Yellow Field Pea Protein (Pisum sativum L.): Extraction Technologies, Functionalities, and Applications
Abstract
:1. Introduction
2. A Comparison of the Chemical Characteristics of Pea Protein and Soybean Protein
2.1. Proximate Analysis
2.2. Amino Acid Profile
2.3. Comparative Nutritional Aspects
2.4. Flavor Components
| Essential Amino Acids | Pea Protein [56] | Soybean Protein [56] | DIAAS (Peas) | DIAAS (Soybeans) | FAO/WHO/UNU [57] |
|---|---|---|---|---|---|
| Emulsions [58] | Milk [58] | ||||
| Threonine | 3.80 | 3.90 | 3.86 | 3.73 | 2.30 |
| Methionine | 0.90 | 1.40 | 0.42 | 1.42 | 1.60 |
| Phenylalanine | 5.70 | 5.50 | 5.95 | 5.30 | 1.36 |
| Histidine | 2.40 | 2.50 | 5.60 | 7.10 | 1.50 |
| Lysine | 6.70 | 5.60 | 7.10 | 5.65 | 4.50 |
| Valine | 4.90 | 5.10 | 4.95 | 4.70 | 3.90 |
| Isoleucine | 4.40 | 4.90 | 4.85 | 4.74 | 3.00 |
| Leucine | 7.60 | 5.60 | 8.74 | 7.46 | 5.90 |
| Tryptophan | 0.90 | 1.30 | 3.23 | 2.82 | 0.60 |
| Non-essential amino acids | |||||
| Serine | 5.40 | 5.20 | |||
| Glycine | 4.00 | 4.40 | |||
| Glutamic acid | 16.40 | 20.50 | |||
| Aspartic acid | 11.80 | 11.90 | |||
| Proline | 4.40 | 4.90 | |||
| Cysteine | 1.20 | 1.00 | 0.6 | ||
| Alanine | 0.71 | 4.20 | |||
| Tyrosine | 4.00 | 3.90 | |||
| Arginine | 7.80 | 8.40 | |||
3. Technologies for Pea Seed Isolation
| Method | Plant Source | Objective | Summary of Finding | Author |
|---|---|---|---|---|
| Dry fractionation | Pea | Using dry milling in combination with air classification to improve protein enrichment | Approx. 50% purity and 77% protein yield were obtained using the method. The native functionality of the protein was preserved. | Pelgrom et al. [7] |
| Peas, beans, chickpeas and lentils | Optimize milling using different settings to achieve maximum detachment of starch granules | Optimal detachment was achieved, but protein content was influenced by the intrinsic properties of the pulse. | Pelgrom et al. [8] | |
| Pea, lentils, and chickpeas | Air classification and electrostatic separation for protein enrichment | Higher protein purity (>60%), improved yield, less energy consumption, and preserved native protein functionality. | Xing et al. [9] | |
| Pea and faba beans | Effect of dehulling on physical, chemical, and technological properties of the fractions | Dehulling slightly increased the protein content of the fine fractions and improved starch enrichment of the coarse fractions. The techno-functional properties were not enhanced with dehulling. | Saldanha do Carmo et al. [72] | |
| Pea | Enhanced pea protein separation using Lorentz force-assisted charge carrier and triboelectric separation. | Protein content was increased by >100%. | Zhu et al. [71] | |
| Pea | Effect of the protein content of pea flour on physicochemical, antinutritional, and functional properties of air-classified protein fractions | Variations in protein content influenced the properties of air-classified pea flour. | Fenn et al. [73] | |
| Pea and chickpea | Determine the effect of relative humidity on particle dispersibility and flowability | Relative humidity above 70% affected the milling and air classification due to reduced particle dispersibility and flowability. | Politiek et al. [74] | |
| Mung bean, field pea, and cowpea | Compare the functional and rheological properties of dry-fractionated ingredients from mung bean, yellow pea, and cowpea | Protein content of the protein-rich fractions was dependent on the air classifier speed. | Schlangen et al. [75] | |
| Wet and aqueous fractionation | ||||
| Aqueous/ultrafiltration | Pea | Mild wet fractionation using water only and continuous ultrafiltration | Method produced high-purity (75%) protein concentrates with improved solubility. | Möller et al. [76] |
| Alkaline extraction and isoelectric point precipitation | Pea | Compare protein functionality of isolates obtained from dry and wet (IP) fractionation | Wet fractionation produced isolates with high protein content, the presence of essential amino acids, and improved emulsification and foaming properties. | Zhu et al. [71] |
| Chickpeas and green peas | Functional properties of protein isolates obtained by AE-IP method combined with modified salt dissolution precipitation | The purity of the globulin fractions was improved to >90%, and the protein composition played a major role in the functional properties. | Chang et al. [28] | |
| Pea | AE-IP extraction in conjunction with lactic acid fermentation | Protein content and yield were improved by 20–30%. | Emkani et al. [42] | |
| Pea | Compare the gelling properties of isolates obtained from different fractionation techniques | Gels from AE-IP in conjunction with ultrafiltration had good gel strength, but weak gels formed with AI alone. | Yang et al. [70] | |
| Pea | Mild wet fractionation coupled with isoelectric precipitation | Method produced both globulins and albumins; functionality was dependent on the dominant protein fraction in a sample. | Möller et al. [77] | |
| Enzyme-assisted extraction method | Pea and flaxseed | Comparison of the properties of protein obtained from different extraction methods | Enzymatic solvent extraction produced high protein quality, and enzymatic extraction produced protein with good emulsifying properties. | Tirgar et al. [78] |
| Pea | Investigate the effect of enzymatic hydrolysis on the techno-functional and sensory properties of pea protein isolates | The different proteases enhanced the properties of the protein and lowered bitterness. | Garcia-arteaga et al. [79] | |
| Osborne fractionation | Commercial pea protein | Fractionation based on solubility in weak salt, water, alcohol, and weak acid or alkaline solution using Osborne fractionation with dialysis | Alkaline-soluble fractions (glutelins) were the most abundant (87.0%) while alcohol-soluble fraction (prolamins) was the lowest in both yield (1.52%) and protein content (57.7%). The other fractions had protein content >79.0%. | Adebiyi and Aluko [34] |
| Pea flour | Fractionation of globulins and albumins using isoelectric point isolation | Albumins and globulins were isolated and showed good foam and emulsification properties, respectively. | Kornet et al. [33] | |
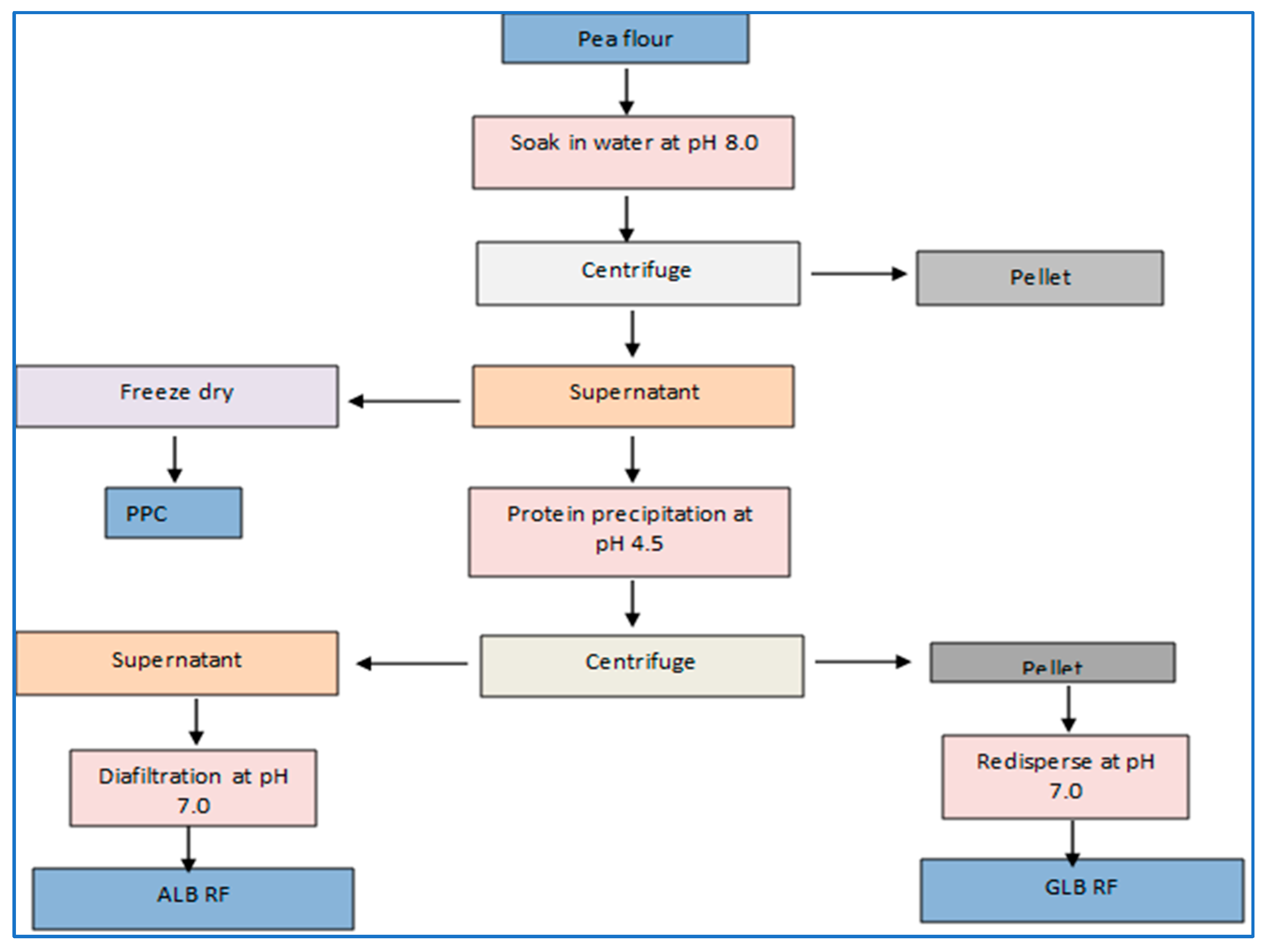

3.1. Dry Fractionation
3.2. Wet Fractionation
3.2.1. Alkaline Solubilization Coupled with Isoelectric pH Precipitation (AE-IP)
3.2.2. Ultrafiltration Processing (UF)
3.2.3. Micellar Precipitation
3.2.4. Salt Extraction—Dialysis
3.2.5. Water Extraction
3.2.6. Enzyme-Assisted Extraction (EAE) Method
3.3. Scaling Up of Laboratory Extraction of Pea Protein Isolates to Industrial Scale
4. Functional Properties of Pea Proteins
4.1. Solubility
4.2. Water-Holding Capacity (WHC) and Oil-Holding Capacity (OHC)
4.3. Foaming Capacity and Stability
4.4. Emulsification Properties
4.5. Gelation Properties
4.6. Digestibility of Pea Protein
4.7. Functional Gap between Laboratory-Prepared Pea Protein Isolates and Commercial Brands
4.8. Potential Processing Technologies Not Yet Applied to Pea Protein
5. Food Applications
6. Concluding Remarks and Future Research Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rasskazova, I.; Kirse-Ozolina, A. Field Pea Pisum sativum L. as a Perspective Ingredient for Vegan Foods: A Review. Res. Rural. Dev. 2020, 35, 125–131. [Google Scholar] [CrossRef]
- Mudryj, A.N.; Yu, N.; Aukema, H.M. Nutritional and Health Benefits of Pulses. Appl. Physiol. Nutr. Metab. 2014, 39, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.; Agarwal, S.; Lieberman, H.; Fulgoni, V. Sources and Amounts of Animal, Dairy, and Plant Protein Intake of US Adults in 2007–2010. Nutrients 2015, 7, 7058–7069. [Google Scholar] [CrossRef] [PubMed]
- Barac, M.; Cabrilo, S.; Pesic, M.; Stanojevic, S.; Zilic, S.; Macej, O.; Ristic, N. Profile and Functional Properties of Seed Proteins from Six Pea (Pisum sativum) Genotypes. Int. J. Mol. Sci. 2010, 11, 4973–4990. [Google Scholar] [CrossRef]
- Choudhury, D.; Singh, S.; Seah, J.S.H.; Yeo, D.C.L.; Tan, L.P. Commercialization of Plant-Based Meat Alternatives. Trends Plant Sci. 2020, 25, 1055–1058. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Boom, R.M.; Schutyser, M.A.I. Food Hydrocolloids Functional Analysis of Mildly Refined Fractions from Yellow Pea. Food Hydrocoll. 2015, 44, 12–22. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Vissers, A.M.; Boom, R.M.; Schutyser, M.A.I. Dry Fractionation for Production of Functional Pea Protein Concentrates. Food Res. Int. 2013, 53, 232–239. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Wang, J.; Boom, R.M.; Schutyser, M.A.I. Pre- and Post-Treatment Enhance the Protein Enrichment from Milling and Air Classification of Legumes. J. Food Eng. 2015, 155, 53–61. [Google Scholar] [CrossRef]
- Xing, Q.; Utami, D.P.; Demattey, M.B.; Kyriakopoulou, K.; de Wit, M.; Boom, R.M.; Schutyser, M.A.I. A Two-Step Air Classification and Electrostatic Separation Process for Protein Enrichment of Starch-Containing Legumes. Innov. Food Sci. Emerg. Technol. 2020, 66, 102480. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein Content and Amino Acid Composition of Commercially Available Plant-Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Malafronte, L.; Ruoff, D.; Gunes, D.Z.; Lequeux, F.; Schmitt, C.; Windhab, E.J. Morphology Development in Single Drop Drying for Native and Aggregated Whey Protein Dispersions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123549. [Google Scholar] [CrossRef]
- Field Pea Breeding at Agriculture and Agri-Food Canada. Available online: https://www.manitobapulse.ca/2020/12 (accessed on 20 October 2023).
- Pulse and Soybean Variety Guide. Available online: https://www.manitobapulse.ca/production/variety-evaluation-guide/ (accessed on 20 October 2023).
- Maharjan, P.; Penny, J.; Partington, D.L.; Panozzo, J.F. Genotype and Environment Effects on the Chemical Composition and Rheological Properties of Field Peas. J. Sci. Food Agric. 2019, 99, 5409–5416. [Google Scholar] [CrossRef] [PubMed]
- Millar, K.A.; Gallagher, E.; Burke, R.; McCarthy, S.; Barry-Ryan, C. Proximate Composition and Anti-Nutritional Factors of Fava-Bean (Vicia faba), Green-Pea and Yellow-Pea (Pisum sativum) Flour. J. Food Compos. Anal. 2019, 82, 103233. [Google Scholar] [CrossRef]
- Kornet, R.; Veenemans, J.; Venema, P.; van der Goot, A.J.; Meinders, M.; Sagis, L.; van der Linden, E. Less Is More: Limited Fractionation Yields Stronger Gels for Pea Proteins. Food Hydrocoll. 2021, 112, 106285. [Google Scholar] [CrossRef]
- García Arteaga, V.; Kraus, S.; Schott, M.; Muranyi, I.; Schweiggert-Weisz, U.; Eisner, P. Screening of Twelve Pea (Pisum sativum L.) Cultivars and Their Isolates Focusing on the Protein Characterization, Functionality, and Sensory Profiles. Foods 2021, 10, 758. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Warkentin, T.D.; Tyler, R.T.; Nickerson, M.T. Physicochemical and Functional Properties of Protein Isolates Obtained from Several Pea Cultivars. Cereal Chem. J. 2017, 94, 89–97. [Google Scholar] [CrossRef]
- Food Safety Authority of Ireland. Information on Nutrition and Health Claims; Food Safety Authority of Ireland: Dublin, Ireland, 2021; Available online: www.fsai.ie (accessed on 20 October 2023).
- Nikolopoulou, D.; Grigorakis, K.; Stasini, M.; Alexis, M.N.; Iliadis, K. Differences in Chemical Composition of Field Pea (Pisum sativum) Cultivars: Effects of Cultivation Area and Year. Food Chem. 2007, 103, 847–852. [Google Scholar] [CrossRef]
- Gao, Z.; Shen, P.; Lan, Y.; Cui, L.; Ohm, J.-B.; Chen, B.; Rao, J. Effect of Alkaline Extraction pH on Structure Properties, Solubility, and Beany Flavor of Yellow Pea Protein Isolate. Food Res. Int. 2020, 131, 109045. [Google Scholar] [CrossRef]
- Pedrosa, M.M.; Varela, A.; Domínguez-Timón, F.; Tovar, C.A.; Moreno, H.M.; Borderías, A.J.; Díaz, M.T. Comparison of Bioactive Compounds Content and Techno-Functional Properties of Pea and Bean Flours and Their Protein Isolates. Plant Foods Hum. Nutr. (Dordr.) 2020, 75, 642–650. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional Attributes of Pea Protein Isolates Prepared Using Different Extraction Methods and Cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Dziuba, J.; Szerszunowicz, I.; Nałecz, D.; Dziuba, M. Proteomic Analysis of Albumin and Globulin Fractions of Pea (Pisum sativum L.) Seeds. Acta Sci. Polonorum. Technol. Aliment. 2014, 13, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, T.W.; Baik, B.-K. Relationship between Proportion and Composition of Albumins, and In Vitro Protein Digestibility of Raw and Cooked Pea Seeds (Pisum sativum L.). J. Sci. Food Agric. 2010, 90, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, J.; Cerletti, P. Proteins of Some Legume Seeds: Soybean, Pea, Fababean and Lupin BT—New and Developing Sources of Food Proteins; Hudson, B.J.F., Ed.; Springer: Boston, MA, USA, 1994; pp. 145–193. [Google Scholar] [CrossRef]
- Tzitzikas, E.N.; Vincken, J.-P.; de Groot, J.; Gruppen, H.; Visser, R.G.F. Genetic Variation in Pea Seed Globulin Composition. J. Agric. Food Chem. 2006, 54, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Lan, Y.; Bandillo, N.; Ohm, J.-B.; Chen, B.; Rao, J. Plant Proteins from Green Pea and Chickpea: Extraction, Fractionation, Structural Characterization and Functional Properties. Food Hydrocoll. 2022, 123, 107165. [Google Scholar] [CrossRef]
- Kaur Dhaliwal, S.; Salaria, P.; Kaushik, P. Pea Seed Proteins: A Nutritional and Nutraceutical Update. In Grain and Seed Proteins Functionality; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Mertens, C.; Dehon, L.; Bourgeois, A.; Verhaeghe-Cartrysse, C.; Blecker, C. Agronomical Factors Influencing the Legumin/Vicilin Ratio in Pea (Pisum sativum L.) Seeds. J. Sci. Food Agric. 2012, 92, 1591–1596. [Google Scholar] [CrossRef]
- Barbana, C.; Boye, J.I. In Vitro Protein Digestibility and Physico-Chemical Properties of Flours and Protein Concentrates from Two Varieties of Lentil (Lens culinaris). Food Funct. 2013, 4, 310–321. [Google Scholar] [CrossRef]
- De Santis, M.A.; Rinaldi, M.; Menga, V.; Codianni, P.; Giuzio, L.; Fares, C.; Flagella, Z. Influence of Organic and Conventional Farming on Grain Yield and Protein Composition of Chickpea Genotypes. Agronomy 2021, 11, 191. [Google Scholar] [CrossRef]
- Kornet, R.; Yang, J.; Venema, P.; van der Linden, E.; Sagis, L.M.C. Optimizing Pea Protein Fractionation to Yield Protein Fractions with a High Foaming and Emulsifying Capacity. Food Hydrocoll. 2022, 126, 107456. [Google Scholar] [CrossRef]
- Adebiyi, A.P.; Aluko, R.E. Functional Properties of Protein Fractions Obtained from Commercial Yellow Field Pea (Pisum sativum L.) Seed Protein Isolate. Food Chem. 2011, 128, 902–908. [Google Scholar] [CrossRef]
- Tang, C.; Sun, X.; Yin, S. Food Hydrocolloids Physicochemical, Functional and Structural Properties of Vicilin-Rich Protein Isolates from Three Phaseolus Legumes: Effect of Heat Treatment. Food Hydrocoll. 2009, 23, 1771–1778. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, C.; Wu, Z.; Zhang, Z.; Xu, C. Comparison of Wheat, Soybean, Rice, and Pea Protein Properties for Effective Applications in Food Products. J. Food Biochem. 2020, 44, e13157. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhang, T.; Jiang, L. Soy Protein: Molecular Structure Revisited and Recent Advances in Processing Technologies. Annu. Rev. Food Sci. Technol. 2021, 12, 119–147. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.A.; de Blas, C.; Cámara, L.; Mateos, G.G. Chemical Composition, Protein Quality and Nutritive Value of Commercial Soybean Meals Produced from Beans from Different Countries: A Meta-Analytical Study. Anim. Feed Sci. Technol. 2020, 267, 114531. [Google Scholar] [CrossRef]
- Damodaran, S.; Paraf, A. Food Proteins and Their Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Zhu, X.; Ning, C.; Li, S.; Xu, P.; Zheng, Y.; Zhou, C. Effects of L-lysine/L-arginine on the Emulsion Stability, Textural, Rheological and Microstructural Characteristics of Chicken Sausages. Int. J. Food Sci. Technol. 2018, 53, 88–96. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Gulewicz, P.; Frias, J.; Gulewicz, K.; Vidal-Valverde, C. Assessment of Protein Fractions of Three Cultivars of Pisum sativum L.: Effect of Germination. Eur. Food Res. Technol. 2008, 226, 1465–1478. [Google Scholar] [CrossRef]
- Emkani, M.; Oliete, B.; Saurel, R. Pea Protein Extraction Assisted by Lactic Fermentation: Impact on Protein Profile and Thermal Properties. Foods 2021, 10, 549. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Eisner, P.; Schweiggert-Weisz, U. Effect of High Moisture Extrusion Cooking on Protein-Protein Interactions of Pea (Pisum sativum L.) Protein Isolates. Int. J. Food Sci. Technol. 2015, 50, 1390–1396. [Google Scholar] [CrossRef]
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for Digestible Indispensable Amino Acid Scores (DIAAS) for Some Dairy and Plant Proteins May Better Describe Protein Quality than Values Calculated Using the Concept for Protein Digestibility-Corrected Amino Acid Scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein Digestibility-Corrected Amino Acid Scores and Digestible Indispensable Amino Acid Scores Differentially Describe Protein Quality in Growing Male Rats. J. Nutr. 2015, 145, 372–379. [Google Scholar] [CrossRef]
- van den Berg, L.A.; Mes, J.J.; Mensink, M.; Wanders, A.J. Protein Quality of Soy and the Effect of Processing: A Quantitative Review. Front. Nutr. 2022, 9, 2148. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Franczyk, A.J.; Medina, G.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of Processing on the In Vitro and In Vivo Protein Quality of Yellow and Green Split Peas (Pisum sativum). J. Agric. Food Chem. 2017, 65, 7790–7796. [Google Scholar] [CrossRef] [PubMed]
- Trindler, C.; Annika Kopf-Bolanz, K.; Denkel, C. Aroma of Peas, Its Constituents and Reduction Strategies—Effects from Breeding to Processing. Food Chem. 2022, 376, 131892. [Google Scholar] [CrossRef]
- Wang, Y.; Guldiken, B.; Tulbek, M.; House, J.D.; Nickerson, M. Impact of Alcohol Washing on the Flavour Profiles, Functionality and Protein Quality of Air Classified Pea Protein Enriched Flour. Food Res. Int. 2020, 132, 109085. [Google Scholar] [CrossRef] [PubMed]
- Hanan, E.; Rudra, S.G.; Sharma, V.; Sagar, V.R.; Sehgal, S. Pea Pod Powder to Enhance the Storage Quality of Buckwheat Bread. Vegetos 2021, 34, 790–799. [Google Scholar] [CrossRef]
- Cui, L.; Kimmel, J.; Zhou, L.; Rao, J.; Chen, B. Identification of Extraction pH and Cultivar Associated Aromatic Compound Changes in Spray Dried Pea Protein Isolate Using Untargeted and Targeted Metabolomic Approaches. J. Agric. Food Res. 2020, 2, 100032. [Google Scholar] [CrossRef]
- García Arteaga, V.; Leffler, S.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Sensory Profile, Functional Properties and Molecular Weight Distribution of Fermented Pea Protein Isolate. Curr. Res. Food Sci. 2021, 4, 1–10. [Google Scholar] [CrossRef]
- Vara-Ubol, S.; Chambers, E.; Chambers, D.H. Sensory Characterisitcs of Chemical Componuds Potentially Associated with Beany Aroma in Foods. J. Sens. Stud. 2004, 19, 15–26. [Google Scholar] [CrossRef]
- Yu, H.; Liu, R.; Hu, Y.; Xu, B. Flavor Profiles of Soymilk Processed with Four Different Processing Technologies and 26 Soybean Cultivars Grown in China. Int. J. Food Prop. 2017, 20 (Suppl. S3), S2887–S2898. [Google Scholar] [CrossRef]
- Lv, Y.-C.; Song, H.-L.; Li, X.; Wu, L.; Guo, S.-T. Influence of Blanching and Grinding Process with Hot Water on Beany and Non-Beany Flavor in Soymilk. J. Food Sci. 2011, 76, S20–S25. [Google Scholar] [CrossRef]
- Liu, J.; Klebach, M.; Visser, M.; Hofman, Z. Amino Acid Availability of a Dairy and Vegetable Protein Blend Compared to Single Casein, Whey, Soy, and Pea Proteins: A Double-Blind, Cross-Over Trial. Nutrients 2019, 11, 2613. [Google Scholar] [CrossRef]
- Joint FAO/WHO/UNU. Expert Consultation on Protein and Amino Acid Requirements in Human. In Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; WHO Technical Report Series; No. 935; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Reynaud, Y.; Buffière, C.; Cohade, B.; Vauris, M.; Liebermann, K.; Hafnaoui, N.; Lopez, M.; Souchon, I.; Dupont, D.; Rémond, D. True Ileal Amino Acid Digestibility and Digestible Indispensable Amino Acid Scores (DIAASs) of Plant-Based Protein Foods. Food Chem. 2021, 338, 128020. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Reducing Off-Flavour in Plant Protein Isolates by Lactic Acid Fermentation. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2020. [Google Scholar]
- Ma, Z.; Boye, J.I.; Azarnia, S.; Simpson, B.K. Volatile Flavor Profile of Saskatchewan Grown Pulses as Affected by Different Thermal Processing Treatments. Int. J. Food Prop. 2016, 19, 2251–2271. [Google Scholar] [CrossRef]
- Schindler, S.; Zelena, K.; Krings, U.; Bez, J.; Eisner, P.; Berger, R.G. Improvement of the Aroma of Pea (Pisum sativum) Protein Extracts by Lactic Acid Fermentation. Food Biotechnol. 2012, 26, 58–74. [Google Scholar] [CrossRef]
- El Youssef, C.; Bonnarme, P.; Fraud, S.; Péron, A.-C.; Helinck, S.; Landaud, S. Sensory Improvement of a Pea Protein-Based Product Using Microbial Co-Cultures of Lactic Acid Bacteria and Yeasts. Foods 2020, 9, 349. [Google Scholar] [CrossRef]
- Yang, M.; Li, N.; Tong, L.; Fan, B.; Wang, L.; Wang, F.; Liu, L. Comparison of Physicochemical Properties and Volatile Flavor Compounds of Pea Protein and Mung Bean Protein-Based Yogurt. LWT 2021, 152, 112390. [Google Scholar] [CrossRef]
- Guldiken, B.; Green, R.; Nickerson, M.T. The Impact of Different Adsorbents on Flavour Characteristics of a Lentil Protein Isolate. Eur. Food Res. Technol. 2021, 247, 593–604. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Jin, Z.; Gu, Z.; Rao, J.; Chen, B. Physicochemical Property Changes and Aroma Differences of Fermented Yellow Pea Flours: Role of Lactobacilli and Fermentation Time. Food Funct. 2021, 12, 6950–6963. [Google Scholar] [CrossRef]
- Frohlich, P.; Young, G.; Borsuk, Y.; Sigvaldson, M.; Bourré, L.; Sopiwnyk, E. Influence of Premilling Thermal Treatments of Yellow Peas, Navy Beans, and Fava Beans on the Flavor and End-product Quality of Tortillas and Pitas. Cereal Chem. 2021, 98, 802–813. [Google Scholar] [CrossRef]
- Zha, F.; Yang, Z.; Rao, J.; Chen, B. Gum Arabic-Mediated Synthesis of Glyco-Pea Protein Hydrolysate via Maillard Reaction Improves Solubility, Flavor Profile, and Functionality of Plant Protein. J. Agric. Food Chem. 2019, 67, 10195–10206. [Google Scholar] [CrossRef]
- Zhou, X.; Cui, H.; Zhang, Q.; Hayat, K.; Yu, J.; Hussain, S.; Tahir, M.U.; Zhang, X.; Ho, C.-T. Taste Improvement of Maillard Reaction Intermediates Derived from Enzymatic Hydrolysates of Pea Protein. Food Res. Int. 2021, 140, 109985. [Google Scholar] [CrossRef]
- Benavides-Paz, Y.L.; Ismail, B.P.; Reineccius, G.A. Monitoring the Aroma Profile During the Production of a Pea Protein Isolate by Alkaline Solubilization Coupled with Isoelectric Precipitation. ACS Food Sci. Technol. 2022, 2, 321–330. [Google Scholar] [CrossRef]
- Yang, J.; Zamani, S.; Liang, L.; Chen, L. Extraction Methods Significantly Impact Pea Protein Composition, Structure and Gelling Properties. Food Hydrocoll. 2021, 117, 106678. [Google Scholar] [CrossRef]
- Zhu, H.-G.; Tang, H.-Q.; Cheng, Y.-Q.; Li, Z.-G.; Tong, L.-T. Novel Electromagnetic Separation Technology for the Production of Pea Protein Concentrate. Innov. Food Sci. Emerg. Technol. 2021, 70, 102668. [Google Scholar] [CrossRef]
- Saldanha do Carmo, C.; Silventoinen, P.; Nordgård, C.T.; Poudroux, C.; Dessev, T.; Zobel, H.; Holtekjølen, A.K.; Draget, K.I.; Holopainen-Mantila, U.; Knutsen, S.H.; et al. Is Dehulling of Peas and Faba Beans Necessary Prior to Dry Fractionation for the Production of Protein- and Starch-Rich Fractions? Impact on Physical Properties, Chemical Composition and Techno-Functional Properties. J. Food Eng. 2020, 278, 109937. [Google Scholar] [CrossRef]
- Fenn, D.; Wang, N.; Maximiuk, L. Physicochemical, Anti-nutritional, and Functional Properties of Air-classified Protein Concentrates from Commercially Grown Canadian Yellow Pea (Pisum sativum) Varieties with Variable Protein Levels. Cereal Chem. 2021, 99, 157–168. [Google Scholar] [CrossRef]
- Politiek, R.G.A.; He, S.; Wilms, P.F.C.; Keppler, J.K.; Bruins, M.E.; Schutyser, M.A.I. Effect of Relative Humidity on Milling and Air Classification Explained by Particle Dispersion and Flowability. J. Food Eng. 2023, 358, 111663. [Google Scholar] [CrossRef]
- Schlangen, M.; Taghian Dinani, S.; Schutyser, M.A.I.; van der Goot, A.J. Dry Fractionation to Produce Functional Fractions from Mung Bean, Yellow Pea and Cowpea Flour. Innov. Food Sci. Emerg. Technol. 2022, 78, 103018. [Google Scholar] [CrossRef]
- Möller, A.C.; Li, J.; van der Goot, A.J.; van der Padt, A. A Water-Only Process to Fractionate Yellow Peas into Its Constituents. Innov. Food Sci. Emerg. Technol. 2022, 75, 102894. [Google Scholar] [CrossRef]
- Möller, A.C.; van der Padt, A.; van der Goot, A.J. Influence of the Fractionation Method on the Protein Composition and Functional Properties. Innov. Food Sci. Emerg. Technol. 2022, 81, 103144. [Google Scholar] [CrossRef]
- Tirgarian, B.; Farmani, J.; Milani, J.M. Enzyme-Assisted Aqueous Extraction of Oil and Protein Hydrolysate from Sesame Seed. J. Food Meas. Charact. 2019, 13, 2118–2129. [Google Scholar] [CrossRef]
- García Arteaga, V.; Apéstegui Guardia, M.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Effect of Enzymatic Hydrolysis on Molecular Weight Distribution, Techno-Functional Properties and Sensory Perception of Pea Protein Isolates. Innov. Food Sci. Emerg. Technol. 2020, 65, 102449. [Google Scholar] [CrossRef]
- Pulse Canada. Available online: https://pulsecanada.com/processing/processing-technology (accessed on 20 October 2023).
- Fernando, S. Production of Protein-Rich Pulse Ingredients through Dry Fractionation: A Review. Food Sci. Technol. 2021, 141, 110961. [Google Scholar] [CrossRef]
- Joyner, J.J.; Yadav, B.K. Microwave Assisted Dehulling of Black Gram (Vigna Mungo L). J. Food Sci. Technol. 2013, 52, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Sunil, C.K.; Chidanand, D.V.; Manoj, D.; Choudhary, P.; Rawson, A. Effect of Ultrasound Treatment on Dehulling Efficiency of Blackgram. J. Food Sci. Technol. 2018, 55, 2504–2513. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; de Wit, M.; Boom, R.M.; Schutyser, M.A.I. Lupine Protein Enrichment by Milling and Electrostatic Separation. Innov. Food Sci. Emerg. Technol. 2016, 33, 596–602. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Vitelli, M.; Rajabzadeh, A.R.; Legge, R.L. Analysis of Protein Enrichment during Single- and Multi-Stage Tribo-Electrostatic Bioseparation Processes for Dry Fractionation of Legume Flour. Sep. Purif. Technol. 2017, 176, 48–58. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Liu, H.; Yoon, A.; Rizvi, S.S.H.; Wang, Q. Changes in Conformation and Quality of Vegetable Protein during Texturization Process by Extrusion. Crit. Rev. Food Sci. Nutr. 2019, 59, 3267–3280. [Google Scholar] [CrossRef]
- Schutyser, M.A.I.; Pelgrom, P.J.M.; van der Goot, A.J.; Boom, R.M. Dry Fractionation for Sustainable Production of Functional Legume Protein Concentrates. Trends Food Sci. Technol. 2015, 45, 327–335. [Google Scholar] [CrossRef]
- Jafari, M.; Rajabzadeh, A.R.; Tabtabaei, S.; Marsolais, F.; Legge, R.L. Physicochemical Characterization of a Navy Bean (Phaseolus vulgaris) Protein Fraction Produced Using a Solvent-Free Method. Food Chem. 2016, 208, 35–41. [Google Scholar] [CrossRef]
- Möller, A.C.; van der Padt, A.; van der Goot, A.J. From Raw Material to Mildly Refined Ingredient—Linking Structure to Composition to Understand Fractionation Processes. J. Food Eng. 2021, 291, 110321. [Google Scholar] [CrossRef]
- Ramirez, J. Ultrafiltration: Methods, Applications and Insights; Environmental Science, Engineering and Technology; Nova Publishers: New York, NY, USA, 2017. [Google Scholar]
- Charcosset, C. 2-Ultrafiltration. In Membrane Processes in Biotechnology and Pharmaceutics; Elsevier: Amsterdam, The Netherlands, 2012; pp. 43–99. [Google Scholar] [CrossRef]
- Etzel, M.R.; Arunkumar, A. Dairy Protein Fractionation and Concentration Using Charged Ultrafiltration Membranes; John Wiley & Sons, Ltd: Chichester, UK, 2015. [Google Scholar] [CrossRef]
- Metsämuuronen, S.; Mänttäri, M.; Nyström, M. Comparison of Analysis Methods for Protein Concentration and Its Use in UF Fractionation of Whey. Desalination 2011, 283, 156–164. [Google Scholar] [CrossRef]
- Ratnaningsih, E.; Reynard, R.; Khoiruddin, K.; Wenten, I.G.; Boopathy, R. Recent Advancements of UF-Based Separation for Selective Enrichment of Proteins and Bioactive Peptides—A Review. Appl. Sci. 2021, 11, 1078. [Google Scholar] [CrossRef]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the Functional Properties of Pea, Chickpea and Lentil Protein Concentrates Processed Using Ultrafiltration and Isoelectric Precipitation Techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Asen, N.D.; Aluko, R.E. Physicochemical and Functional Properties of Membrane-Fractionated Heat-Induced Pea Protein Aggregates. Front. Nutr. 2022, 9, 852225. [Google Scholar] [CrossRef]
- Berghout, J.A.M.; Marmolejo-Garcia, C.; Berton-Carabin, C.C.; Nikiforidis, C.V.; Boom, R.M.; van der Goot, A.J. Aqueous Fractionation Yields Chemically Stable Lupin Protein Isolates. Food Res. Int. 2015, 72, 82–90. [Google Scholar] [CrossRef]
- Zhu, H.-G.; Tang, H.-Q.; Cheng, Y.-Q.; Li, Z.-G.; Tong, L.-T. Potential of Preparing Meat Analogue by Functional Dry and Wet Pea (Pisum sativum) Protein Isolate. LWT 2021, 148, 111702. [Google Scholar] [CrossRef]
- Görgüç, A.; Özer, P.; Yılmaz, F.M. Simultaneous Effect of Vacuum and Ultrasound Assisted Enzymatic Extraction on the Recovery of Plant Protein and Bioactive Compounds from Sesame Bran. J. Food Compos. Anal. 2020, 87, 103424. [Google Scholar] [CrossRef]
- Yang, J.; Liu, G.; Zeng, H.; Chen, L. Effects of High Pressure Homogenization on Faba Bean Protein Aggregation in Relation to Solubility and Interfacial Properties. Food Hydrocoll. 2018, 83, 275–286. [Google Scholar] [CrossRef]
- Amat, T.; Assifaoui, A.; Buczkowski, J.; Silva, J.V.C.; Schmitt, C.; Saurel, R. Interplay between Soluble and Insoluble Protein/Calcium/Phytic Acid Complexes in Dispersions of Faba Bean and Pea Protein Concentrates around Neutral pH. Food Hydrocoll. 2024, 147, 109273. [Google Scholar] [CrossRef]
- Awosika, T.; Aluko, R.E. Enzymatic Pea Protein Hydrolysates Are Active Trypsin and Chymotrypsin Inhibitors. Foods 2019, 8, 200. [Google Scholar] [CrossRef]
- Awosika, T.O.; Aluko, R.E. Inhibition of the in Vitro Activities of A-amylase, A-glucosidase and Pancreatic Lipase by Yellow Field Pea (Pisum sativum L.) Protein Hydrolysates. Int. J. Food Sci. Tech. 2019, 54, 2021–2034. [Google Scholar] [CrossRef]
- Asen, N.D.; Aluko, R.E. Effect of Heat Treatment on Yellow Field Pea (Pisum sativum) Protein Concentrate Coupled with Membrane Ultrafiltration on Emulsification Properties of the Isolated >50 kDa Proteins. Membranes 2023, 13, 767. [Google Scholar] [CrossRef]
- Hansen, L.; Bu, F.; Ismail, B.P. Structure-Function Guided Extraction and Scale-Up of Pea Protein Isolate Production. Foods 2022, 11, 3773. [Google Scholar] [CrossRef]
- Aluko, R.E. Antihypertensive Peptides from Food Proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Manzanilla-Valdez, M.L.; Wang, Y.; Mondor, M.; Hernández-Álvarez, A.J. Micellar Precipitation and Reverse Micelle Extraction of Plant Proteins. In Green Protein Processing Technologies from Plants: Novel Extraction and Purification Methods for Product Development; Hernández-Álvarez, A.J., Mondor, M., Nosworthy, M.G., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 237–263. [Google Scholar] [CrossRef]
- Mondor, M.; Hernandez-alvarez, A.J. Processing Technologies to Produce Plant Protein Concentrates and Isolates. In Plant Protein Foods; Springer International Publishing: Cham, Switzerland, 2022; pp. 61–108. [Google Scholar]
- Tanger, C.; Engel, J.; Kulozik, U. Influence of Extraction Conditions on the Conformational Alteration of Pea Protein Extracted from Pea Flour. Food Hydrocoll. 2020, 107, 105949. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse Proteins: Processing, Characterization, Functional Properties and Applications in Food and Feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation Properties of Salt-Extracted Pea Protein Isolate Induced by Heat Treatment: Effect of Heating and Cooling Rate. Food Chem. 2011, 124, 1011–1016. [Google Scholar] [CrossRef]
- Geerts, M.E.J.; Mienis, E.; Nikiforidis, C.V.; van der Padt, A.; van der Goot, A.J. Mildly Refined Fractions of Yellow Peas Show Rich Behaviour in Thickened Oil-in-Water Emulsions. Innov. Food Sci. Emerg. Technol. 2017, 41, 251–258. [Google Scholar] [CrossRef]
- Görgüç, A.; Özer, P.; Yılmaz, F.M. Microwave-assisted Enzymatic Extraction of Plant Protein with Antioxidant Compounds from the Food Waste Sesame Bran: Comparative Optimization Study and Identification of Metabolomics Using LC/Q-TOF/MS. J. Food Process. Preserv. 2020, 44, e14304. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-Innovative Technologies for Extraction of Proteins for Human Consumption from Renewable Protein Sources of Plant Origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the Plant Protein Extraction: Mechanism and Recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Sari, Y.W.; Bruins, M.E.; Sanders, J.P.M. Enzyme Assisted Protein Extraction from Rapeseed, Soybean, and Microalgae Meals. Ind. Crops Prod. 2013, 43, 78–83. [Google Scholar] [CrossRef]
- Liu, J.; Gasmalla, M.A.A.; Li, P.; Yang, R. Enzyme-Assisted Extraction Processing from Oilseeds: Principle, Processing and Application. Innov. Food Sci. Emerg. Technol. 2016, 35, 184–193. [Google Scholar] [CrossRef]
- Das, R. Multienzyme Modification of Hemp Protein for Functional Peptides Synthesis. J. Food Process. 2015, 2015, 1–5. [Google Scholar] [CrossRef]
- Schmidt, F.; Blankart, M.; Wanger, J.; Scharfe, M.; Scheuerer, T.; Hinrichs, J. Upscaling of Alkaline Pea Protein Extraction from Dry Milled and Pre-Treated Peas from Laboratory to Pilot Scale: Optimization of Process Parameters for Higher Protein Yields. J. Food Meas. Charact. 2022, 16, 4904–4913. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying Properties of Canola and Flaxseed Protein Isolates Produced by Isoelectric Precipitation and Salt Extraction. Food Res. Int. 2011, 44, 2991–2998. [Google Scholar] [CrossRef]
- Burger, T.G.; Singh, I.; Mayfield, C.; Baumert, J.L.; Zhang, Y. Comparison of Physicochemical and Emulsifying Properties of Commercial Pea Protein Powders. J. Sci. Food Agric. 2021, 102, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Aryee, A.N.A.; Agyei, D.; Udenigwe, C.C. Impact of Selected Process Parameters on Solubility and Heat Stability of Pea Protein Isolate. In Proteins in Food Processing; Yada, R., Ed.; Elsevier Science & Technology: Amsterdam, The Netherlands, 2018; pp. 27–46. [Google Scholar]
- Tang, X.; Shen, Y.; Zhang, Y.; Schilling, M.W.; Li, Y. Parallel Comparison of Functional and Physicochemical Properties of Common Pulse Proteins. LWT 2021, 146, 111594. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N. Relationship between Protein Characteristics and Film-Forming Properties of Kidney Bean, Field Pea and Amaranth Protein Isolates. Int. J. Food Sci. Technol. 2015, 50, 1033–1043. [Google Scholar] [CrossRef]
- Pelegrine, D.H.G.; Gasparetto, C.A. Whey Proteins Solubility as Function of Temperature and pH. LWT Food Sci. Technol. 2005, 38, 77–80. [Google Scholar] [CrossRef]
- Kimura, A.; Fukuda, T.; Zhang, M.; Motoyama, S.; Maruyama, N.; Utsumi, S. Comparison of Physicochemical Properties of 7S and 11S Globulins from Pea, Fava Bean, Cowpea, and French Bean with Those of Soybean—French Bean 7S Globulin Exhibits Excellent Properties. J. Agric. Food Chem. 2008, 56, 10273–10279. [Google Scholar] [CrossRef]
- Liang, H.-N.; Tang, C.-H. pH-Dependent Emulsifying Properties of Pea (Pisum sativum L.) Proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- Hall, A.E.; Moraru, C.I. Structure and Function of Pea, Lentil and Faba Bean Proteins Treated by High Pressure Processing and Heat Treatment. LWT 2021, 152, 112349. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, Y.; McClements, D.J.; Liu, X.; Wang, P.; Liu, F. Improving Pea Protein Functionality by Combining High-Pressure Homogenization with an Ultrasound-Assisted Maillard Reaction. Food Hydrocoll. 2021, 126, 107441. [Google Scholar] [CrossRef]
- Zhi, Z.; Yan, L.; Li, H.; Dewettinck, K.; Van der Meeren, P.; Liu, R.; Van Bockstaele, F. A Combined Approach for Modifying Pea Protein Isolate to Greatly Improve Its Solubility and Emulsifying Stability. Food Chem. 2021, 38, 0131832. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Chand, J. Food Hydrocolloids Structural and Functional Characterization of Kidney Bean and Field Pea Protein Isolates: A Comparative Study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Boye, J.I. Comparative Study of the Effects of Processing on the Nutritional, Physicochemical and Functional Properties of Lentil. J. Food Process. Preserv. 2017, 41, e12824. [Google Scholar] [CrossRef]
- Nieto Nieto, T.V.; Wang, Y.; Ozimek, L.; Chen, L. Improved Thermal Gelation of Oat Protein with the Formation of Controlled Phase-Separated Networks Using Dextrin and Carrageenan Polysaccharides. Food Res. Int. 2016, 82, 95–103. [Google Scholar] [CrossRef]
- Zhan, F.; Tang, X.; Sobhy, R.; Li, B.; Chen, Y. Structural and Rheology Properties of Pea Protein Isolate-stabilised Emulsion Gel: Effect of Crosslinking with Transglutaminase. Int. J. Food Sci. Technol. 2021, 57, 974–982. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Deng, L.; Dai, T.; Liu, C.; Chen, J. High Energy Media Mill Modified Pea Dietary Fiber: Physicochemical Property and Its Mechanism in Stabilizing Pea Protein Beverage. Food Hydrocoll. 2024, 147, 109392. [Google Scholar] [CrossRef]
- Erdoğdu, Ö.; Görgüç, A.; Yılmaz, F.M. Functionality Enhancement of Pea Protein Powder via High-Intensity Ultrasound: Screening in-Vitro Digestion, o/w Emulsion Properties and Testing in Gluten-Free Bread. Plant Foods Hum. Nutr. 2023, 78, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.X.; Tan, C.-C.; Dharmawan, J.; Leong, S.S. Effects of Ethanol Washing on Off-Flavours Removal and Protein Functionalities of Pea Protein Concentrate. Food Bioprod. Process. 2023, 141, 73–80. [Google Scholar] [CrossRef]
- Batbayar, B.; Kryachko, Y.; Nickerson, M.T.; Korber, D.R.; Tanaka, T. Solid-State and Submerged Fermentation Effects on Functional Properties of Pea Protein-Enriched Flour. Cereal Chem. 2023, 100, 1092–1105. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, M.-H.; Bi, X.; Du, J. Physicochemical Properties, Structural Characteristics and In Vitro Digestion of Brown Rice–Pea Protein Isolate Blend Treated by Microbial Transglutaminase. Food Hydrocoll. 2023, 141, 108673. [Google Scholar] [CrossRef]
- Tang, Y.R.; Stone, A.K.; Wang, Y.; Jafarian, Z.; Zhou, L.; Kimmel, J.; House, J.D.; Tanaka, T.; Nickerson, M.T. Effects of Enzyme Treatments on the Functionality of Commercial Pea and Pea Blended Protein Ingredients. Food Biosci. 2023, 53, 102838. [Google Scholar] [CrossRef]
- Zhao, P.; Li, N.; Chen, L.; Guo, Y.; Huang, Y.; Tong, L.; Wang, L.; Fan, B.; Wang, F.; Liu, L. Effects of Oat β-Glucan on the Textural and Sensory Properties of Low-Fat Set Type Pea Protein Yogurt. Molecules 2023, 28, 3067. [Google Scholar] [CrossRef]
- Zayas, J.F. Foaming Properties of Proteins. In Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 260–309. [Google Scholar] [CrossRef]
- Kristensen, H.T.; Denon, Q.; Tavernier, I.; Gregersen, S.B.; Hammershøj, M.; Van der Meeren, P.; Dewettinck, K.; Dalsgaard, T.K. Improved Food Functional Properties of Pea Protein Isolate in Blends and Co-Precipitates with Whey Protein Isolate. Food Hydrocoll. 2021, 113, 106556. [Google Scholar] [CrossRef]
- Rullier, B.; Novales, B.; Axelos, M.A.V. Effect of Protein Aggregates on Foaming Properties of β-Lactoglobulin. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 96–102. [Google Scholar] [CrossRef]
- Du, M.; Xie, J.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, Physicochemical Characteristics and Functional Properties of Mung Bean Protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Malomo, S.A.; He, R.; Aluko, R.E. Structural and Functional Properties of Hemp Seed Protein Products. J. Food Sci. 2014, 79, C1512–C1521. [Google Scholar] [CrossRef]
- Taherian, A.R.; Mondor, M.; Labranche, J.; Drolet, H.; Ippersiel, D.; Lamarche, F. Comparative Study of Functional Properties of Commercial and Membrane Processed Yellow Pea Protein Isolates. Food Res. Int. 2011, 44, 2505–2514. [Google Scholar] [CrossRef]
- Chao, D.; Aluko, R.E. Modification of the Structural, Emulsifying, and Foaming Properties of an Isolated Pea Protein by Thermal Pretreatment. CyTA J. Food 2018, 16, 357–366. [Google Scholar] [CrossRef]
- Saldanha do Carmo, C.; Nunes, A.N.; Silva, I.; Maia, C.; Poejo, J.; Ferreira-Dias, S.; Nogueira, I.; Bronze, R.; Duarte, C.M.M. Formulation of Pea Protein for Increased Satiety and Improved Foaming Properties. RSC Adv. 2016, 6, 6048–6057. [Google Scholar] [CrossRef]
- Chang, L.; Chen, B.; Rao, J. Synergistic Effect of pH-Shift and Controlled Heating on Improving Foaming Properties of Pea Vicilin and Its Adsorption Behavior at the Air-Water Interface. Food Hydrocoll. 2023, 145, 109022. [Google Scholar] [CrossRef]
- Xie, J.; Huang, W.; Wu, X. Effects of Tea Saponin on the Foaming Properties of Pea Protein. Food Funct. 2023, 14, 4339–4353. [Google Scholar] [CrossRef]
- Shen, Q.; Zheng, W.; Han, F.; Zuo, J.; Dai, J.; Tang, C.; Song, R.; Li, B.; Chen, Y. Air-Water Interfacial Properties and Quantitative Description of Pea Protein Isolate-Tween 20. Food Hydrocoll. 2023, 139, 108568. [Google Scholar] [CrossRef]
- Kapoor, R.; Karabulut, G.; Mundada, V.; Feng, H. Non-Thermal Ultrasonic Contact Drying of Pea Protein Isolate Suspensions: Effects on Physicochemical and Functional Properties. Int. J. Biol. Macromol. 2023, 253, 126816. [Google Scholar] [CrossRef]
- Cui, L.; Bandillo, N.; Wang, Y.; Ohm, J.-B.; Chen, B.; Rao, J. Functionality and Structure of Yellow Pea Protein Isolate as Affected by Cultivars and Extraction pH. Food Hydrocoll. 2020, 108, 106008. [Google Scholar] [CrossRef]
- McClements, D.J.; Lu, J.; Grossmann, L. Proposed Methods for Testing and Comparing the Emulsifying Properties of Proteins from Animal, Plant, and Alternative Sources. Colloids Interfaces 2022, 6, 19. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Nickerson, M.T. Food Proteins: A Review on Their Emulsifying Properties Using a Structure–Function Approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef]
- Liu, Q.; Dai, Y.; Hou, H.; Wang, W.; Ding, X.; Zhang, H.; Li, X.; Dong, H. Changes in the Structure and Emulsification Properties of Pea Protein Isolate during Grinding. Food Sci. Technol. 2020, 133, 110066. [Google Scholar] [CrossRef]
- Peng, W.; Kong, X.; Chen, Y.; Zhang, C.; Yang, Y.; Hua, Y. Effects of Heat Treatment on the Emulsifying Properties of Pea Proteins. Food Hydrocoll. 2016, 52, 301–310. [Google Scholar] [CrossRef]
- Gao, K.; Zha, F.; Yang, Z.; Rao, J.; Chen, B. Structure Characteristics and Functionality of Water-Soluble Fraction from High-Intensity Ultrasound Treated Pea Protein Isolate. Food Hydrocoll. 2022, 125, 107409. [Google Scholar] [CrossRef]
- Sha, L.; Koosis, A.O.; Wang, Q.; True, A.D.; Xiong, Y.L. Interfacial Dilatational and Emulsifying Properties of Ultrasound-Treated Pea Protein. Food Chem. 2021, 350, 129271. [Google Scholar] [CrossRef]
- Wang, W.; Sun, R.; Ji, S.; Xia, Q. Effects of κ-Carrageenan on the Emulsifying Ability and Encapsulation Properties of Pea Protein Isolate-Grape Seed Oil Emulsions. Food Chem. 2024, 435, 137561. [Google Scholar] [CrossRef]
- Lao, Y.; Ye, Q.; Wang, Y.; Vongsvivut, J.; Selomulya, C. Quantifying the Effects of Pre-Roasting on Structural and Functional Properties of Yellow Pea Proteins. Food Res. Int. 2023, 172, 113180. [Google Scholar] [CrossRef]
- Cai, W.; Huang, W.; Chen, L. Soluble Pea Protein Aggregates Form Strong Gels in the Presence of κ-Carrageenan. ACS Food Sci. Technol. 2021, 1, 1605–1614. [Google Scholar] [CrossRef]
- Moreno, H.M.; Domínguez-Timón, F.; Díaz, M.T.; Pedrosa, M.M.; Borderías, A.J.; Tovar, C.A. Evaluation of Gels Made with Different Commercial Pea Protein Isolate: Rheological, Structural and Functional Properties. Food Hydrocoll. 2020, 99, 105375. [Google Scholar] [CrossRef]
- Arntfield, S.D.; Maskus, H.D. Peas and Other Legume Proteins. In Handbook on Food Proteins; Woodhead Publishing: Cambridge, UK, 2011; pp. 234–266. [Google Scholar]
- Barac, M.B.; Pesic, M.B.; Stanojevic, S.P.; Kostic, A.Z.; Bivolarevic, V. Comparative Study of the Functional Properties of Three Legume Seed Isolates: Adzuki, Pea and Soy Bean. J. Food Sci. Technol. 2015, 52, 2779–2787. [Google Scholar] [CrossRef]
- Xia, S.; Xue, Y.; Xue, C.; Jiang, X.; Li, J. Structural and Rheological Properties of Meat Analogues from Haematococcus pluvialis Residue-Pea Protein by High Moisture Extrusion. LWT 2022, 154, 112756. [Google Scholar] [CrossRef]
- Bu, F.; Feyzi, S.; Nayak, G.; Mao, Q.; Kondeti, V.S.S.K.; Bruggeman, P.; Chen, C.; Ismail, B.P. Investigation of Novel Cold Atmospheric Plasma Sources and Their Impact on the Structural and Functional Characteristics of Pea Protein. Innov. Food Sci. Emerg. Technol. 2023, 83, 103248. [Google Scholar] [CrossRef]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition Report of an FAO Expert Consultation. Report of an FAO Expert Consultation; FAO Food: Rome, Italy, 2013. [Google Scholar]
- Guillin, F.M.; Gaudichon, C.; Guérin-Deremaux, L.; Lefranc-Millot, C.; Airinei, G.; Khodorova, N.; Benamouzig, R.; Pomport, P.-H.; Martin, J.; Calvez, J. Real Ileal Amino Acid Digestibility of Pea Protein Compared to Casein in Healthy Humans: A Randomized Trial. Am. J. Clin. Nutr. 2022, 115, 353–363. [Google Scholar] [CrossRef]
- Röhe, I.; Göbel, T.W.; Goodarzi Boroojeni, F.; Zentek, J. Effect of Feeding Soybean Meal and Differently Processed Peas on the Gut Mucosal Immune System of Broilers. Poult. Sci. 2017, 96, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Rivera del Rio, A.; Boom, R.M.; Janssen, A.E.M. Effect of Fractionation and Processing Conditions on the Digestibility of Plant Proteins as Food Ingredients. Foods 2022, 11, 870. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Boye, J.I.; Hu, X. In Vitro Digestibility, Protein Composition and Techno-Functional Properties of Saskatchewan Grown Yellow Field Peas (Pisum sativum L.) as Affected by Processing. Food Res. Int. 2017, 92, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Çabuk, B.; Nosworthy, M.G.; Stone, A.K.; Korber, D.R.; Tanaka, T.; House, J.D.; Nickerson, M.T. Effect of Fermentation on the Protein Digestibility and Levels of Non-Nutritive Compounds of Pea Protein Concentrate. Food Technol. Biotechnol. 2018, 56, 257. [Google Scholar] [CrossRef]
- Skalickova, S.; Ridoskova, A.; Slama, P.; Skladanka, J.; Skarpa, P.; Smykalova, I.; Horacek, J.; Dostalova, R.; Horky, P. Effect of Lactic Fermentation and Cooking on Nutrient and Mineral Digestibility of Peas. Front. Nutr. 2022, 9, 838963. [Google Scholar] [CrossRef]
- Compton, M.; Willis, S.; Rezaie, B.; Humes, K. Food Processing Industry Energy and Water Consumption in the Pacific Northwest. Innov. Food Sci. Emerg. Technol. 2018, 47, 371–383. [Google Scholar] [CrossRef]
- Yao, S.; Li, W.; Martin, G.J.O.; Ashokkumar, M. An Investigation into the Mechanism of Alkaline Extraction-Isoelectric Point Precipitation (AE-IEP) of High-Thiol Plant Proteins. Appl. Sci. 2023, 13, 6469. [Google Scholar] [CrossRef]
- Du, T.; Xu, J.; Zhu, S.; Yao, X.; Guo, J.; Lv, W. Effects of Spray Drying, Freeze Drying, and Vacuum Drying on Physicochemical and Nutritional Properties of Protein Peptide Powder from Salted Duck Egg White. Front. Nutr. 2022, 9, 1026903. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, F.; Cao, J.; Huang, Y.; Li, C.; Li, J.; Ren, X. Effects of Hydrodynamic Cavitation at Different pH Values on the Physicochemical Properties and Aggregation Behavior of Soybean Glycinin. LWT 2022, 163, 113615. [Google Scholar] [CrossRef]
- Gong, Q.; Liu, C.; Tian, Y.; Zheng, Y.; Wei, L.; Cheng, T.; Wang, Z.; Guo, Z.; Zhou, L. Effect of Cavitation Jet Technology on Instant Solubility Characteristics of Soymilk Flour: Based on the Change of Protein Conformation in Soymilk. Ultrason. Sonochem. 2023, 96, 106421. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G.; Lenza, E.; Maresca, P. Main Factors Regulating Microbial Inactivation by High-Pressure Homogenization: Operating Parameters and Scale of Operation. Chem. Eng. Sci. 2009, 64, 520–532. [Google Scholar] [CrossRef]
- Martynenko, A.; Astatkie, T.; Satanina, V. Novel Hydrothermodynamic Food Processing Technology. J. Food Eng. 2015, 152, 8–16. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Cavitation Technology-the Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Appl. Sci. 2019, 9, 766. [Google Scholar] [CrossRef]
- Boukid, F. Plant-Based Meat Analogues: From Niche to Mainstream. Eur. Food Res. Technol. 2021, 247, 297–308. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.; Corke, H.; Gul, K.; Gan, R.; Fang, Y. The Health Benefits, Functional Properties, Modifications, and Applications of Pea (Pisum sativum L.) Protein: Current Status, Challenges, and Perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef]
- Banki, N.M.; Salihu, A.; Muhammad, A.; Bala, S.M. Optimization and Characterization of Rice–Pigeon Pea Flour Blend Using Extrusion Cooking Process. Legume Sci. 2021, 3, e73. [Google Scholar] [CrossRef]
- Chan, E.; Masatcioglu, T.M.; Koksel, F. Effects of Different Blowing Agents on Physical Properties of Extruded Puffed Snacks Made from Yellow Pea and Red Lentil Flours. J. Food Process Eng. 2019, 42, e12989. [Google Scholar] [CrossRef]
- Jebalia, I.; Maigret, J.-E.; Réguerre, A.-L.; Novales, B.; Guessasma, S.; Lourdin, D.; Della Valle, G.; Kristiawan, M. Morphology and Mechanical Behaviour of Pea-Based Starch-Protein Composites Obtained by Extrusion. Carbohydr. Polym. 2019, 223, 115086. [Google Scholar] [CrossRef]
- Webb, D.; Plattner, B.J.; Donald, E.; Funk, D.; Plattner, B.S.; Alavi, S. Role of Chickpea Flour in Texturization of Extruded Pea Protein. J. Food Sci. 2020, 85, 4180–4187. [Google Scholar] [CrossRef] [PubMed]
- Saint-Eve, A.; Granda, P.; Legay, G.; Cuvelier, G.; Delarue, J. Consumer Acceptance and Sensory Drivers of Liking for High Plant Protein Snacks. J. Sci. Food Agric. 2019, 99, 3983–3991. [Google Scholar] [CrossRef] [PubMed]
- Morales-Polanco, E.; Campos-Vega, R.; Gaytán-Martínez, M.; Enriquez, L.G.; Loarca-Piña, G. Functional and Textural Properties of a Dehulled Oat (Avena sativa L) and Pea (Pisum sativum) Protein Isolate Cracker. Food Sci. Technol. 2017, 86, 418–423. [Google Scholar] [CrossRef]
- Kim, T.; Miller, R.; Laird, H.; Riaz, M.N. Beef Flavor Vegetable Hamburger Patties with High Moisture Meat Analogs (HMMA) with Pulse Proteins—Peas, Lentils, and Faba Beans. Food Sci. Nutr. 2021, 9, 4048–4056. [Google Scholar] [CrossRef]
- De Angelis, D.; Kaleda, A.; Pasqualone, A.; Vaikma, H.; Tamm, M.; Tammik, M.-L.; Squeo, G.; Summo, C. Physicochemical and Sensorial Evaluation of Meat Analogues Produced from Dry-Fractionated Pea and Oat Proteins. Foods 2020, 9, 1754. [Google Scholar] [CrossRef]
- Shin, J.-S.; Kim, B.-H.; Baik, M.-Y. Applicable Plant Proteins and Dietary Fibers for Simulate Plant-Based Yogurts. Foods 2021, 10, 2305. [Google Scholar] [CrossRef]
- Klost, M.; Drusch, S. Structure Formation and Rheological Properties of Pea Protein-Based Gels. Food Hydrocoll. 2019, 94, 622–630. [Google Scholar] [CrossRef]
- Yousseef, M.; Lafarge, C.; Valentin, D.; Lubbers, S.; Husson, F. Fermentation of Cow Milk and/or Pea Milk Mixtures by Different Starter Cultures: Physico-Chemical and Sensorial Properties. LWT Food Sci. Technol. 2016, 69, 430–437. [Google Scholar] [CrossRef]
- Akkam, Y.; Rababah, T.; Costa, R.; Almajwal, A.; Feng, H.; Laborde, J.E.A.; Abulmeaty, M.M.; Razak, S. Pea Protein Nanoemulsion Effectively Stabilizes Vitamin d in Food Products: A Potential Supplementation during the COVID-19 Pandemic. Nanomaterials 2021, 11, 887. [Google Scholar] [CrossRef]
- Hadidi, M.; Motamedzadegan, A.; Jelyani, A.Z.; Khashadeh, S. Nanoencapsulation of Hyssop Essential Oil in Chitosan-Pea Protein Isolate Nano-Complex. Food Sci. Technol. 2021, 144, 111254. [Google Scholar] [CrossRef]

| Cultivar | Dry Matter (%) | Protein (%) | Ash (%) | Fat (%) | Protein Yield (g/kg) |
|---|---|---|---|---|---|
| Navarro | 93.0 ± 0.0 ab | 83.5 ± 0.4 c | 5.3 ± 0.3 cd | 5.9 ± 0.0 c | 33.8 |
| Dolores | 93.5 ± 0.1 ab | 89.5 ± 0.2 a | 5.4 ± 0.1 cd | 4.7 ± 0.1 d | 54.4 |
| Greenwich | 93.8 ± 0.0 ab | 83.6 ± 0.4 c | 6.0 ± 0.6 c | 9.0 ± 0.2 a | 34.8 |
| Bluetime | 94.4 ± 0.0 a | 84.1 ± 0.0 bc | 6.4 ± 0.4 c | 8.4 ± 0.3 a | 42.2 |
| Ostinato | 94.1 ± 0.0 a | 86.0 ± 0.5 b | 7.6 ± 0.4 b | 7.1 ± 0.4 b | 38.6 |
| Kalifa | 93.0 ± 0.0 ab | 86.9 ± 0.9 b | 5.9 ± 0.1 c | 7.0 ± 0.5 b | 46.2 |
| Salamanca | 93.7 ± 0.6 ab | 85.0 ± 0.3 bc | 6.1 ± 1.0 c | 8.7 ± 0.6 a | 42.2 |
| Florida | 92.5 ± 0.0 b | 87.4 ± 1.1 b | 5.6 ± 0.1 cd | 7.4 ± 0.7 b | 59.2 |
| RLPY 141091 | 93.4 ± 0.0 ab | 90.3 ± 0.0 a | 8.5 ± 0.7 a | 7.3 ± 0.8 b | 53.6 |
| Orchestra | 92.8 ± 0.3 b | 87.1 ± 0.1 b | 6.7 ± 1.1 c | 6.2 ± 0.9 c | 62.2 |
| Astronaute | 96.0 ± 0.2 a | 86.4 ± 0.1 b | 5.4 ± 0.1 cd | 7.8 ± 0.1 b | 42.1 |
| Croft | 92.5 ± 0.1 b | 86.7 ± 0.6 b | 6.2 ± 0.1 c | 7.8 ± 0.1 b | 47.3 |
| Classification | Content | Protein Fraction | Polypeptide | Svedberg Unit | Features | Author |
|---|---|---|---|---|---|---|
| Globulins | 55–65% | Hexameric/quarternary legumin (300–600 kDa) | Six paired α and β (60–80 kDa) | 11S | α and β subunits linked by disulfide linkage | Gueguen and Cerletti [26]; Lam et al. [18]; Tzitzikas et al. [27] |
| Trimeric vicilin (175–180 kDa) | α, β, and γ (14–20 kDa) | 7S | Non-covalent bonds between subunits and glycosylation | Chang et al. [28]; Kaur Dhaliwal et al. [29] | ||
| Trimeric convicilin (210 kDa) | ~70 kDa | 8S | 80% amino acid homology with 7S | Kaur Dhaliwal et al. [29]; Mertens et al. [30] | ||
| Albumins | 18–25% | Pea albumins | PA1a (5.8 kDa) | 2S | 53 amino acids and high Cys | Barbana and Boye [31]; De Santis et al. [32]; Kornet et al. [33]; Park et al. [25] |
| PA1b (4.0 kDa) | 2S | 37 amino acids and high Cys | ||||
| Lectins | n/a | n/a | n/a | |||
| Lipoxygenase | 90–100 kDa | n/a | n/a | |||
| Protease inhibitors | n/a | n/a | ||||
| Natural pigments (anthocyanins and tannins) | n/a | n/a | ||||
| Prolamin | 4–5% | n/a | n/a | n/a | High Glu and Pro | Adebiyi and Aluko [34] |
| Glutelin | 3–4% | n/a | n/a | n/a | n/a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asen, N.D.; Aluko, R.E.; Martynenko, A.; Utioh, A.; Bhowmik, P. Yellow Field Pea Protein (Pisum sativum L.): Extraction Technologies, Functionalities, and Applications. Foods 2023, 12, 3978. https://doi.org/10.3390/foods12213978
Asen ND, Aluko RE, Martynenko A, Utioh A, Bhowmik P. Yellow Field Pea Protein (Pisum sativum L.): Extraction Technologies, Functionalities, and Applications. Foods. 2023; 12(21):3978. https://doi.org/10.3390/foods12213978
Chicago/Turabian StyleAsen, Nancy D., Rotimi E. Aluko, Alex Martynenko, Alphonsus Utioh, and Pankaj Bhowmik. 2023. "Yellow Field Pea Protein (Pisum sativum L.): Extraction Technologies, Functionalities, and Applications" Foods 12, no. 21: 3978. https://doi.org/10.3390/foods12213978
APA StyleAsen, N. D., Aluko, R. E., Martynenko, A., Utioh, A., & Bhowmik, P. (2023). Yellow Field Pea Protein (Pisum sativum L.): Extraction Technologies, Functionalities, and Applications. Foods, 12(21), 3978. https://doi.org/10.3390/foods12213978









