A Review of Plant-Based Drinks Addressing Nutrients, Flavor, and Processing Technologies
Abstract
:1. Introduction
2. Classification of Plant-Based Drinks
3. Plant-Based Drink Nutrients
3.1. Protein
3.2. Dietary Fiber
3.3. Fats and Fat-Soluble Ingredients
3.4. Vitamins and Minerals
3.5. Bioactive Molecules
4. Key Substances That Contribute to Aroma in Plant-Based Drink
4.1. Aldehyde Compounds
4.2. Ketone and Alcohol Compounds
4.3. Ester and Acid Compounds
4.4. Pyrazines and Other Compounds
5. Flavor Formation Pathways
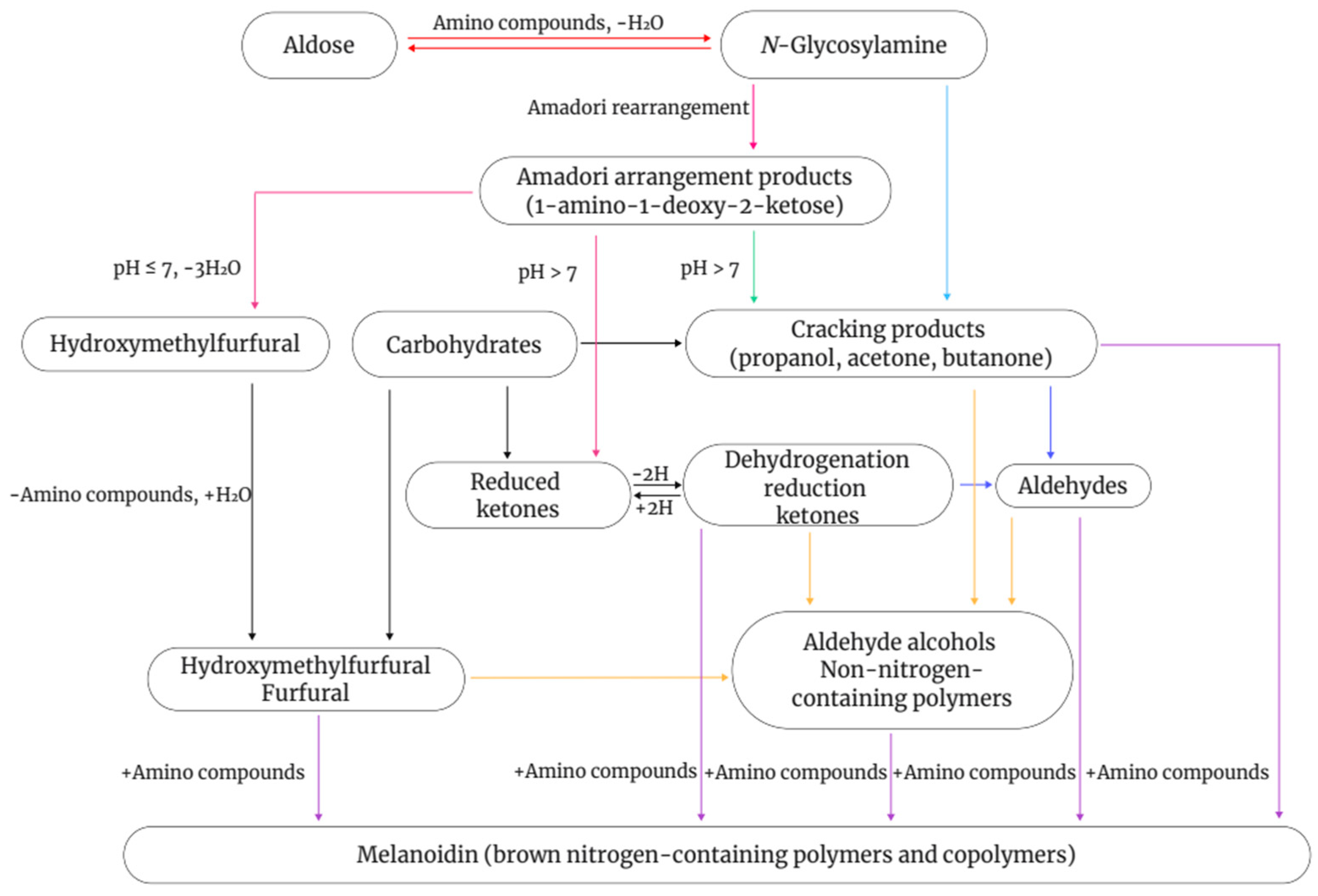
5.1. Maillard Reaction
5.2. Oxidative Degradation of Fats
6. Processing Technology
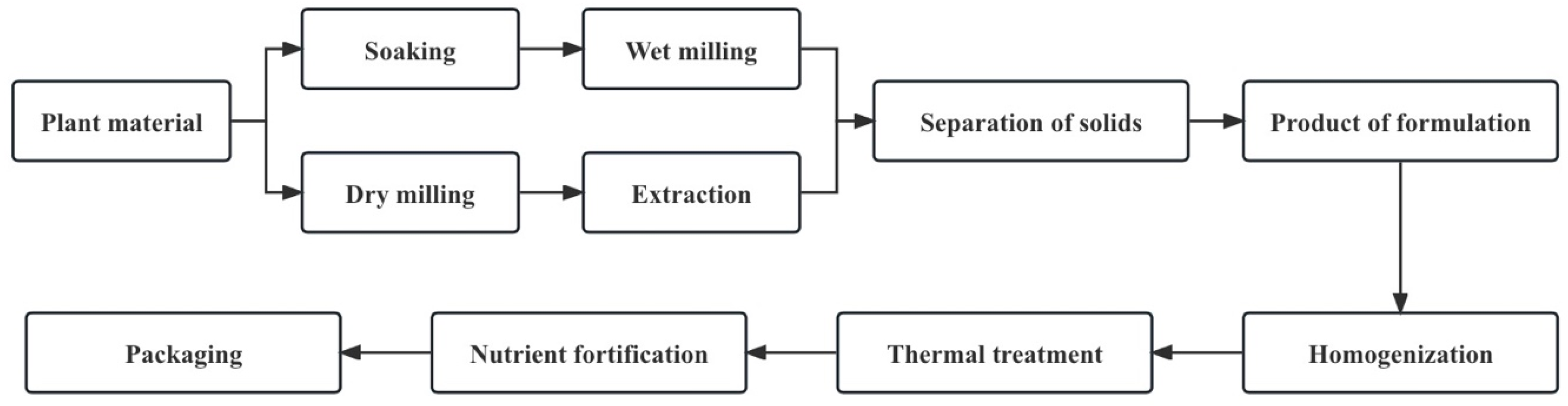
6.1. Pre-Treatment
6.2. Extraction
6.3. Enzymatic Processing
6.4. Fermentation
6.5. Germination
6.6. Separation
6.7. Product Formulation
6.8. Homogenization
6.9. Heat Treatment
6.10. Packaging and Shelf Life
7. Challenges Facing Plant-Based Drink Processing
7.1. Plant Cell Wall Tissue Limits the Dissolution of Endogenous Nutrients
7.2. The Challenge of Efficiently Removing Consumer Health Risk Factors from Plant Sources
7.3. The Control of System Stability
7.4. Adjustment of System Flavor
7.5. Control of Spoilage
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reyes-Jurado, F.; Soto-Reyes, N.; Dávila-Rodríguez, M.; Lorenzo-Leal, A.; Jiménez-Munguía, M.; Mani-López, E.; López-Malo, A. Plant-based milk alternatives: Types, processes, benefits, and characteristics. Food Rev. Int. 2021, 6, 1–32. [Google Scholar] [CrossRef]
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based milks: A review of the science underpinning their design, fabrication, and performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kang, S.; Zheng, Y.; Shao, J.; Zhao, H.; An, Y.; Cao, G.; Li, Q.; Yue, X.; Yang, M. Comparative metabolomics analysis of donkey colostrum and mature milk using ultra-high-performance liquid tandem chromatography quadrupole time-of-flight mass spectrometry. J. Dairy Sci. 2020, 103, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, W.; Wu, J.; Zheng, Y.; Shao, J.; Li, Q.; Kang, S.; Zhang, Z.; Yue, X.; Yang, M. Quantitative lipidomics reveals alterations in donkey milk lipids according to lactation. Food Chem. 2020, 310, 125866. [Google Scholar] [CrossRef]
- Haas, R.; Schnepps, A.; Pichler, A.; Meixner, O. Cow milk versus plant-based milk substitutes: A comparison of product image and motivational structure of consumption. Sustainability 2019, 11, 5046. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Pimentel, M. Sustainability of meat-based and plant-based diets and the environment. Am. J. Clin. Nutr. 2003, 78, 660S–663S. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. A brief review of the science behind the design of healthy and sustainable plant-based foods. NPJ Sci. Food 2021, 5, 17. [Google Scholar] [CrossRef]
- Grossmann, L.; McClements, D.J. The science of plant-based foods: Approaches to create nutritious and sustainable plant-based cheese analogs. Trends Food Sci. Technol. 2021, 118, 207–229. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Shen, X.; Abdlla, R.; Liu, L.; Yue, X.; Li, Q. Metabolomics-based comparative study of breast colostrum and mature breast milk. Food Chem. 2022, 384, 132491. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Q.; Kang, S.; Cao, X.; Zheng, Y.; Wu, J.; Wu, R.; Shao, J.; Yang, M.; Yue, X. Characterization and comparison of lipids in bovine colostrum and mature milk based on UHPLC-QTOF-MS lipidomics. Food Res. Int. 2020, 136, 109490. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, H.; Chen, J.; Abdlla, R.; Liu, A.; Song, W.; Zhang, J.; Zhang, X.; Yue, X.; Li, Q. Novel insights into whey protein differences between donkey and bovine milk. Food Chem. 2021, 365, 130397. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.A.; Kumar, S.; Kumar, V.; Sharma, R. Milk Analog: Plant based alternatives to conventional milk, production, potential and health concerns. Crit. Rev. Food Sci. Nutr. 2020, 60, 3005–3023. [Google Scholar] [CrossRef] [PubMed]
- Qamar, S.; Manrique, Y.J.; Parekh, H.; Falconer, J.R. Nuts, cereals, seeds and legumes proteins derived emulsifiers as a source of plant protein beverages: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2742–2762. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for special dietary needs: Non-dairy plant-based milk substitutes and fermented dairy-type products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Jeske, S.; Zannini, E.; Arendt, E.K. Evaluation of physicochemical and glycaemic properties of commercial plant-based milk substitutes. Plant Foods Hum. Nutr. 2017, 72, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, G. The protein digestibility–corrected amino acid score. J. Nutr. 2000, 130, 1865S–1867S. [Google Scholar] [CrossRef]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 24 September 2023).
- Giri, S.; Mangaraj, S. Processing influences on composition and quality attributes of soymilk and its powder. Food Eng. Rev. 2012, 4, 149–164. [Google Scholar] [CrossRef]
- Belewu, M.; Belewu, K. Comparative physico-chemical evaluation of tiger-nut, soybean and coconut milk sources. Int. J. Agric. Biol. 2007, 5, e787. [Google Scholar]
- Zhou, S.; Jia, Q.; Cui, L.; Dai, Y.; Li, R.; Tang, J.; Lu, J. Physical–Chemical and Sensory Quality of Oat Milk Produced Using Different Cultivars. Foods 2023, 12, 1165. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Chen, Y.; Wang, X.; Chen, H.; Deng, Q. Effect of Different Temperatures on the Storage Stability of Flaxseed Milk. Foods 2023, 12, 3571. [Google Scholar] [CrossRef] [PubMed]
- Seong, G.-U.; Moon, K.-D.; Park, D.-S.; Shin, D.; Lee, S.-B.; Lee, J.-Y.; Cho, J.-H.; Park, S.; Kim, J.; Kim, J. Quality characteristics of plant-based rice milk prepared with different rice varieties. Korean J. Food Preserv. 2022, 29, 395–406. [Google Scholar] [CrossRef]
- Zheng, B.; Zhou, H.; McClements, D.J. Nutraceutical-fortified plant-based milk analogs: Bioaccessibility of curcumin-loaded almond, cashew, coconut, and oat milks. LWT 2021, 147, 111517. [Google Scholar] [CrossRef]
- Xie, A.-J.; Lee, D.-J.; Lim, S.-T. Characterization of resistant waxy maize dextrins prepared by simultaneous debranching and crystallization followed by acidic or enzymatic hydrolysis. Food Hydrocoll. 2021, 121, 106942. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.-M.; Xie, A.-J.; Zhu, C.-Y.; Qin, G.-Y. Dietary fiber extraction from defatted corn hull by hot-compressed water. Pol. J. Food Nutr. Sci. 2018, 68. [Google Scholar] [CrossRef]
- Xie, A.-J.; Yin, H.-S.; Liu, H.-M.; Zhu, C.-Y.; Yang, Y.-J. Chinese quince seed gum and poly (N, N-diethylacryl amide-co-methacrylic acid) based pH-sensitive hydrogel for use in drug delivery. Carbohydr. Polym. 2018, 185, 96–104. [Google Scholar] [CrossRef]
- Xie, A.; Zhu, C.; Hua, L. Food gum based hydrogel polymers. MOJ Food Process. Technol. 2017, 4, 192–196. [Google Scholar]
- Wang, L.; Liu, H.-M.; Zhu, C.-Y.; Xie, A.-J.; Ma, B.-J.; Zhang, P.-Z. Chinese quince seed gum: Flow behaviour, thixotropy and viscoelasticity. Carbohydr. Polym. 2019, 209, 230–238. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.-M.; Xie, A.-J.; Wang, X.-D.; Zhu, C.-Y.; Qin, G.-Y. Chinese quince (Chaenomeles sinensis) seed gum: Structural characterization. Food Hydrocoll. 2018, 75, 237–245. [Google Scholar] [CrossRef]
- Xie, A.; Zhao, S.; Liu, Z.; Yue, X.; Shao, J.; Li, M.; Li, Z. Polysaccharides, proteins, and their complex as microencapsulation carriers for delivery of probiotics: A review on carrier types and encapsulation techniques. Int. J. Biol. Macromol. 2023, 124784. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Q.; Dong, H.; Zhao, S.; Ning, J.; Bai, X.; Yue, X.; Xie, A. Pilose antler polypeptides enhance chemotherapy effects in triple-negative breast cancer by activating the adaptive immune system. Int. J. Biol. Macromol. 2022, 222, 2628–2638. [Google Scholar] [CrossRef] [PubMed]
- Swallah, M.S.; Fan, H.; Wang, S.; Yu, H.; Piao, C. Prebiotic impacts of soybean residue (Okara) on eubiosis/dysbiosis condition of the gut and the possible effects on liver and kidney functions. Molecules 2021, 26, 326. [Google Scholar] [CrossRef]
- Yanai, H.; Katsuyama, H.; Hamasaki, H.; Abe, S.; Tada, N.; Sako, A. Effects of dietary fat intake on HDL metabolism. J. Clin. Med. Res. 2015, 7, 145. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Bao, Y.; Liu, X.; Zhang, H. Conjugated linoleic acid conversion by six Lactobacillus plantarum strains cultured in MRS broth supplemented with sunflower oil and soymilk. J. Food Sci. 2012, 77, M330–M336. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.; Benjakul, S. Coconut milk and coconut oil: Their manufacture associated with protein functionality. J. Food Sci. 2018, 83, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Institute, N.F. Frida Food Data. Available online: http://frida.fooddata.dk (accessed on 25 September 2023).
- Hussain, M.; Sun, Y.; Pan, Y.; Liu, L.; Zhang, X.; Wang, Q.; Lin, S.; Qayum, A.; Hussain, K.; Li, X. Formulation, invitro digestive study, and comparative fatty acid analysis of walnut oil-based infant formula, with human milk, animal milk, and commercial infant formula. Innov. Food Sci. Emerg. Technol. 2023, 84, 103279. [Google Scholar] [CrossRef]
- Isanga, J.; Zhang, G. Production and evaluation of some physicochemical parameters of peanut milk yoghurt. LWT-Food Sci. Technol. 2009, 42, 1132–1138. [Google Scholar] [CrossRef]
- Caroprese, M.; Marzano, A.; Marino, R.; Gliatta, G.; Muscio, A.; Sevi, A. Flaxseed supplementation improves fatty acid profile of cow milk. J. Dairy Sci. 2010, 93, 2580–2588. [Google Scholar] [CrossRef]
- Vanga, S.K.; Raghavan, V. How well do plant based alternatives fare nutritionally compared to cow’s milk? J. Food Sci. Technol. 2018, 55, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Rafferty, K. The settling problem in calcium-fortified soybean drinks. J. Am. Diet. Assoc. 2006, 106, 1753. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Dowell, M.S.; Rafferty, K.; Bierman, J. Bioavailability of the calcium in fortified soy imitation milk, with some observations on method. Am. J. Clin. Nutr. 2000, 71, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Romulo, A. Nutritional Contents and Processing of Plant-Based Milk: A Review. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Online, 17–18 November 2022; p. 012054. [Google Scholar]
- Ferragut, V.; Valencia-Flores, D.C.; Pérez-González, M.; Gallardo, J.; Hernández-Herrero, M. Quality characteristics and shelf-life of ultra-high pressure homogenized (UHPH) almond beverage. Foods 2015, 4, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.Y.; Volcheck, G.W. Food allergy: Common causes, diagnosis, and treatment. Mayo Clin. Proc. 2015, 90, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Sancheti, H.; Liu, Z.; Cadenas, E. Mitochondrial function in ageing: Coordination with signalling and transcriptional pathways. J. Physiol. 2016, 594, 2025–2042. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.-X.; Lu, Z.-Z.; Luyengi, L.; Lee, S.K.; Pezzuto, J.M.; Farnsworth, N.R.; Thompson, L.U.; Fong, H.H. Isolation and characterization of flaxseed (Linum usitatissimum) constituents. Pharm. Biol. 1999, 37, 1–7. [Google Scholar] [CrossRef]
- Decloedt, A.I.; Van Landschoot, A.; Watson, H.; Vanderputten, D.; Vanhaecke, L. Plant-based beverages as good sources of free and glycosidic plant sterols. Nutrients 2017, 10, 21. [Google Scholar] [CrossRef]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C.-Y.O. Polyphenol content and antioxidant activity of California almonds depend on cultivar and harvest year. Food Chem. 2010, 122, 819–825. [Google Scholar] [CrossRef]
- Bernat, N.; Cháfer, M.; Chiralt, A.; Laparra, J.M.; González-Martínez, C. Almond milk fermented with different potentially probiotic bacteria improves iron uptake by intestinal epithelial (Caco-2) cells. Int. J. Food Stud. 2015, 4, 49–60. [Google Scholar] [CrossRef]
- Pelvan, E.; Olgun, E.Ö.; Karadağ, A.; Alasalvar, C. Phenolic profiles and antioxidant activity of Turkish Tombul hazelnut samples (natural, roasted, and roasted hazelnut skin). Food Chem. 2018, 244, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Pasli, A.A.; Ozcelik, B.; Capanoglu, E. Evaluating the in vitro bioaccessibility of phenolics and antioxidant activity during consumption of dried fruits with nuts. LWT-Food Sci. Technol. 2014, 56, 284–289. [Google Scholar] [CrossRef]
- Fitrotin, U.; Utami, T.; Hastuti, P.; Santoso, U. Antioxidant properties of fermented sesame milk using Lactobacillus plantarum Dad 13. Int. Res. J. Biol. Sci. 2015, 4, 56–61. [Google Scholar]
- Bopitiya, D.; Madhujith, T. Antioxidant activity and total phenolic content of sesame (Sesamum indicum L.) seed oil. Trop. Agric. Res. 2013, 24, 296. [Google Scholar]
- Hamza, A.H.; Mahmoud, R.H. Soy milk and Sesame seeds (Phytoestrogens) Ameliorate cardiotoxcity induced by adriamycin in experimental animals. Am. J. Res. Commun. 2013, 1, 1–15. [Google Scholar]
- Alu’datt, M.H.; Rababah, T.; Ereifej, K.; Alli, I. Distribution, antioxidant and characterisation of phenolic compounds in soybeans, flaxseed and olives. Food Chem. 2013, 139, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Abou-Dobara, M.; Nawal, M. Functional rice rayeb milk: Chemical, microbiological and sensory properties. J. Nutr. Health Sci 2018, 5, 1–12. [Google Scholar]
- Moretto, L.; Tonolo, F.; Folda, A.; Scalcon, V.; Bindoli, A.; Bellamio, M.; Feller, E.; Rigobello, M.P. Comparative analysis of the antioxidant capacity and lipid and protein oxidation of soy and oats beverages. Food Prod. Process. Nutr. 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Grossmann, L.; Kinchla, A.J.; Nolden, A.; McClements, D.J. Standardized methods for testing the quality attributes of plant-based foods: Milk and cream alternatives. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2206–2233. [Google Scholar] [CrossRef]
- Schifferstein, H.N.; Verlegh, P.W. The role of congruency and pleasantness in odor-induced taste enhancement. Acta Psychol. 1996, 94, 87–105. [Google Scholar] [CrossRef]
- Noble, A.C. Taste-aroma interactions. Trends Food Sci. Technol. 1996, 7, 439–444. [Google Scholar] [CrossRef]
- Frank, R.A.; Byram, J. Taste–smell interactions are tastant and odorant dependent. Chem. Senses 1988, 13, 445–455. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Snow, N.H.; Slack, G.C. Head-space analysis in modern gas chromatography. TrAC Trends Anal. Chem. 2002, 21, 608–617. [Google Scholar] [CrossRef]
- Lv, Y.C.; Song, H.L.; Li, X.; Wu, L.; Guo, S.T. Influence of blanching and grinding process with hot water on beany and non-beany flavor in soymilk. J. Food Sci. 2011, 76, S20–S25. [Google Scholar] [CrossRef] [PubMed]
- Saison, D.; De Schutter, D.P.; Uyttenhove, B.; Delvaux, F.; Delvaux, F.R. Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chem. 2009, 114, 1206–1215. [Google Scholar] [CrossRef]
- Curioni, P.; Bosset, J. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Yu, H.; Liu, R.; Hu, Y.; Xu, B. Flavor profiles of soymilk processed with four different processing technologies and 26 soybean cultivars grown in China. Int. J. Food Prop. 2017, 20, S2887–S2898. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Chang, Y.H. Volatile compound, physicochemical, and antioxidant properties of beany flavor-removed soy protein isolate hydrolyzates obtained from combined high temperature pre-treatment and enzymatic hydrolysis. Prev. Nutr. Food Sci. 2016, 21, 338. [Google Scholar] [CrossRef]
- Jensen, P.N.; Sørensen, G.; Engelsen, S.B.; Bertelsen, G. Evaluation of quality changes in walnut kernels (Juglans regia L.) by Vis/NIR spectroscopy. J. Agric. Food Chem. 2001, 49, 5790–5796. [Google Scholar] [CrossRef]
- Raftaniamiri, Z.; Khandelwal, P.; Aruna, B. Development of acidophilus milk via selected probiotics & prebiotics using artificial neural network. Adv. Biosci. Biotechnol. 2010, 1, 224–231. [Google Scholar]
- Chetschik, I.; Granvogl, M.; Schieberle, P. Comparison of the key aroma compounds in organically grown, raw West-African peanuts (Arachis hypogaea) and in ground, pan-roasted meal produced thereof. J. Agric. Food Chem. 2008, 56, 10237–10243. [Google Scholar] [CrossRef] [PubMed]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects. J. Funct. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- De Girolamo, A.; Lattanzio, V.; Schena, R.; Visconti, A.; Pascale, M. Effect of alkaline cooking of maize on the content of fumonisins B1 and B2 and their hydrolysed forms. Food Chem. 2016, 192, 1083–1089. [Google Scholar] [CrossRef]
- Kaneko, S.; Kumazawa, K.; Nishimura, O. Studies on the key aroma compounds in soy milk made from three different soybean cultivars. J. Agric. Food Chem. 2011, 59, 12204–12209. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, Q.; Zhao, H.; Zhao, M.; Yang, B. Volatile compounds of Cantonese sausage released at different stages of processing and storage. Food Chem. 2010, 121, 319–325. [Google Scholar] [CrossRef]
- Combet, E.; Eastwood, D.C.; Burton, K.S.; Henderson, J. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef] [PubMed]
- Pripis-Nicolau, L.; De Revel, G.; Bertrand, A.; Maujean, A. Formation of flavor components by the reaction of amino acid and carbonyl compounds in mild conditions. J. Agric. Food Chem. 2000, 48, 3761–3766. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Boye, J.I.; Azarnia, S.; Simpson, B.K. Volatile flavor profile of Saskatchewan grown pulses as affected by different thermal processing treatments. Int. J. Food Prop. 2016, 19, 2251–2271. [Google Scholar] [CrossRef]
- Degon, J.G.; Zheng, C.; Elkhedir, A.; Yang, B.; Zhou, Q.; Li, W. Effect of microwave pre-treatment on physical quality, bioactive compounds, safety risk factor, and storage stability of peanut butter. Oil Crop Sci. 2021, 6, 137–144. [Google Scholar] [CrossRef]
- Van Boekel, M. Kinetic aspects of the Maillard reaction: A critical review. Food/Nahr. 2001, 45, 150–159. [Google Scholar] [CrossRef]
- Martins, S.I.; Jongen, W.M.; Van Boekel, M.A. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Ellis, G. The maillard reaction. In Advances in Carbohydrate Chemistry; Elsevier: Amsterdam, The Netherlands, 1959; Volume 14, pp. 63–134. [Google Scholar]
- Fu, M.-X.; Wells-Knecht, K.J.; Blackledge, J.A.; Lyons, T.J.; Thorpe, S.R.; Baynes, J.W. Glycation, glycoxidation, and cross-linking of collagen by glucose: Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes 1994, 43, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Van Boekel, M. Effect of heating on Maillard reactions in milk. Food Chem. 1998, 62, 403–414. [Google Scholar] [CrossRef]
- Dai, X.; Wang, P.; Wu, W.; Wang, H.; Xu, Q.; Li, Z. Separation of Dimethyl Sulfide from Wort by Multi-Layer Centrifugal Film Method. Foods 2022, 11, 2901. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S.; Elmore, J.S. Control of the Maillard reaction during the cooking of food. Control. Mail. Pathw. Gener. Flavors 2010, 14, 143–155. [Google Scholar]
- Matsui, K.; Takemoto, H.; Koeduka, T.; Ohnishi, T. 1-Octen-3-ol is formed from its glycoside during processing of soybean [Glycine max (L.) Merr.] seeds. J. Agric. Food Chem. 2018, 66, 7409–7416. [Google Scholar] [CrossRef]
- Lokuruka, M. Effects of processing on soybean nutrients and potential impact on consumer health: An overview. Afr. J. Food Agric. Nutr. Dev. 2011, 11, 4. [Google Scholar] [CrossRef]
- Klensporf, D.; Jeleñ, H.H. Analysis of volatile aldehydes in oat flakes by SPME-GC/MS. Pol. J. Food Nutr. Sci. 2005, 14, 389. [Google Scholar]
- Matsumura, S.; Takahashi, S.; Nishikitani, M.; Kubota, K.; Kobayashi, A. The role of diglycosides as tea aroma precursors: Synthesis of tea diglycosides and specificity of glycosidases in tea leaves. J. Agric. Food Chem. 1997, 45, 2674–2678. [Google Scholar] [CrossRef]
- Hüsnü, K.; Başer, C.; Demirci, F. Chemistry of essential oils. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2007; pp. 43–86. [Google Scholar] [CrossRef]
- Taurino, M.; De Domenico, S.; Bonsegna, S.; Santino, A. The hydroperoxide lyase branch of the oxylipin pathway and green leaf volatiles in plant/insect interaction. J. Plant Biochem. Physiol. 2013, 1, 2. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.A.; Tavares, S.R.; Ramos, V.; De Barros, E.G. Hexanal production and TBA number are reduced in soybean [Glycine max (L.) Merr.] seeds lacking lipoxygenase isozymes 2 and 3. J. Agric. Food Chem. 1993, 41, 103–106. [Google Scholar] [CrossRef]
- Lee, J.; Min, S.; Choe, E.; Min, D. Formation of volatile compounds in soy flour by singlet oxygen oxidation during storage under light. J. Food Sci. 2003, 68, 1933–1937. [Google Scholar] [CrossRef]
- Bradley, D.; Lee, H.; Min, D. Singlet oxygen detection in skim milk by electron spin resonance spectroscopy. J. Food Sci. 2003, 68, 491–494. [Google Scholar] [CrossRef]
- Padma, M.; Jagannadarao, P.; Edukondalu, L.; Ravibabu, G.; Aparna, K. Physico-chemical analysis of milk prepared from broken rice. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 426–428. [Google Scholar] [CrossRef]
- Gajdoš Kljusurić, J.; Benković, M.; Bauman, I. Classification and processing optimization of barley milk production using NIR spectroscopy, particle size, and total dissolved solids analysis. J. Chem. 2015, 2015, 896051. [Google Scholar] [CrossRef]
- Bridges, M. Moo-ove over, cow’s milk: The rise of plant-based dairy alternatives. Pract. Gastroenterol. 2018, 21, 20–27. [Google Scholar]
- Xiao, X.; Zou, P.-R.; Hu, F.; Zhu, W.; Wei, Z.-J. Updates on Plant-Based Protein Products as an Alternative to Animal Protein: Technology, Properties, and Their Health Benefits. Molecules 2023, 28, 4016. [Google Scholar] [CrossRef]
- Boukid, F.; Hassoun, A.; Zouari, A.; Tülbek, M.Ç.; Mefleh, M.; Aït-Kaddour, A.; Castellari, M. Fermentation for Designing Innovative Plant-Based Meat and Dairy Alternatives. Foods 2023, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.N.; Bansal, S.; Jaiswal, A.K.; Singh, R. Plant based dairy analogues: An emerging food. Agri. Res. Tech. 2017, 10, 2471–6774. [Google Scholar] [CrossRef]
- Martins, C.P.; Cavalcanti, R.N.; Rocha, R.S.; Esmerino, E.A.; Freitas, M.Q.; Pimentel, T.C.; Silva, M.C.; Cruz, A.G. Microwave heating impacts positively on the physical properties of orange juice-milk beverage. Int. J. Dairy Technol. 2022, 75, 129–138. [Google Scholar] [CrossRef]
- Torki-Harchegani, M.; Ghanbarian, D.; Pirbalouti, A.G.; Sadeghi, M. Dehydration behaviour, mathematical modelling, energy efficiency and essential oil yield of peppermint leaves undergoing microwave and hot air treatments. Renew. Sustain. Energy Rev. 2016, 58, 407–418. [Google Scholar] [CrossRef]
- Varghese, T.; Pare, A. Effect of microwave assisted extraction on yield and protein characteristics of soymilk. J. Food Eng. 2019, 262, 92–99. [Google Scholar] [CrossRef]
- Wong, W.L.E.; Gupta, M. Using microwave energy to synthesize light weight/energy saving magnesium based materials: A review. Technologies 2015, 3, 1–18. [Google Scholar] [CrossRef]
- Jacob, M.; Jaros, D.; Rohm, H. Recent advances in milk clotting enzymes. Int. J. Dairy Technol. 2011, 64, 14–33. [Google Scholar] [CrossRef]
- Penha, C.B.; Santos, V.D.P.; Speranza, P.; Kurozawa, L.E. Plant-based beverages: Ecofriendly technologies in the production process. Innov. Food Sci. Emerg. Technol. 2021, 72, 102760. [Google Scholar] [CrossRef]
- Vong, W.C.; Liu, S.-Q. Biovalorisation of okara (soybean residue) for food and nutrition. Trends Food Sci. Technol. 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Pandey, S.; Poonia, A. Plant-based milk substitutes: A novel non-dairy source. Innov. Food Technol. Curr. Perspect. Future Goals 2020, 63–71. [Google Scholar] [CrossRef]
- Rosenthal, A.; Deliza, R.; Cabral, L.M.; Cabral, L.C.; Farias, C.A.; Domingues, A.M. Effect of enzymatic treatment and filtration on sensory characteristics and physical stability of soymilk. Food Control 2003, 14, 187–192. [Google Scholar] [CrossRef]
- Sahoo, A.; Gaikwad, V.; Ranveer, R.; Dandge, P.; Waghmare, S. Improvement of shelf life of soymilk using immobilized protease of Oerskovia xanthineolytica NCIM 2839. 3 Biotech 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Karamać, M.; Kosińska-Cagnazzo, A.; Kulczyk, A. Use of different proteases to obtain flaxseed protein hydrolysates with antioxidant activity. Int. J. Mol. Sci. 2016, 17, 1027. [Google Scholar] [CrossRef] [PubMed]
- Görgüç, A.; Bircan, C.; Yılmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Jeske, S.; Zannini, E.; Arendt, E.K. Past, present and future: The strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res. Int. 2018, 110, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Rasika, D.; Vidanarachchi, J.K.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ranadheera, C.S. Probiotic delivery through non-dairy plant-based food matrices. Agriculture 2021, 11, 599. [Google Scholar] [CrossRef]
- Hou, J.-W.; Yu, R.-C.; Chou, C.-C. Changes in some components of soymilk during fermentation with bifidobacteria. Food Res. Int. 2000, 33, 393–397. [Google Scholar] [CrossRef]
- Rekha, C.; Vijayalakshmi, G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. 2010, 109, 1198–1208. [Google Scholar] [CrossRef]
- Wansutha, S.; Yuenyaow, L.; Jantama, K.; Jantama, S.S. Antioxidant activities of almond milk fermented with lactic acid bacteria. Thaiphesatchasan 2018, 42, 115–119. [Google Scholar]
- Cruz, N.; Capellas, M.; Hernández, M.; Trujillo, A.; Guamis, B.; Ferragut, V. Ultra high pressure homogenization of soymilk: Microbiological, physicochemical and microstructural characteristics. Food Res. Int. 2007, 40, 725–732. [Google Scholar] [CrossRef]
- Penha, C.B.; Falcão, H.G.; Ida, E.I.; Speranza, P.; Kurozawa, L.E. Enzymatic pretreatment in the extraction process of soybean to improve protein and isoflavone recovery and to favor aglycone formation. Food Res. Int. 2020, 137, 109624. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Shah, N.P. Changes in antioxidant capacity, isoflavone profile, phenolic and vitamin contents in soymilk during extended fermentation. LWT-Food Sci. Technol. 2014, 58, 454–462. [Google Scholar] [CrossRef]
- Criste, A.D.; Urcan, A.C.; Coroian, C.O.; Copolovici, L.; Copolovici, D.M.; Burtescu, R.F.; Oláh, N.K. Plant-Based Beverages from Germinated and Ungerminated Seeds, as a Source of Probiotics, and Bioactive Compounds with Health Benefits—Part 1: Legumes. Agriculture 2023, 13, 1185. [Google Scholar] [CrossRef]
- Aboulfazli, F.; Shori, A.B.; Baba, A.S. Effects of the replacement of cow milk with vegetable milk on probiotics and nutritional profile of fermented ice cream. LWT 2016, 70, 261–270. [Google Scholar] [CrossRef]
- Oghbaei, M.; Prakash, J. Effect of primary processing of cereals and legumes on its nutritional quality: A comprehensive review. Cogent Food Agric. 2016, 2, 1136015. [Google Scholar] [CrossRef]
- Nirmala, M.; Rao, M.S.; Muralikrishna, G. Carbohydrates and their degrading enzymes from native and malted finger millet (Ragi, Eleusine coracana, Indaf-15). Food Chem. 2000, 69, 175–180. [Google Scholar] [CrossRef]
- Saxena, S.; Vasudevan, H.; Saini, S.; Sasmal, S. Comparative Nutritional Assessment of Millet-Based Milk Produced by Ultrasound, Germination, and a Combined Approach. ACS Food Sci. Technol. 2023, 3, 600–607. [Google Scholar] [CrossRef]
- Özer, B.H.; Kirmaci, H.A. Functional milks and dairy beverages. Int. J. Dairy Technol. 2010, 63, 1–15. [Google Scholar] [CrossRef]
- BALCI, A.T.; Wilbey, R.A. High pressure processing of milk-the first 100 years in the development of a new technology. Int. J. Dairy Technol. 1999, 52, 149–155. [Google Scholar] [CrossRef]
- Poliseli-Scopel, F.H.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Comparison of ultra high pressure homogenization and conventional thermal treatments on the microbiological, physical and chemical quality of soymilk. LWT-Food Sci. Technol. 2012, 46, 42–48. [Google Scholar] [CrossRef]
- Zamora, A.; Guamis, B. Opportunities for ultra-high-pressure homogenisation (UHPH) for the food industry. Food Eng. Rev. 2015, 7, 130–142. [Google Scholar] [CrossRef]
- Mollakhalili-Meybodi, N.; Nejati, R.; Sayadi, M.; Nematollahi, A. Novel nonthermal food processing practices: Their influences on nutritional and technological characteristics of cereal proteins. Food Sci. Nutr. 2022, 10, 1725–1744. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.J.D. Method for Preparing a Beanless-Flavor Soymilk and/or Okara Using Carbon Dioxide in a State of Sublimation. Google Patents EP1830658A2 12 September 2007. [Google Scholar]
- Poliseli-Scopel, F.H.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Sterilization and aseptic packaging of soymilk treated by ultra high pressure homogenization. Innov. Food Sci. Emerg. Technol. 2014, 22, 81–88. [Google Scholar] [CrossRef]
- Rysstad, G.; Kolstad, J. Extended shelf life milk—Advances in technology. Int. J. Dairy Technol. 2006, 59, 85–96. [Google Scholar] [CrossRef]
- Panchal, H.; Patel, J.; Chaudhary, S. A comprehensive review of solar milk pasteurization system. J. Sol. Energy Eng. 2018, 140, 010801. [Google Scholar] [CrossRef]
- McCarthy, K.; Parker, M.; Ameerally, A.; Drake, S.; Drake, M. Drivers of choice for fluid milk versus plant-based alternatives: What are consumer perceptions of fluid milk? J. Dairy Sci. 2017, 100, 6125–6138. [Google Scholar] [CrossRef] [PubMed]
- Watts, S. A mini review on technique of milk pasteurization. J. Pharmacogn. Phytochem. 2016, 5, 99–101. [Google Scholar]
- Gentès, M.C.; Caron, A.; Champagne, C.P. Potential applications of pulsed electric field in cheesemaking. Int. J. Dairy Technol. 2022, 75, 270–288. [Google Scholar] [CrossRef]
- Ross, A.I.; Griffiths, M.W.; Mittal, G.S.; Deeth, H.C. Combining nonthermal technologies to control foodborne microorganisms. Int. J. Food Microbiol. 2003, 89, 125–138. [Google Scholar] [CrossRef]
- Silva, A.R.; Silva, M.M.; Ribeiro, B.D. Health issues and technological aspects of plant-based alternative milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef]
- Zhao, W.; Tang, Y.; Lu, L.; Chen, X.; Li, C. Pulsed electric fields processing of protein-based foods. Food Bioprocess Technol. 2014, 7, 114–125. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Tian, W.-L.; Mo, H.-Z.; Zhang, Y.-L.; Zhao, X.-Z. Effects of pulsed electric field processing on quality characteristics and microbial inactivation of soymilk. Food Bioprocess Technol. 2013, 6, 1907–1916. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Chen, Q.; Liu, X.-H.; Chen, Z.-X. Inactivation of soybean lipoxygenase in soymilk by pulsed electric fields. Food Chem. 2008, 109, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Mu, K.; Arntfield, S.D.; Nickerson, M.T. Changes in levels of enzyme inhibitors during soaking and cooking for pulses available in Canada. J. Food Sci. Technol. 2017, 54, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Pataro, G.; Ferrari, G. Limitations of pulsed electric field utilization in food industry. In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–310. [Google Scholar]
- Korhonen, H. Technology options for new nutritional concepts. Int. J. Dairy Technol. 2002, 55, 79–88. [Google Scholar] [CrossRef]
- Wattanayon, W.; Udompijitkul, P.; Kamonpatana, P. Ohmic heating of a solid-liquid food mixture in an electrically conductive package. J. Food Eng. 2021, 289, 110180. [Google Scholar] [CrossRef]
- Saxena, J.; Ahmad Makroo, H.; Srivastava, B. E ffect of ohmic heating on Polyphenol Oxidase (PPO) inactivation and color change in sugarcane juice. J. Food Process Eng. 2017, 40, e12485. [Google Scholar] [CrossRef]
- Saini, A.; Morya, S. A Review based study on Soymilk: Focuses on production technology, Prospects and Progress Scenario in last Decade. Pharma Innov. 2021, 5, 10. [Google Scholar]
- Lu, L.; Zhao, L.; Zhang, C.; Kong, X.; Hua, Y.; Chen, Y. Comparative effects of ohmic, induction cooker, and electric stove heating on soymilk trypsin inhibitor inactivation. J. Food Sci. 2015, 80, C495–C503. [Google Scholar] [CrossRef]
- Castro, I.; Macedo, B.; Teixeira, J.; Vicente, A. The effect of electric field on important food-processing enzymes: Comparison of inactivation kinetics under conventional and ohmic heating. J. Food Sci. 2004, 69, C696–C701. [Google Scholar] [CrossRef]
- Shim, J.; Lee, S.H.; Jun, S. Modeling of ohmic heating patterns of multiphase food products using computational fluid dynamics codes. J. Food Eng. 2010, 99, 136–141. [Google Scholar] [CrossRef]
- Makroo, H.; Rastogi, N.; Srivastava, B. Ohmic heating assisted inactivation of enzymes and microorganisms in foods: A review. Trends Food Sci. Technol. 2020, 97, 451–465. [Google Scholar] [CrossRef]
- Aidoo, H.; Sakyi-Dawson, E.; Tano-Debrah, K.; Saalia, F.K. Development and characterization of dehydrated peanut–cowpea milk powder for use as a dairy milk substitute in chocolate manufacture. Food Res. Int. 2010, 43, 79–85. [Google Scholar] [CrossRef]
- Zungur Bastıoğlu, A.; Tomruk, D.; Koç, M.; Ertekin, F.K. Spray dried melon seed milk powder: Physical, rheological and sensory properties. J. Food Sci. Technol. 2016, 53, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, Z.; Taip, F.S.; Mustapa Kamal, S.M.; Abdul Rahman, R.Z. Effect of sodium caseinate concentration and sonication amplitude on the stability and physical characteristics of homogenized coconut milk. J. Food Process. Preserv. 2018, 42, e13773. [Google Scholar] [CrossRef]
- Yazici, F.; Alvarez, V.; Mangino, M.; Hansen, P. Formulation and processing of a heat stable calcium-fortified soy milk. J. Food Sci. 1997, 62, 535–538. [Google Scholar] [CrossRef]
- Kwok, K.; Qin, W.; Tsang, J. Heat inactivation of trypsin inhibitors in soymilk at ultra-high temperatures. J. Food Sci. 1993, 58, 859–862. [Google Scholar] [CrossRef]
- Sizer, C. Aseptic packaging of soymilk. In Food Uses of Whole Oil and Protein Seeds; Lucas, E.W., Erickson, D.R., Nip, W.K., Eds.; American Oil Chemists’ Society: Champaign, IL, USA, 1989; pp. 98–101, Ch. 6. [Google Scholar]
- Han, T.B. Technology of Soymilk and Some Derivatives. 1958. Available online: https://library.wur.nl/WebQuery/wurpubs/fulltext/180242 (accessed on 25 September 2023).
- Arise, A.K.; Malomo, S.A.; Jacob, O.A.; Olagunju, O.F.; Esan, O.T. Comparative studies of yoghurt produced from animal and selected imitation vegetable milk. Nutrire 2022, 47, 33. [Google Scholar] [CrossRef]
- SV, R.; Shameena Beegum, P.; Pandiselvam, R.; Manikantan, M.; Hebbar, K. Plant-Based Milk Alternatives: Nutritional Potential and Challenges. In Conceptualizing Plant-Based Nutrition: Bioresources, Nutrients Repertoire and Bioavailability; Springer: Berlin/Heidelberg, Germany, 2022; pp. 91–106. [Google Scholar]
- Anand, S.P.; Awasti, N. Novel Dairy-Based Drinks: Changing Scenario. Dairy Process. Adv. Res. Appl. 2020, 301–325. [Google Scholar] [CrossRef]
- Valencia-Flores, D.C.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Comparing the effects of ultra-high-pressure homogenization and conventional thermal treatments on the microbiological, physical, and chemical quality of almond beverages. J. Food Sci. 2013, 78, E199–E205. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Colosimo, R.; Warren, F.J.; Edwards, C.H.; Ryden, P.; Dyer, P.S.; Finnigan, T.J.; Wilde, P.J. Comparison of the behavior of fungal and plant cell wall during gastrointestinal digestion and resulting health effects: A review. Trends Food Sci. Technol. 2021, 110, 132–141. [Google Scholar] [CrossRef]
- Burton, R.A.; Fincher, G.B. Evolution and development of cell walls in cereal grains. Front. Plant Sci. 2014, 5, 456. [Google Scholar] [CrossRef] [PubMed]
- Bocker, R.; Silva, E.K. Innovative technologies for manufacturing plant-based non-dairy alternative milk and their impact on nutritional, sensory and safety aspects. Future Foods 2022, 5, 100098. [Google Scholar] [CrossRef]
- Chain, E.P.o.C.i.t.F.; Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C. Evaluation of the health risks related to the presence of cyanogenic glycosides in foods other than raw apricot kernels. EFSA J. 2019, 17, e05662. [Google Scholar]
- Abd Rahim, M.H.; Hazrin-Chong, N.H.; Harith, H.H.; Wan, W.A.A.Q.I.; Sukor, R. Roles of fermented plant-, dairy-and meat-based foods in the modulation of allergic responses. Food Sci. Hum. Wellness 2023, 12, 691–701. [Google Scholar] [CrossRef]
- Liang, J.; Han, B.-Z.; Nout, M.R.; Hamer, R.J. Effects of soaking, germination and fermentation on phytic acid, total and in vitro soluble zinc in brown rice. Food Chem. 2008, 110, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, S.; Venkatachalam, M.; Mistry, A.M.; Lapsley, K.; Sathe, S.K. Almond (Prunus dulcis L.) protein quality. Plant Foods Hum. Nutr. 2005, 60, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Sarangapany, A.K.; Murugesan, A.; Annamalai, A.S.; Balasubramanian, A.; Shanmugam, A. An overview on ultrasonically treated plant-based milk and its properties–A Review. Appl. Food Res. 2022, 2, 100130. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.; Zhang, C.; Kong, X.; Chen, Y.; Hua, Y. Formation mechanism of hexanal and (E)-2-hexenal during soybean [Glycine max (L.) Merr] processing based on the subcellular and molecular levels. J. Agric. Food Chem. 2021, 70, 289–300. [Google Scholar] [CrossRef]
- Trawatha, S.; TeKrony, D.; Hildebrand, D. Relationship of Soybean Seed Quality to Fatty Acid and C6-Aldehyde Levels during Storage. Crop Sci. 1995, 35, 1415–1422. [Google Scholar] [CrossRef]
- Noordermeer, M.A.; Van Der Goot, W.; Van Kooij, A.J.; Veldsink, J.W.; Veldink, G.A.; Vliegenthart, J.F. Development of a biocatalytic process for the production of C6-aldehydes from vegetable oils by soybean lipoxygenase and recombinant hydroperoxide lyase. J. Agric. Food Chem. 2002, 50, 4270–4274. [Google Scholar] [CrossRef]
- Zhang, C.; Hua, Y.; Li, X.; Kong, X.; Chen, Y. Key volatile off-flavor compounds in peas (Pisum sativum L.) and their relations with the endogenous precursors and enzymes using soybean (Glycine max) as a reference. Food Chem. 2020, 333, 127469. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Q.; Zhang, N.; Bak, K.H.; Soladoye, O.P.; Aluko, R.E.; Fu, Y.; Zhang, Y. Insights into formation, detection and removal of the beany flavor in soybean protein. Trends Food Sci. Technol. 2021, 112, 336–347. [Google Scholar] [CrossRef]
- Rico-Munoz, E.; dos Santos, J.L.P. The fungal problem in thermal processed beverages. Curr. Opin. Food Sci. 2019, 29, 80–87. [Google Scholar] [CrossRef]
- Agalloco, J.; Akers, J. The future of aseptic processing. In Advanced Aseptic Processing Technology; CRC Press: Thames, UK, 2016; pp. 465–469. [Google Scholar]
| Classification | Raw Material Sources | References |
|---|---|---|
| Nut-based plant drinks | Almonds, pistachios, walnuts, cashews, etc. | [7,15] |
| Soy-based plant drinks | Peas, chickpeas, soybeans, peanuts, cowpeas, etc. | [7] |
| Seed-based plant drinks | Sesame, flax, hemp, pumpkin seeds, etc. | [7,15] |
| Cereal-based plant drinks | Millet, corn, barley, sorghum, wheat, etc. | [7,15] |
| Pseudo-cereal-based plant drinks | Quinoa, moss bran, etc. | [7] |
| Drink Type | Protein (g) | Total Fat (g) | Ash (g) | Total Carbohydrates (g) | Fiber (g) | References |
|---|---|---|---|---|---|---|
| Soy | 2.78 | 1.96 | 0.75 | 3 | <0.75 | [20,21] |
| Coconut | 2.02 | 21.3 | 0.97 | 2.81 | - | [20,22] |
| Oat | 0.8 | 2.75 | 0.79 | 5.1 | <0.75 | [20,23] |
| Flaxseed | - | 1.04 | - | 0.42 | - | [20,24] |
| Rice | 0.42 | 1.04 | 0.34 | 9.58 | - | [20,25] |
| Cashew | 2.2 | 5.29 | - | 5.73 | 0.4 | [20,26] |
| Almond | 0.66 | 1.56 | 0.6 | 0.67 | <0.75 | [20,26] |
| Drink Type | Main Fatty Acid Components % | Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| Saturated Fatty Acid | Unsaturated Fatty Acid | |||||||
| C12:0 | C14:0 | C16:0 | C18:0 | C18:1n − 9 | C18:2n − 6 | C18:3n − 3 | ||
| Soy | - | - | 9.8 | 3.7 | 21.9 | 53.7 | 9.9 | [37] |
| Coconut | 50.0 | 17.3 | 7.5 | 2.7 | 0.01 | 0.77 | - | [38] |
| Oat | - | - | 5.09 | 1.81 | 45.1 | 23.7 | 0.234 | [39] |
| Walnut | - | - | 8.0 | 3.0 | 18.0 | 59.0 | 6.0 | [40] |
| Peanut | - | - | 16.63 | 4.88 | 42.63 | 15.56 | - | [41] |
| Flaxseed | - | 4.59 | 20.4 | 6.65 | 27.59 | 24.24 | 7.01 | [42] |
| Almond | - | - | 14.6 | 10.8 | 54.0 | 15.4 | - | [39] |
| Drink Type | Calcium (mg) | Iron (mg) | Phosphorus (mg) | Magnesium (mg) | Potassium (mg) | Sodium (mg) | Zinc (mg) | Reference |
|---|---|---|---|---|---|---|---|---|
| Soy | 155 | 0.37 | 46 | 17.5 | 118 | 39 | 0.26 | [20,21] |
| Coconut | 18 | 3.3 | 96 | 46 | 220 | 13 | 0.56 | [20,22] |
| Oat | 148 | 0.26 | 89 | 5.9 | 148 | 42 | 0.09 | [20,23] |
| Flaxseed | 125 | 0.15 | 62 | - | - | 33 | - | [20,24] |
| Rice | 125 | 0.3 | 62 | - | - | 42 | - | [20,25] |
| Cashew | 9 | 1.51 | 66 | 35 | 84 | 51 | 1.26 | [20,26] |
| Almond | 158 | 0.12 | 19 | 8.2 | 49 | 59 | 0.08 | [20,26] |
| Drink Type | Thiamin (mg) | Riboflavin (mg) | Vitamin B-6 (mg) | Folate (µg) | Vitamin B-12 (µg) | Retinol (µg) | Vitamin D (µg) | Reference |
|---|---|---|---|---|---|---|---|---|
| Soy | 0.044 | 0.331 | 0.036 | 16 | 1.33 | 89 | 4.63 | [20,21] |
| Coconut | 0.022 | - | 0.028 | 14 | - | - | - | [20,22] |
| Oat | 0.04 | 0.281 | 0.006 | <6 | 0.51 | 85 | 1.7 | [20,23] |
| Flaxseed | - | - | - | - | 0.62 | - | 1.05 | [20,24] |
| Rice | - | - | - | - | 0.62 | - | 1.05 | [20,25] |
| Cashew | - | 0.015 | 0.053 | - | - | - | - | [20,26] |
| Almond | 0.005 | 0.083 | <0.01 | <6 | 0.45 | 61’ | 1.59 | [20,26] |
| Drink Type | Total Phenolic Compounds | β-Sitosterol (mg/100 mL) | β-Sitosterol-β-D-Glucoside (mg/100 mL) | Campesterol (µg/100 mL) | Stigmasterol (µg/100 mL) | Reference |
|---|---|---|---|---|---|---|
| Almond | 1.24 mg GAE/L | 2.5 ± 0.1 | 13 ± 2 | 62 ± 4 | 1915 ± 109 | [51,52,53] |
| Hazelnut | 130.42 mg GAE/100 mL | - | - | - | - | [54,55] |
| Sesame | 4 mg GAE/g | - | - | - | - | [56,57] |
| Soy | 8.79 mg GAE/100 g | 2.5 ± 0.5 | 4.9 ± 2.1 | 1290 ± 291 | 998 ± 111 | [51,58,59] |
| Rice | 122.05 mg GAE/100 mL | 0.51 ± 0.07 | 2.4 ± 0.6 | 260 ± 28 | 234 ± 23 | [51,60] |
| Cashew | - | 2.7 ± 0.4 | >60 | 279 ± 44 | 15 ± 1 | [51] |
| Oat | 15 mg GAE/100 mL | 2.1 ± 0.2 | 26 ± 4 | 475 ± 30 | 182 ± 16 | [51,61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, A.; Dong, Y.; Liu, Z.; Li, Z.; Shao, J.; Li, M.; Yue, X. A Review of Plant-Based Drinks Addressing Nutrients, Flavor, and Processing Technologies. Foods 2023, 12, 3952. https://doi.org/10.3390/foods12213952
Xie A, Dong Y, Liu Z, Li Z, Shao J, Li M, Yue X. A Review of Plant-Based Drinks Addressing Nutrients, Flavor, and Processing Technologies. Foods. 2023; 12(21):3952. https://doi.org/10.3390/foods12213952
Chicago/Turabian StyleXie, Aijun, Yushi Dong, Zifei Liu, Zhiwei Li, Junhua Shao, Mohan Li, and Xiqing Yue. 2023. "A Review of Plant-Based Drinks Addressing Nutrients, Flavor, and Processing Technologies" Foods 12, no. 21: 3952. https://doi.org/10.3390/foods12213952
APA StyleXie, A., Dong, Y., Liu, Z., Li, Z., Shao, J., Li, M., & Yue, X. (2023). A Review of Plant-Based Drinks Addressing Nutrients, Flavor, and Processing Technologies. Foods, 12(21), 3952. https://doi.org/10.3390/foods12213952









