- Hewitson, T.D.; Wigg, B.; Becker, G.J. Tissue preparation for histochemistry: Fixation, embedding, and antigen retrieval for light microscopy. Methods Mol. Biol. 2010, 611, 3–18. [Google Scholar] [CrossRef]
- Hira, V.; de Jong, A.L.; Ferro, K.; Khurshed, M.; Molenaar, R.J.; Van Noorden, C. Comparison of different methodologies and cryostat versus paraffin sections for chromogenic immunohistochemistry. Acta Histochem. 2019, 121, 125–134. [Google Scholar] [CrossRef]
- Pham, N.A.; Morrison, A.; Schwock, J.; Aviel-Ronen, S.; Iakovlev, V.; Tsao, M.S.; Ho, J.; Hedley, D.W. Quantitative image analysis of immunohistochemical stains using a CMYK color model. Diagn. Pathol. 2007, 2, 8. [Google Scholar] [CrossRef]
- De Freitas, M.C.; Ricci-Vitor, A.L.; Freire, R.V.; Caperuto, E.C.; Vanderlei, L.C.; Lira, F.S.; Rossi, F.E. Oral adenosine 5′-triphosphate supplementation improved hemodynamic and autonomic parameters after exercise in hypertensive women. J. Exerc. Rehabil. 2018, 14, 671. [Google Scholar] [CrossRef]
- dos Santos Nunes de Moura, H.P.; Jäger, R.; Purpura, M.; Rathmacher, J.A.; Fuller Jr, J.C.; Rossi, F.E. Dose response of acute ATP supplementation on strength training performance. Front. Sports Act. Living 2021, 8, 780459. [Google Scholar] [CrossRef]
- Jun, Y.W.; Kant, M.; Coskun, E.; Kato, T.A.; Jaruga, P.; Palafox, E.; Dizdaroglu, M.; Kool, E.T. Possible genetic risks from heat-damaged DNA in food. ACS Cent. Sci. 2023, 9, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Berg, T.; Singh, J.; Hardacre, A.; Boland, M.J. The role of cotyledon cell structure during in vitro digestion of starch in navy beans. Carbohydr. Polym. 2012, 87, 1678–1688. [Google Scholar] [CrossRef]
- Pineda-Vadillo, C.; Nau, F.; Guérin-Dubiard, C.; Bourlieu, C.; Capozzi, F.; Bordoni, A.; Dupont, D. In vivo digestion of egg products enriched with DHA: Effect of the food matrix on DHA bioavailability. Foods 2021, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.E.; Espinosa, Y.G.; Watson, R.L.; Spyropoulos, F.; Norton, I.T. Functional food microstructures for macronutrient release and delivery. Food Funct. 2015, 6, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-Y.; Zhao, Y.; Zhang, N.; Wang, K.-N.; Tian, M.; Pan, Q.; Lin, W. Ratiometric fluorescence imaging for the distribution of nucleic acid content in living cells and human tissue sections. Anal. Chem. 2021, 93, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Herbel, W.; Montag, A. Nucleostoffe in proteinreichen Lebensmitteln. Leb. Unter Forsch 1987, 185, 119–122. [Google Scholar] [CrossRef]
- Lassek, E.; Montag, A. Nucleostoffe in kohlenhydratreichen Lebensmitteln. Leb. Unters Forsch 1990, 190, 17–21. [Google Scholar] [CrossRef]
- Kokabi, M.; Sui, J.; Gandotra, N.; Pournadali Khamseh, A.; Scharfe, C.; Javanmard, M. Nucleic acid quantification by multi-frequency impedance cytometry and machine learning. Biosensors 2023, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Lowenthal, M.S.; Quittman, E.; Phinney, K.W. Absolute quantification of RNA or DNA using acid hydrolysis and mass spectrometry. Anal. Chem. 2019, 91, 14569–14576. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Lüthy, J. Quantitative competitive (QC) PCR for quantification of porcine DNA. Meat Sci. 2001, 57, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.R.; Desjardins, P.R. Quantitation of DNA and RNA with absorption and fluorescence spectroscopy. Curr. Protoc. Mol. Biol. 2006, 76, Appendix 3:Appendix 3D. [Google Scholar] [CrossRef]

| Stage of dietNA Analysis | Parameter | Paraffin-Embedding | Cryosectioning |
|---|---|---|---|
| Sample fixation | Duration time | 2–4 days | - |
| Fixative and solvents | Formaldehyde, xylene, ethanol | Optional saturation with sucrose solution | |
| Blocks forming | Duration time | >1 h (depending on the number of samples) | <1 h (depending on the number of samples) |
| Molds | Metal or plastic | Plastic | |
| Embedding medium | Soft or hard paraffin wax | Freezing medium such as optimal cutting temperature (OCT) compound | |
| Solidification | Paraffin-embedded samples in molds left on ice | Freezing samples in molds in liquid nitrogen | |
| Additional equipment | Thermostat for paraffin wax | - | |
| Sections cutting | Duration time | >1 h (depending on the number of samples) | <1 h (depending on the number of samples) |
| Cutting device | Microtome | Cryostat with an integrated microtome | |
| Cutting temperature | Room temperature | −20 °C (but depends on the type of tissue) | |
| Sections storage temperature | Room temperature | −80 °C | |
| Slides coating | Mayer’s albumin adhesive | Poly-L-lysine, gelatin, or agarose | |
| Additional equipment | Section dryer hot plate | - | |
| Staining | Duration time | >1 h (depending on the number of samples) | <1 h (depending on the number of samples) |
| Dewaxing and rehydrating | Required | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kościelak, A.; Koziara, Z.; Maria, A.P.; Płatek, R.; Bartoszek, A. Microscopic Imaging to Visualize the Distribution of Dietary Nucleic Acids in Food Products of Various Origins. Foods 2023, 12, 3942. https://doi.org/10.3390/foods12213942
Kościelak A, Koziara Z, Maria AP, Płatek R, Bartoszek A. Microscopic Imaging to Visualize the Distribution of Dietary Nucleic Acids in Food Products of Various Origins. Foods. 2023; 12(21):3942. https://doi.org/10.3390/foods12213942
Chicago/Turabian StyleKościelak, Anna, Zuzanna Koziara, Ana Pons Maria, Rafał Płatek, and Agnieszka Bartoszek. 2023. "Microscopic Imaging to Visualize the Distribution of Dietary Nucleic Acids in Food Products of Various Origins" Foods 12, no. 21: 3942. https://doi.org/10.3390/foods12213942






