Lacticaseibacillus Strains Isolated from Raw Milk: Screening Strategy for Their Qualification as Adjunct Culture in Cheesemaking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Milk Sampling
2.2. Strain Isolation and Screening
2.2.1. Raw Milk Spontaneous Fermentation
2.2.2. Bacteria Culturing and Isolation
2.2.3. DNA Extraction and Strain Identification
2.2.4. Strain Genotypic Characterization
2.3. Strain Phenotypic Characterization
2.3.1. Evaluation of the Strain Resistance to Cheesemaking Stresses
2.3.2. Evaluation of Metabolic Capabilities
Carbon Source Utilization and Chemical Sensitivity
Volatile Compounds
2.4. Statistical Analysis
3. Results
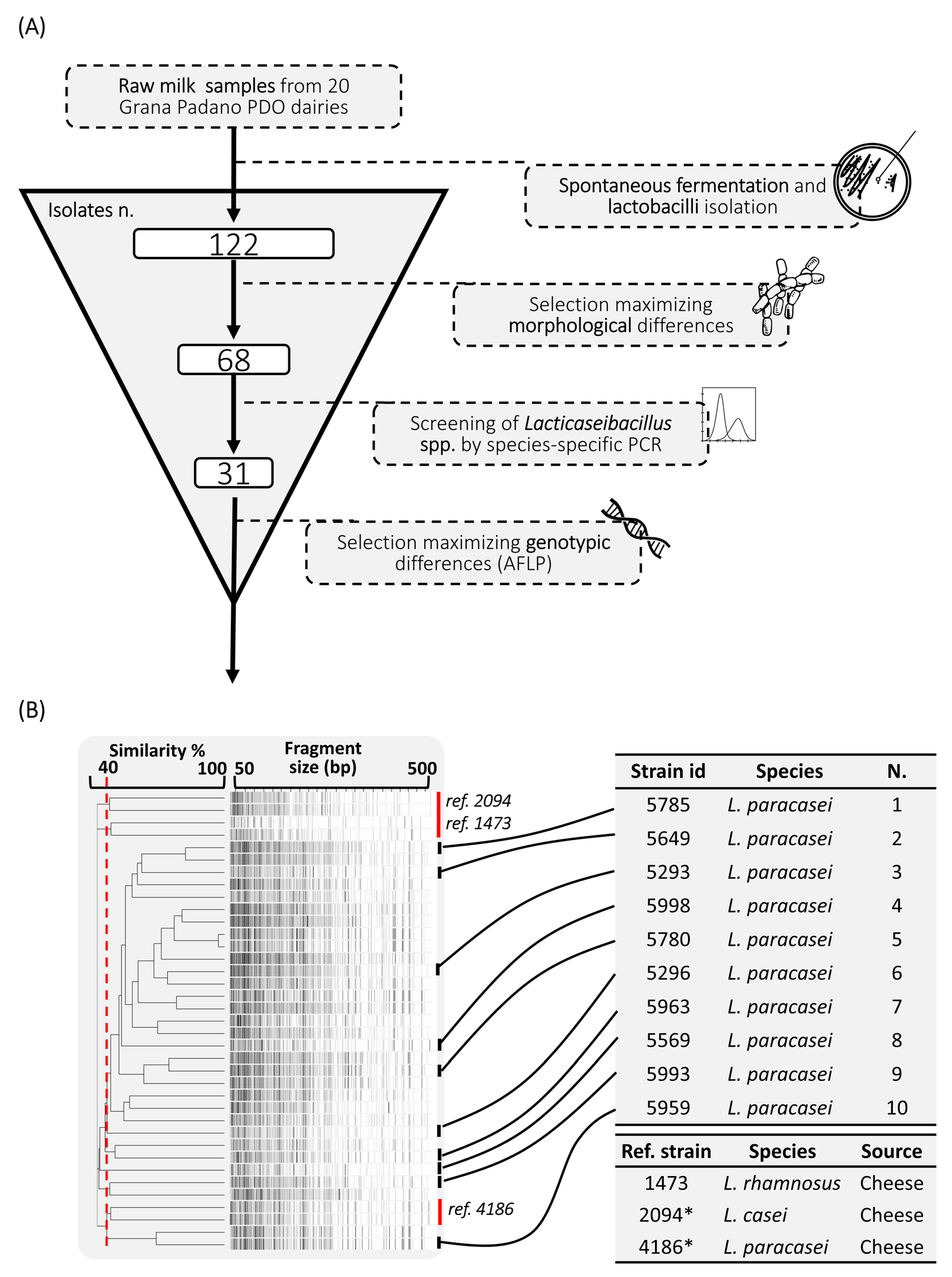
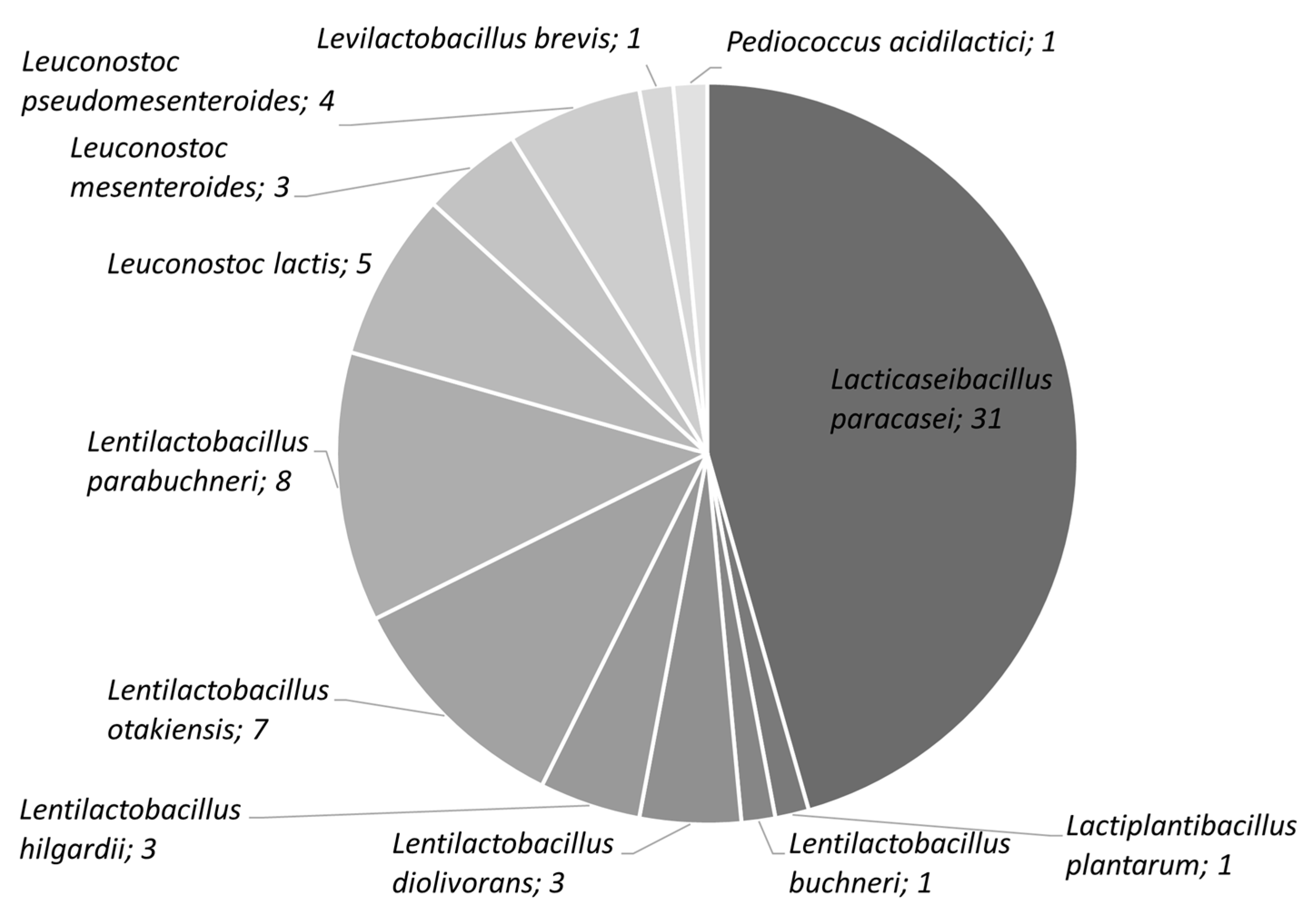
3.1. Screening of the Isolates
3.2. Phenotypic Tests
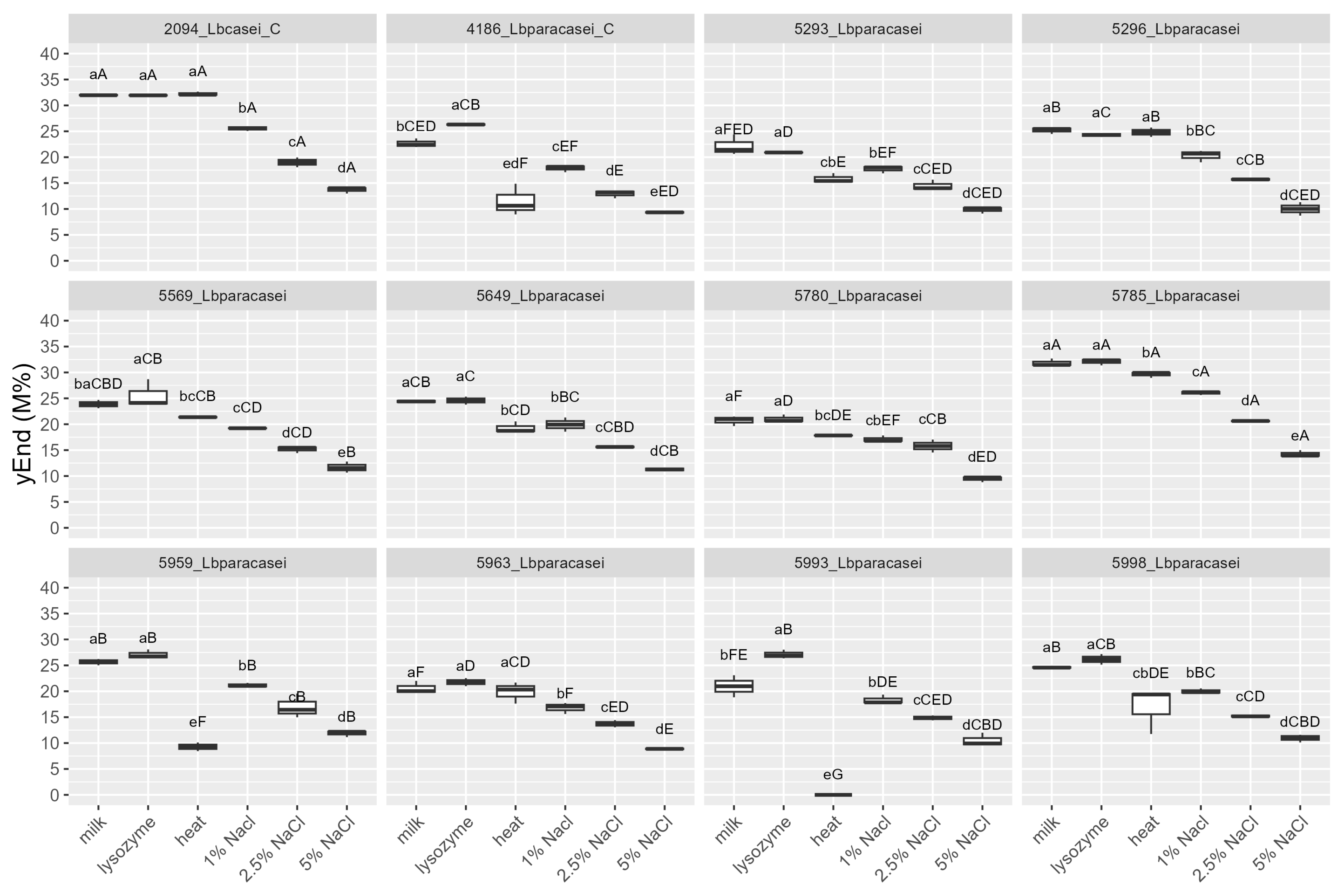
3.2.1. Resistance to Cheesemaking Stresses
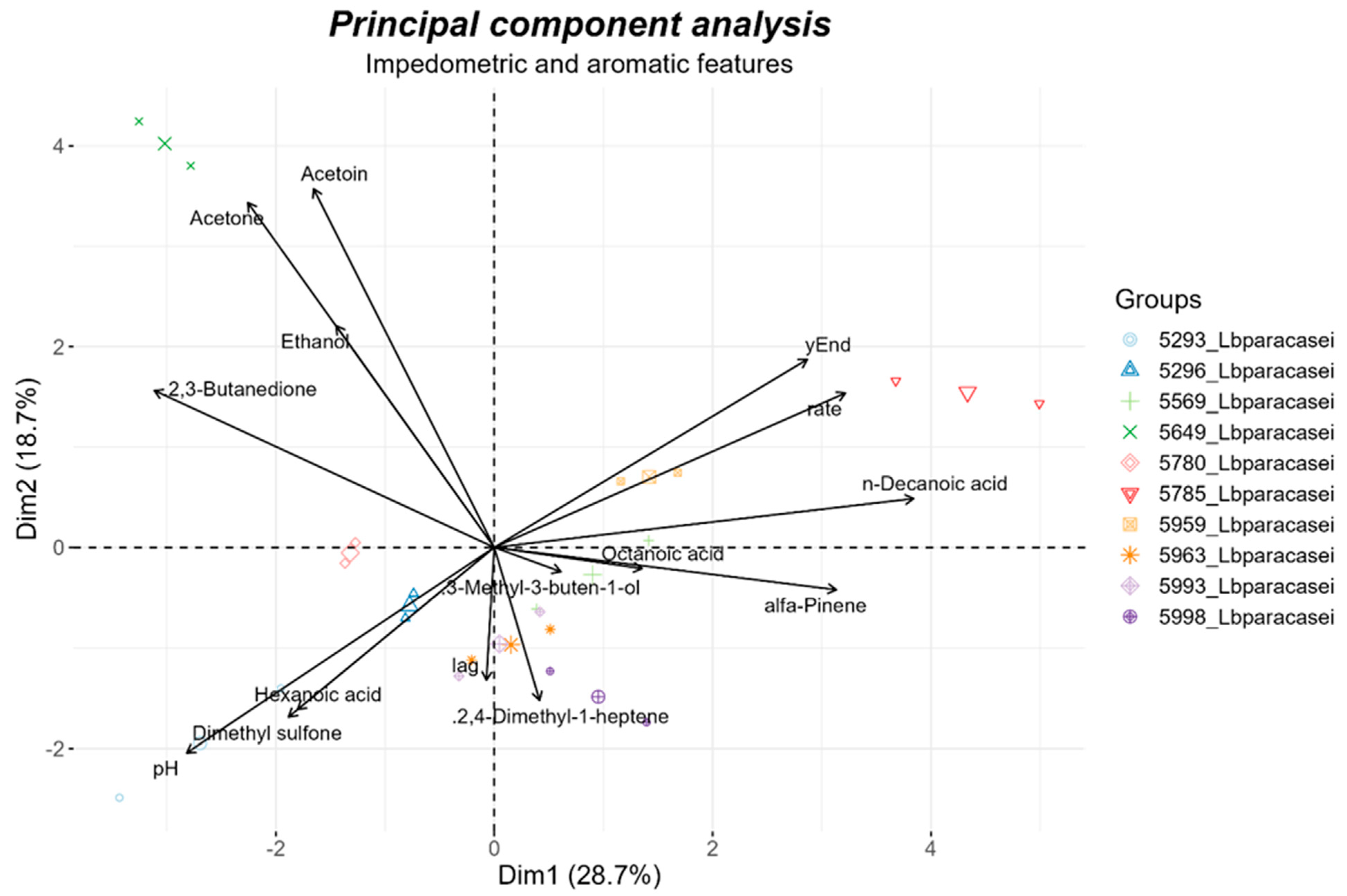
3.2.2. Aromatic Compounds Production
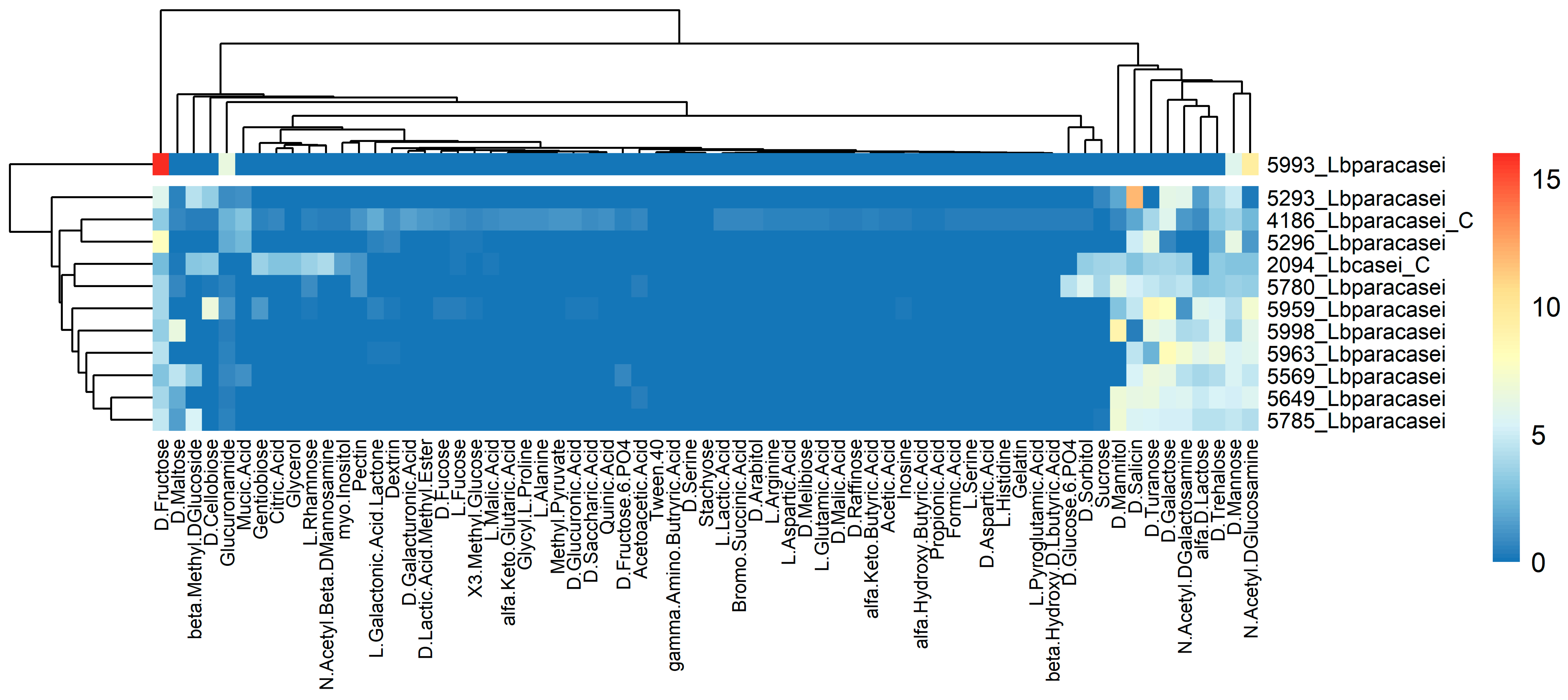
3.2.3. Carbon Source and Sensitivity Assays
4. Discussion
4.1. Strains’ Resistance to Cheesemaking Stresses
4.2. Strains’ Metabolic Capabilities
4.3. Adjunct Culture Implementation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gatti, M.; Bottari, B.; Lazzi, C.; Neviani, E.; Mucchetti, G. Invited Review: Microbial Evolution in Raw-Milk, Long-Ripened Cheeses Produced Using Undefined Natural Whey Starters. J. Dairy Sci. 2014, 97, 573–591. [Google Scholar] [CrossRef]
- Giraffa, G. The Microbiota of Grana Padano Cheese. A Review. Foods 2021, 10, 2632. [Google Scholar] [CrossRef]
- Blaya, J.; Barzideh, Z.; LaPointe, G. Symposium Review: Interaction of Starter Cultures and Nonstarter Lactic Acid Bacteria in the Cheese Environment. J. Dairy Sci. 2018, 101, 3611–3629. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Toh, H.; Oshima, K.; Nakano, A.; Takahata, M.; Murakami, M.; Takaki, T.; Nishiyama, H.; Igimi, S.; Hattori, M.; Morita, H. Genomic Adaptation of the Lactobacillus Casei Group. PLoS ONE 2013, 8, e75073. [Google Scholar] [CrossRef]
- Bettera, L.; Levante, A.; Bancalari, E.; Bottari, B.; Gatti, M. Lactic Acid Bacteria in Cow Raw Milk for Cheese Production: Which and How Many? Front. Microbiol. 2023, 13, 1092224. [Google Scholar] [CrossRef]
- Bottari, B.; Levante, A.; Neviani, E.; Gatti, M. How the Fewest Become the Greatest. L. Casei’s Impacton Long Ripened Cheeses. Front. Microbiol. 2018, 9, 2014–2019. [Google Scholar] [CrossRef]
- Bottari, B.; Levante, A.; Bancalari, E.; Sforza, S.; Bottesini, C.; Prandi, B.; De Filippis, F.; Ercolini, D.; Nocetti, M.; Gatti, M. The Interrelationship Between Microbiota and Peptides During Ripening as a Driver for Parmigiano Reggiano Cheese Quality. Front. Microbiol. 2020, 11, 581658. [Google Scholar] [CrossRef]
- Buron-Moles, G.; Chailyan, A.; Dolejs, I.; Forster, J.; Mikš, M.H. Uncovering Carbohydrate Metabolism through a Genotype-Phenotype Association Study of 56 Lactic Acid Bacteria Genomes. Appl. Microbiol. Biotechnol. 2019, 103, 3135–3152. [Google Scholar] [CrossRef]
- Levante, A.; Lazzi, C.; Vatsellas, G.; Chatzopoulos, D.; Dionellis, V.S.; Makrythanasis, P.; Neviani, E.; Folli, C. Genome Sequencing of Five Lacticaseibacillus Strains and Analysis of Type I and II Toxin-Antitoxin System Distribution. Microorganisms 2021, 9, 648. [Google Scholar] [CrossRef]
- Alessandria, V.; Ferrocino, I.; De Filippis, F.; Fontana, M.; Rantsiou, K.; Ercolini, D.; Cocolin, L. Microbiota of an Italian Grana-like Cheese during Manufacture and Ripening, Unraveled by 16S rRNA-Based Approaches. Appl. Environ. Microbiol. 2016, 82, 3988–3995. [Google Scholar] [CrossRef] [PubMed]
- Lazzi, C.; Povolo, M.; Locci, F.; Bernini, V.; Neviani, E.; Gatti, M. Can the Development and Autolysis of Lactic Acid Bacteria Influence the Cheese Volatile Fraction? The Case of Grana Padano. Int. J. Food Microbiol. 2016, 233, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Levante, A.; De Filippis, F.; La Storia, A.; Gatti, M.; Neviani, E.; Ercolini, D.; Lazzi, C. Metabolic Gene-Targeted Monitoring of Non-Starter Lactic Acid Bacteria during Cheese Ripening. Int. J. Food Microbiol. 2017, 257, 276–284. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Mancini, L.; Fox, P.F. Pros and Cons for Using Non-Starter Lactic Acid Bacteria (NSLAB) as Secondary/Adjunct Starters for Cheese Ripening. Trends Food Sci. Technol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Levante, A.; Bancalari, E.; Tambassi, M.; Lazzi, C.; Neviani, E.; Gatti, M. Phenotypic Diversity of Lactobacillus Casei Group Isolates as a Selection Criterion for Use as Secondary Adjunct Starters. Microorganisms 2020, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Cuffia, F.; Bergamini, C.V.; Hynes, É.R.; Wolf, I.V.; Perotti, M.C. Evaluation of Autochthonous Cultures to Improve the Cheese Flavor: A Case Study in Hard Cheese Model. Food Sci. Technol. Int. 2020, 26, 173–184. [Google Scholar] [CrossRef]
- Bancalari, E.; Savo Sardaro, M.L.; Levante, A.; Marseglia, A.; Caligiani, A.; Lazzi, C.; Neviani, E.; Gatti, M. An Integrated Strategy to Discover Lactobacillus Casei Group Strains for Their Potential Use as Aromatic Starters. Food Res. Int. 2017, 100, 682–690. [Google Scholar] [CrossRef]
- Kouadri Boudjelthia, N.; Belabbas, M.; Bekenniche, N.; Monnoye, M.; Gérard, P.; Riazi, A. Probiotic Properties of Lactic Acid Bacteria Newly Isolated from Algerian Raw Cow’s Milk. Microorganisms 2023, 11, 2091. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Mari, E.; Guerrini, S.; Granchi, L. Gamma-Aminobutyric Acid (GABA) Production in Fermented Milk by Lactic Acid Bacteria Isolated from Spontaneous Raw Milk Fermentation. Int. Dairy J. 2022, 127, 105284. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Mari, E.; Guerrini, S.; Granchi, L. Selection of Yeast and Lactic Acid Bacteria Strains, Isolated from Spontaneous Raw Milk Fermentation, for the Production of a Potential Probiotic Fermented Milk. Fermentation 2022, 8, 407. [Google Scholar] [CrossRef]
- Chessa, L.; Paba, A.; Dupré, I.; Daga, E.; Fozzi, M.C.; Comunian, R. A Strategy for the Recovery of Raw Ewe’s Milk Microbiodiversity to Develop Natural Starter Cultures for Traditional Foods. Microorganisms 2023, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, E.; Settanni, L.; Cavazza, A.; Poznanski, E. Biodiversity and Technological Potential of Wild Lactic Acid Bacteria from Raw Cows’ Milk. Int. Dairy J. 2009, 19, 3–11. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A Medium for the Cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Sato, T.; Weightman, A.J.; Martin, T.A.; Fry, J.C.; Hiom, S.J.; Wade, W.G. Design and Evaluation of Useful Bacterium-Specific PCR Primers That Amplify Genes Coding for Bacterial 16S rRNA. Appl. Environ. Microbiol. 1998, 64, 795–799. [Google Scholar] [CrossRef]
- Bertani, G.; Savo Sardaro, M.L.; Neviani, E.; Lazzi, C. AFLP Protocol Comparison for Microbial Diversity Fingerprinting. J. Appl. Genet. 2019, 60, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Bikandi, J.; Millán, R.S.; Rementeria, A.; Garaizar, J. In Silico Analysis of Complete Bacterial Genomes: PCR, AFLP-PCR and Endonuclease Restriction. Bioinformatics 2004, 20, 798–799. [Google Scholar] [CrossRef]
- Bancalari, E.; Bernini, V.; Bottari, B.; Neviani, E. Application of Impedance Microbiology for Evaluating Potential Acidifying Performances of Starter Lactic Acid Bacteria to Employ in Milk Transformation. Front. Microbiol. 2016, 7, 1628. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Troiani, L.; Levante, A.; Bonicci, V.; Velia, P.D.; dal Palù, A.; Neviani, E.; Bernini, V.; Lazzi, C. Zooming into the Metabolic Biodiversity of a Microbial Core Community of Food Origin. mySystems, 2023, submitted.
- Acin-Albiac, M.; Filannino, P.; Gobbetti, M.; Di Cagno, R. Microbial High Throughput Phenomics: The Potential of an Irreplaceable Omics. Comput. Struct. Biotechnol. J. 2020, 18, 2290–2299. [Google Scholar] [CrossRef]
- Dubey, A.; Malla, M.A.; Kumar, A. Taxonomical and Functional Bacterial Community Profiling in Disease-Resistant and Disease-Susceptible Soybean Cultivars. Braz. J. Microbiol. 2022, 53, 1355–1370. [Google Scholar] [CrossRef]
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Kolde, R. Pheatmap: Pretty Heatmaps; R package version; R Core Team: Vienna, Austria, 2012; Volume 1, p. 726. [Google Scholar]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. Environ. Int. 2009, 35, 253–258. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Package ‘factoextra’. Extract and Visualize the Results of Multivariate Data Analyses; R Core Team: Vienna, Austria, 2017; Volume 76. [Google Scholar]
- Wei, T.; Simko, V. R package “corrplot”: Visualization of a Correlation Matrix; (Version 0.84); R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Fitzsimons, N.; Cogan, T.; Condon, S.; Beresford, T. Phenotypic and Genotypic Characterization of Non-Starter Lactic Acid Bacteria in Mature Cheddar Cheese. Appl. Environ. Microbiol. 1999, 65, 3418–3426. [Google Scholar] [CrossRef] [PubMed]
- Bove, C.G.; De Dea Lindner, J.; Lazzi, C.; Gatti, M.; Neviani, E. Evaluation of Genetic Polymorphism among Lactobacillus Rhamnosus Non-Starter Parmigiano Reggiano Cheese Strains. Int. J. Food Microbiol. 2011, 144, 569–572. [Google Scholar] [CrossRef]
- Solieri, L.; Bianchi, A.; Giudici, P. Inventory of Non Starter Lactic Acid Bacteria from Ripened Parmigiano Reggiano Cheese as Assessed by a Culture Dependent Multiphasic Approach. Syst. Appl. Microbiol. 2012, 35, 270–277. [Google Scholar] [CrossRef]
- Temmerman, R.; Huys, G.; Swings, J. Identification of Lactic Acid Bacteria: Culture-Dependent and Culture-Independent Methods. Trends Food Sci. Technol. 2004, 15, 348–359. [Google Scholar] [CrossRef]
- Minervini, F.; Calasso, M. Lactobacillus casei group. In Encyclopedia of Dairy Sciences, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 2021; Volume 4. [Google Scholar]
- Zúñiga, M.; Champomier-Verges, M.; Zagorec, M.; Pérez-Martínez, G. Structural and Functional Analysis of the Gene Cluster Encoding the Enzymes of the Arginine Deiminase Pathway of Lactobacillus Sake. J. Bacteriol. 1998, 180, 4154–4159. [Google Scholar] [CrossRef]
- Barbieri, G.; Bolzoni, L.; Careri, M.; Mangia, A.; Parolari, G.; Spagnoli, S.; Virgili, R. Study of the Volatile Fraction of Parmesan Cheese. J. Agric. Food Chem. 1994, 42, 1170–1176. [Google Scholar] [CrossRef]
- Hajikhani, B.; Goudarzi, M.; Kakavandi, S.; Amini, S.; Zamani, S.; van Belkum, A.; Goudarzi, H.; Dadashi, M. The Global Prevalence of Fusidic Acid Resistance in Clinical Isolates of Staphylococcus Aureus: A Systematic Review and Meta-Analysis. Antimicrob. Resist. Infect. Control 2021, 10, 75. [Google Scholar] [CrossRef]
- Stogios, P.J.; Savchenko, A. Molecular Mechanisms of Vancomycin Resistance. Protein Sci. 2020, 29, 654–669. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]
- Zarzecka, U.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Starter Cultures as a Reservoir of Antibiotic Resistant Microorganisms. LWT 2020, 127, 109424. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). EFSA Statement on the Requirements for Whole Genome Sequence Analysis of Microorganisms Intentionally Used in the Food Chain. EFS2 2021, 19, e06506. [Google Scholar] [CrossRef]
- Nunziata, L.; Brasca, M.; Morandi, S.; Silvetti, T. Antibiotic Resistance in Wild and Commercial Non-Enterococcal Lactic Acid Bacteria and Bifidobacteria Strains of Dairy Origin: An Update. Food Microbiol. 2022, 104, 103999. [Google Scholar] [CrossRef]
- Bancalari, E.; Montanari, C.; Levante, A.; Alinovi, M.; Neviani, E.; Gardini, F.; Gatti, M. Lactobacillus Paracasei 4341 as Adjunct Culture to Enhance Flavor in Short Ripened Caciotta-Type Cheese. Food Res. Int. 2020, 135, 109284. [Google Scholar] [CrossRef] [PubMed]
- Decadt, H.; Weckx, S.; De Vuyst, L. The Rotation of Primary Starter Culture Mixtures Results in Batch-to-Batch Variations during Gouda Cheese Production. Front. Microbiol. 2023, 14, 1128394. [Google Scholar] [CrossRef] [PubMed]
- Consorzio Tutela Grana Padano Disciplinare Grana Padano DOP; Official Journal of the European Union: Brussels, Belgum, 1996.
- McSweeney, P.L.H.; Fox, P.F.; Cotter, P.D.; Everett, D.W. Cheese: Chemistry, Physics & Microbiology, 4th ed.; Elsevier, Ed.; Academic Press: London, UK, 2017. [Google Scholar]
- Morandi, S.; Battelli, G.; Silvetti, T.; Goss, A.; Cologna, N.; Brasca, M. How the Biodiversity Loss in Natural Whey Culture Is Affecting Ripened Cheese Quality? The Case of Trentingrana Cheese. LWT 2019, 115, 108480. [Google Scholar] [CrossRef]
- Bettera, L.; Dreier, M.; Schmidt, R.S.; Gatti, M.; Berthoud, H.; Bachmann, H.-P. Selective Enrichment of the Raw Milk Microbiota in Cheese Production: Concept of a Natural Adjunct Milk Culture. Front. Microbiol. 2023, 14, 1154508. [Google Scholar] [CrossRef]
- Bachmann, H.-P. Cheese Analogues: A Review. Int. Dairy J. 2001, 11, 505–515. [Google Scholar] [CrossRef]
- Harper, A.R.; Dobson, R.C.J.; Morris, V.K.; Moggré, G. Fermentation of Plant-based Dairy Alternatives by Lactic Acid Bacteria. Microb. Biotechnol. 2022, 15, 1404–1421. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of Agri-Food Waste: A Promising Route for the Production of Aroma Compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef]
- Waggott, A.; Davies, I.W. Identification of Organic Pollutants Using Linear Temperature Programmed Retention Indices (LTPRIs). 1984. Available online: https://dwi-content.s3.eu-west-2.amazonaws.com/wp-content/uploads/2020/10/27110228/dwi0382.pdf (accessed on 22 October 2023).
- Muresan, S.; Eillebrecht, M.A.J.L.; de Rijk, T.C.; de Jonge, H.G.; Leguijt, T.; Nijhuis, H.H. Aroma Profile Development of Intermediate Chocolate productsI. Volatile Constituents of Block-Milk. Food Chem. 2000, 68, 167–174. [Google Scholar] [CrossRef]
- Bonastre, J.; Grenier, P. Contribution à l’étude de La Polarité Des Phases Stationnaires En Chromatographie Gaz-Liquide. III. Calcul Des Coefficients d’activité Relatifs et Des Indices de Rétention de Quelques Alcools Aliphatiques. Bull. Société Chim. Fr. 1968, 1, 118–125. [Google Scholar]
- Umano, K.; Shoji, A.; Hagi, Y.; Shibamoto, T. Volatile Constituents of Peel of Quince Fruit, Cydonia Oblonga Miller. J. Agric. Food Chem. 1986, 34, 593–596. [Google Scholar] [CrossRef]
- Umano, K.; Shibamoto, T. A New Method of Headspace Sampling: Grapefruit Volatiles. In Proceedings of the 10th International Congress of Essential Oils, Fragrances and Flavors, Washington, DC, USA, 16–20 November 1986; Elsevier Ltd.: New York, NY, USA, 1988; pp. 981–998. [Google Scholar] [CrossRef]
- Umano, K.; Hagi, Y.; Shibamoto, T. Volatile Chemicals Identified in Extracts from Newly Hybrid Citrus, Dekopon (Shiranuhi mandarin Suppl. J.). J. Agric. Food Chem. 2002, 50, 5355–5359. [Google Scholar] [CrossRef]
- Yuhong, T. Research Advances on the Essential Oils from Leaves of Eucalyptus. Food Ferment. Ind. 2007, 33, 139. [Google Scholar]
- Kim, T.H.; Kim, T.H.; Shin, J.H.; Yu, E.J.; Kim, Y.-S.; Lee, H.J. Characteristics of Aroma-Active Compounds in the Pectin-Elicited Suspension Culture of Zanthoxylum Piperitum (Prickly ash). Biotechnol. Lett. 2002, 24, 551–556. [Google Scholar] [CrossRef]
- Soria, A.C.; Martínez-Castro, I.; Sanz, J. Some Aspects of Dynamic Headspace Analysis of Volatile Components in Honey. Food Res. Int. 2008, 41, 838–848. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, H.J.; Yook, H.S.; Kim, K.S.; Rhee, M.S.; Ryu, G.H.; Byun, M.W. Color, Flavor, and Sensory Characteristics of Gamma-Irradiated Salted and Fermented Anchovy Sauce. Radiat. Phys. Chem. 2004, 69, 179–187. [Google Scholar] [CrossRef]
- Chung, H.Y.; Yung, I.K.S.; Ma, W.C.J.; Kim, J.-S. Analysis of Volatile Components in Frozen and Dried Scallops (Patinopecten yessoensis) by Gas Chromatography/Mass Spectrometry. Food Res. Int. 2002, 35, 43–53. [Google Scholar] [CrossRef]
- Peng, C.T. Prediction of Retention Indices: V. Influence of Electronic Effects and Column Polarity on Retention Index. J. Chromatogr. A 2000, 903, 117–143. [Google Scholar] [CrossRef]
- Welke, J.E.; Manfroi, V.; Zanus, M.; Lazarotto, M.; Alcaraz Zini, C. Characterization of the Volatile Profile of Brazilian Merlot Wines through Comprehensive Two Dimensional Gas Chromatography Time-of-Flight Mass Spectrometric Detection. J. Chromatogr. A 2012, 1226, 124–139. [Google Scholar] [CrossRef] [PubMed]





| Strain Id | MRS Broth | UPSM |
|---|---|---|
| 2094_Lbcasei_C | 9.15 | 9.77 |
| 4186_Lbparacasei_C | 9.23 | 8.19 |
| 5293_Lbparacasei | 8.62 | 7.96 |
| 5296_Lbparacasei | 8.91 | 8.78 |
| 5569_Lbparacasei | 8.96 | 8.55 |
| 5649_Lbparacasei | 9.28 | 8.93 |
| 5780_Lbparacasei | 9.38 | 8.81 |
| 5785_Lbparacasei | 9.03 | 8.52 |
| 5959_Lbparacasei | 9.09 | 8.46 |
| 5963_Lbparacasei | 9.37 | 8.83 |
| 5993_Lbparacasei | 9.22 | 7.98 |
| 5998_Lbparacasei | 8.12 | 8.64 |
| Estimation | Formula | C Source Utilization | Chemical Sensitivity |
|---|---|---|---|
| Average Well Color Development (AWCD) | ODi = raw OD value of each well from 1 to 9 column R = OD value of negative control well n = number of substrates analyzed (71) | ODi = raw OD value of each well from 10 to 12 columns R = half of the OD value of positive control well n = number of substrates analyzed (23) | |
| Absorbance Ratios |
| Grana Padano PDO Dairy | Province of Origin | Lactobacilli (log CFU/mL) | pH | Initial Isolates (n) (tot. = 122) | Strains after Morphological Screening (n) (tot. = 68) | Strains Belonging to Lacticaseibacillus spp. (n) (tot. = 31) | Strains after AFLP Screening (n) (tot. = 10) | Odor Evaluation | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Expired Milk | Acidic (Yogurt-like) | Cheese | Notes | ||||||||
| D1 | Bergamo | 7.39 | 4.40 | 7 | 4 | 3 | 1 | 0 | 4 | 3 | High gas formation, fizzy |
| D2 | Brescia | 5.90 | 4.60 | 6 | 3 | 1 | 1 | 0 | 4 | 2 | Slight fruity notes |
| D3 | Brescia | 7.08 | 4.52 | 8 | 3 | 2 | - | 2 | 2 | 3 | - |
| D4 | Brescia | 7.58 | 4.59 | 4 | 3 | 1 | 1 | 0 | 2 | 5 | - |
| D5 | Cremona | 6.34 | 4.53 | 5 | 3 | 2 | 1 | 2 | 2 | 2 | - |
| D6 | Cremona | 7.67 | 4.60 | 6 | 4 | 1 | - | 0 | 2 | 5 | - |
| D7 | Cuneo | 6.62 | 4.50 | 6 | 3 | 2 | - | 0 | 2 | 3 | - |
| D8 | Lodi | 7.70 | 4.50 | 6 | 4 | 2 | - | 0 | 2 | 5 | - |
| D9 | Lodi | 7.95 | 4.69 | 8 | 5 | 2 | 1 | 0 | 3 | 2 | High gas formation, fizzy |
| D10 | Mantova | 7.75 | 4.60 | 5 | 4 | 1 | - | 0 | 2 | 3 | - |
| D11 | Mantova | 7.59 | 4.50 | 8 | 3 | 2 | - | 0 | 0 | 5 | - |
| D12 | Padova | 6.94 | 4.40 | 7 | 4 | 2 | 1 | 0 | 4 | 2 | High gas formation, fizzy |
| D13 | Pavia | 6.82 | 4.50 | 4 | 3 | - | - | 0 | 5 | 1 | Slight fruity notes |
| D14 | Piacenza | 7.39 | 4.50 | 7 | 3 | 2 | 1 | 0 | 2 | 5 | - |
| D15 | Piacenza | 5.76 | 4.56 | 7 | 3 | 0 | - | 0 | 2 | 4 | - |
| D16 | Trento | 7.95 | 4.45 | 5 | 3 | 1 | 1 | 0 | 3 | 5 | - |
| D17 | Verona | 6.53 | 4.50 | 5 | 3 | 1 | 1 | 0 | 3 | 3 | - |
| D18 | Verona | 7.53 | 4.50 | 5 | 4 | 3 | - | 0 | 3 | 4 | - |
| D19 | Vicenza | 8.55 | 4.59 | 6 | 3 | 1 | - | 0 | 4 | 5 | - |
| D20 | Vicenza | 7.46 | 4.31 | 7 | 3 | 2 | 1 | 0 | 1 | 5 | - |
| Compound Class | Aromatic Compound | Odor Type (a) | Strain ID (b) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2094_C | 4186_C | 5293 | 5296 | 5569 | 5649 | 5780 | 5785 | 5959 | 5963 | 5993 | 5998 | |||
| Acids | Acetic acid | sharp pungent sour vinegar | 0.215 ± 0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Hexanoic acid | sour fatty sweet cheese | 0.049 0.034 | 0.003 ± 0 | 0.007 ± 0.001 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Decanoic acid | sour, creamy, buttery, fatty | n.d. | 0.005 ± 0 | n.d. | 0.002 ± 0.001 | 0.003 ± 0 | n.d. | n.d. | 0.011 ± 0.005 | 0.004 ± 0.001 | 0.004 ± 0.001 | 0.003 ± 0 | 0.003 ± 0.001 | |
| Octanoic acid | fatty waxy rancid oily vegetable cheesy | 0.021 ± 0.014 | 0.007 ± 0 | 0.005 ± 0 | 0.005 ± 0.002 | 0.006 ± 0.001 | n.d. | 0.026 ± 0.005 | 0.017 ± 0.007 | 0.007 ± 0.002 | 0.007 ± 0.001 | 0.006 ± 0 | 0.006 ± 0.002 | |
| Acids sum | 0.285 | 0.015 | 0.012 | 0.007 | 0.009 | n.d. | 0.026 | 0.028 | 0.011 | 0.011 | 0.009 | 0.009 | ||
| Alcohols | 2-Furanmethanol | alcoholic chemical musty sweet caramel bread coffee | n.d. | 0.004 ± 0.001 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ethanol | strong alcoholic ethereal medical | 0.003 ± 0.002 | 0.002 ± n.d. | 0.001 ± 0 | n.d. | 0.001 ± 0 | 0.006 ± 0.001 | 0.01 ± 0.001 | 0.003 ± 0 | 0.001 ± 0 | 0.001 ± 0.001 | 0.001 ± 0 | n.d. | |
| Alcohols sum | 0.003 | 0.006 | 0.001 | n.d. | 0.001 | 0.006 | 0.01 | 0.003 | 0.001 | 0.001 | 0.001 | n.d. | ||
| Ketons | 2,3-Butanedione | sweet, creamy, buttery, pungent caramellic nuance | n.d. | n.d. | 0.001 ± 0 | 0.001 ± 0 | n.d. | 0.002 ± 0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ketons | 2-Heptanone | cheese, fruity, ketonic, green banana, with a creamy nuance | n.d. | 0.002 ± 0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Acetoin | sweet buttery creamy dairy milky fatty | 0.114 ± 0.073 | 0.015 ± 0.001 | n.d. | n.d. | n.d. | 0.037 ± 0.003 | n.d. | n.d. | 0.014 ± 0 | n.d. | n.d. | n.d. | |
| Acetone | solvent ethereal apple pear | n.d. | n.d. | n.d. | n.d. | n.d. | 0.011 ± 0.002 | 0.001 ± 0 | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Dimethyl sulfone | sulfurous burnt | 0.011 ± 0.01 | 0.001 ± 0 | 0.001 ± 0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Ketons sum | 0.125 | 0.019 | 0.003 | 0.001 | n.d. | 0.049 | 0.001 | n.d. | 0.014 | n.d. | n.d. | n.d. | ||
| Others | 2,4-Dimethyl-1-heptene | absent | n.d. | 0.005 ± 0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.002 ± 0.001 |
| 3-Methyl-3-buten-1-ol | sweet fruity | n.d. | n.d. | n.d. | n.d. | 0.001 ± 0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| alpha-Pinene | Woody, piney, and turpentine-like, a fresh herbal lift | n.d. | n.d. | 0.001 ± 0 | 0.001 ± 0 | 0.001 ± 0 | n.d. | n.d. | 0.004 ± 0.001 | 0.002 ± 0.001 | 0.003 ± 0.001 | n.d. | 0.003 ± 0.001 | |
| Others sum | n.d. | 0.005 | 0.001 | 0.001 | 0.002 | n.d. | n.d. | 0.004 | 0.002 | 0.003 | n.d. | 0.005 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettera, L.; Levante, A.; Bancalari, E.; Bottari, B.; Cirlini, M.; Neviani, E.; Gatti, M. Lacticaseibacillus Strains Isolated from Raw Milk: Screening Strategy for Their Qualification as Adjunct Culture in Cheesemaking. Foods 2023, 12, 3949. https://doi.org/10.3390/foods12213949
Bettera L, Levante A, Bancalari E, Bottari B, Cirlini M, Neviani E, Gatti M. Lacticaseibacillus Strains Isolated from Raw Milk: Screening Strategy for Their Qualification as Adjunct Culture in Cheesemaking. Foods. 2023; 12(21):3949. https://doi.org/10.3390/foods12213949
Chicago/Turabian StyleBettera, Luca, Alessia Levante, Elena Bancalari, Benedetta Bottari, Martina Cirlini, Erasmo Neviani, and Monica Gatti. 2023. "Lacticaseibacillus Strains Isolated from Raw Milk: Screening Strategy for Their Qualification as Adjunct Culture in Cheesemaking" Foods 12, no. 21: 3949. https://doi.org/10.3390/foods12213949





