Gellan Gum and Polyvinyl Alcohol Based Triple-Layer Films Enriched with Alhagi sparsifolia Flower Extract: Preparation, Characterization, and Application of Dried Shrimp Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of A. sparsifolia Flowers
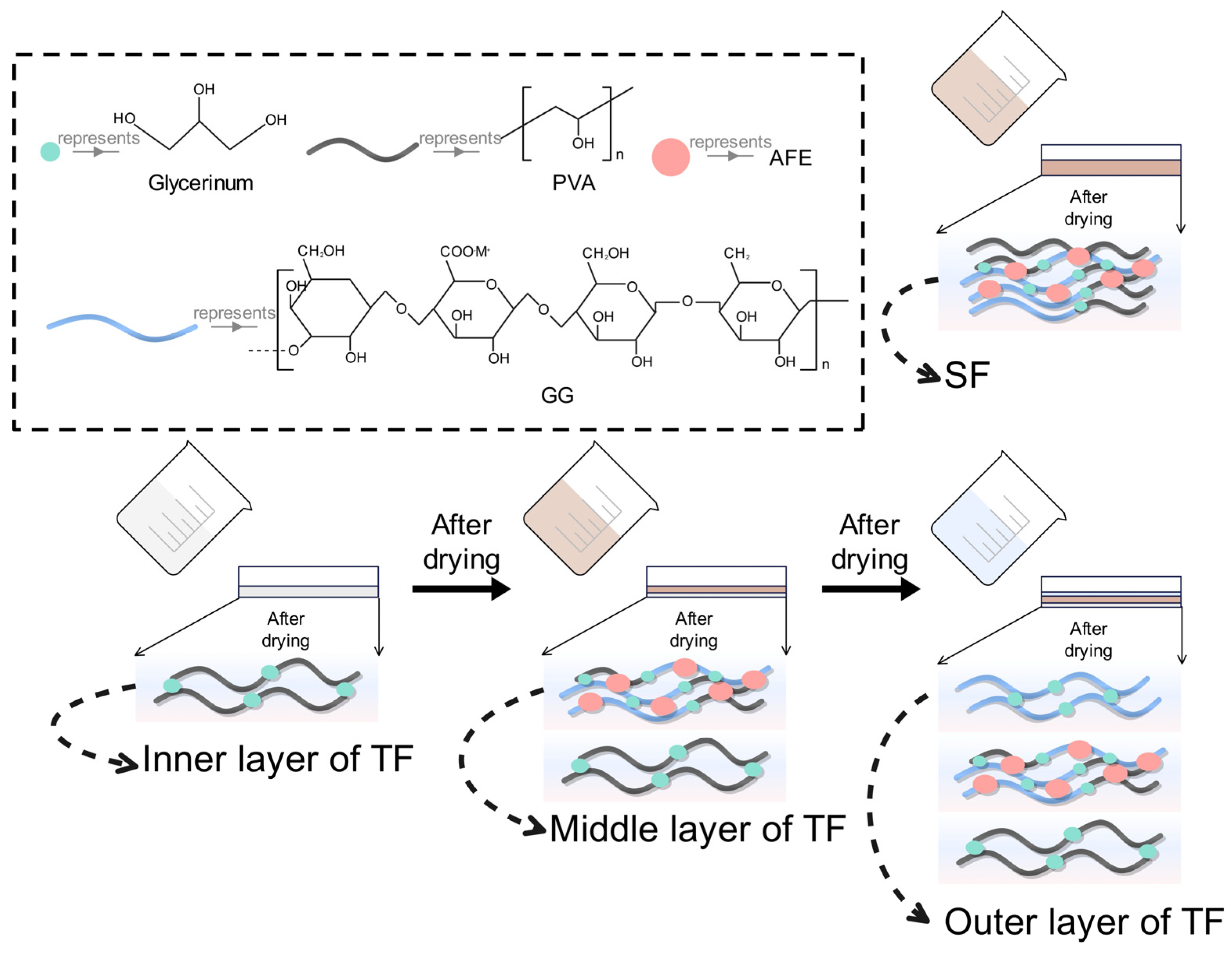
2.3. Preparation of SFs and TFs
2.4. Film Characterization
2.4.1. Colour Measurements
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.4.4. Mechanical Properties
2.4.5. Barrier Properties
2.4.6. Moisture Content and Water Solubility of Film
2.4.7. Optical Transmittance
2.4.8. Anti-Oxidative Slow-Release Properties
2.5. Experiment on Application in Dried Shrimp Storage
2.5.1. Preparation, Packaging, and Storage Method of Dried Shrimp
2.5.2. Protein Oxidation of Dried Shrimp
2.5.3. Lipid Oxidation of Dried Shrimp
2.6. Statistical Analysis
3. Results and Discussion
3.1. Visual Changes
3.2. Morphology Structure
3.3. FTIR Analysis
3.4. Optical Transmittance
3.5. Moisture Content and Water Solubility of Films
3.6. Barrier Properties of Films
3.7. Mechanical Properties of Films
3.8. Anti-Oxidative Capacity Slow-Release Properties
3.9. Application of Films
3.9.1. Lipid Oxidation of Dried Shrimp
3.9.2. Protein Oxidation of Dried Shrimp
3.10. Partial Least Squares Discrimination Analysis (PLS-DA) of Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Liu, X.; Liao, W.; Wang, Q.; Xia, W. Chitosan/bacterial cellulose films incorporated with tea polyphenol nanoliposomes for silver carp preservation. Carbohydr. Polym. 2022, 297, 120048. [Google Scholar] [CrossRef] [PubMed]
- Sripahco, T.; Khruengsai, S.; Pripdeevech, P. Biodegradable antifungal films from nanocellulose-gellan gum incorporated with Anethum graveolens essential oil for bread packaging. Int. J. Biol. Macromol 2023, 243, 125244. [Google Scholar] [CrossRef] [PubMed]
- Grzebieniarz, W.; Biswas, D.; Roy, S.; Jamróz, E. Advances in biopolymer-based multi-layer film preparations and food packaging applications. Food Packag. Shelf Life 2023, 35, 101033. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, H.; McClements, D.J. Impact of dispersion conditions and coacervation on fibril formation in gellan gum-potato protein mixtures. Food Hydrocoll. 2023, 145, 109153. [Google Scholar] [CrossRef]
- Yang, Z.; Li, C.; Wang, T.; Li, Z.; Zou, X.; Huang, X.; Zhai, X.; Shi, J.; Shen, T.; Gong, Y.; et al. Novel gellan gum-based probiotic film with enhanced biological activity and probiotic viability: Application for fresh-cut apples and potatoes. Int. J. Biol. Macromol. 2023, 239, 124128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, D.; Jin, T.Z.; Chen, W.; He, Q.; Zou, Z.; Zhao, H.; Ye, X.; Guo, M. Preparation and characterization of gellan gum-chitosan polyelectrolyte complex films with the incorporation of thyme essential oil nanoemulsion. Food Hydrocoll. 2021, 114, 106570. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Z.; Li, X.; Wu, Z.; Liu, Y.; Hu, J.; Zhang, C.; Chen, J.; Zhou, Y.; Rao, J.; et al. Facile preparation of biocompatible and antibacterial water-soluble films using polyvinyl alcohol/carboxymethyl chitosan blend fibers via centrifugal spinning. Carbohydr. Polym. 2023, 317, 121062. [Google Scholar] [CrossRef] [PubMed]
- Zulkiflee, I.; Fauzi, M.B. Gelatin-polyvinyl alcohol film for tissue engineering: A concise review. Biomedicines 2021, 9, 979. [Google Scholar] [CrossRef]
- Zeng, J.; Song, Y.; Fan, X.; Luo, J.; Song, J.; Xu, J.; Xue, C. Effect of lipid oxidation on quality attributes and control technologies in dried aquatic animal products: A critical review. Crit. Rev. Food Sci. Nutr. 2023, 19, 1–22. [Google Scholar] [CrossRef]
- Neamah, N.F. A pharmacological evaluation of aqueous extract of Alhagi maurorum. Glob. J. Pharmacol. 2012, 6, 41–46. [Google Scholar]
- Maimaitimin, K.; Jiang, Z.; Aierken, A.; Shayibuzhati, M.; Zhang, X. Hepatoprotective effect of Alhagi sparsifolia against Alcoholic Liver injury in mice. Braz. J. Pharm. Sci. 2018, 54, 03. [Google Scholar] [CrossRef]
- Guo, D.; Xue, W.J.; Zou, G.A.; Aisa, H.A. Chemical composition of Alhagi sparsifolia flowers. Chem. Nat. Compd. 2016, 52, 1095–1097. [Google Scholar] [CrossRef]
- Jamróz, E.; Cabaj, A.; Tkaczewska, J.; Kawecka, A.; Krzyściak, P.; Szuwarzyński, M.; Mazur, T.; Juszczak, L. Incorporation of curcumin extract with lemongrass essential oil into the middle layer of triple-layered films based on furcellaran/chitosan/gelatin hydrolysates–In vitro and in vivo studies on active and intelligent properties. Food Chem. 2023, 402, 134476. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Liu, D.; Zhang, C.; Yi, H.; Liu, D. Preparation, characterization, and application of edible antibacterial three-layer films based on gelatin–chitosan–corn starch–incorporated nisin. Food Packag. Shelf Life 2022, 34, 100980. [Google Scholar] [CrossRef]
- Kim, S.; Kim, B.S.; Bai, J.; Chang, Y. Antibacterial κ-carrageenan/konjac glucomannan-based edible hydrogel film containing Salmonella phage PBSE191 and its application in chicken meat. LWT 2023, 180, 114707. [Google Scholar] [CrossRef]
- Lian, H.; Shi, J.; Zhang, X.; Peng, Y. Effect of the added polysaccharide on the release of thyme essential oil and structure properties of chitosan based film. Food Packag. Shelf Life 2020, 23, 100467. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, X.; Zang, M.; Wang, L.; Li, X.; Yue, Y.; Liu, B. Insights into the characteristics and molecular transformation of lipids in Litopenaeus vannamei during drying from combined lipidomics. J. Food Compos. Anal. 2022, 114, 104809. [Google Scholar] [CrossRef]
- Wang, L.; Zang, M.; Zhao, X.; Cheng, X.; Li, X.; Bai, J. Lipid oxidation and free radical formation of shrimp (Penaeus vannamei) during hot air drying. J. Food Meas. Charact. 2023, 17, 3493–3504. [Google Scholar] [CrossRef]
- Gan, M.; Guo, C.; Liao, W.; Liu, X.; Wang, Q. Development and characterization of chitosan/bacterial cellulose/pullulan bilayer film with sustained release curcumin. Int. J. Biol. Macromol. 2023, 226, 301–311. [Google Scholar] [CrossRef]
- Li, Y.; Tang, X.; Zhu, L. Bilayer pH-sensitive colorimetric indicator films based on zein/gellan gum containing black rice (Oryza sativa L.) extracts for monitoring of largemouth bass (Micropterus salmoides) fillets freshness. Int. J. Biol. Macromol. 2022, 223, 1268–1277. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Thermoprocessed starch-polyester bilayer films as affected by the addition of gellan or xanthan gum. Food Hydrocoll. 2021, 113, 106509. [Google Scholar] [CrossRef]
- Zhao, X.; Han, Z.; Zhang, S.; Abuduaini, G.; Wen, X.; Liu, T.; Cheng, Z. Preparation of PVA/Tremella polysaccharide and soy protein isolate complex/ε-polylysine active membrane and its application in blueberry preservation. Food Packag. Shelf Life 2023, 40, 101163. [Google Scholar] [CrossRef]
- Azadi, A.; Rafieian, F.; Sami, M.; Rezaei, A. Fabrication, characterization and antimicrobial activity of chitosan/tragacanth gum/polyvinyl alcohol composite films incorporated with cinnamon essential oil nanoemulsion. Int. J. Biol. Macromol. 2023, 245, 125225. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Choi, S.W.; Lee, N.; Chang, H.J. Antimicrobial Activity of Chitosan/Gelatin/Poly (Vinyl Alcohol) Ternary Blend Film Incorporated with Duchesnea indica Extract in Strawberry Applications. Foods 2022, 11, 3963. [Google Scholar] [CrossRef]
- Wang, F.; Wen, Y.; Bai, T. Thermal behavior of polyvinyl alcohol–gellan gum–Al 3+ composite hydrogels with improved network structure and mechanical property. J. Therm. Anal. Calorim. 2017, 127, 2447–2457. [Google Scholar] [CrossRef]
- Sudhamani, S.R.; Prasad, M.S.; Sankar, K.U. DSC and FTIR studies on gellan and polyvinyl alcohol (PVA) blend films. Food Hydrocoll. 2003, 17, 245–250. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C. A double-layer smart film based on gellan gum/modified anthocyanin and sodium carboxymethyl cellulose/starch/Nisin for application in chicken breast. Int. J. Biol. Macromol. 2023, 232, 123464. [Google Scholar] [CrossRef]
- Akhila, K.; Sultana, A.; Ramakanth, D.; Gaikwad, K.K. Monitoring freshness of chicken using intelligent pH indicator packaging film composed of polyvinyl alcohol/guar gum integrated with Ipomoea coccinea extract. Food Biosci. 2023, 52, 102397. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, B.; Zheng, T.; Zhao, C.; Gao, Y.; Wu, W.; Fan, Y.; Wang, X.; Qiu, M.; Fan, J. Structural characteristics, rheological properties, and antioxidant and anti-glycosylation activities of pectin polysaccharides from Arabica coffee husks. Foods 2023, 12, 423. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, F.; Xu, Y.; Hu, Y.; Liu, W.; Yang, Z.; Yu, Z.; Xiong, G.; Zhou, Y.; Xiao, Y. Development of antioxidant and smart NH3-sensing packaging film by incorporating bilirubin into κ-carrageenan matrix. J. Sci. Food Agric. 2023, 103, 7030–7039. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Min, S.J.; Biswas, D.; Rhim, J.W. Pullulan/chitosan-based functional film incorporated with curcumin-integrated chitosan nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130898. [Google Scholar] [CrossRef]
- Rufato, K.B.; Souza, P.R.; de Oliveira, A.C.; Berton, S.B.; Sabino, R.M.; Muniz, E.C.; Popat, K.C.; Radovanovic, E.; Kipper, M.J.; Martins, A.F. Antimicrobial and cytocompatible chitosan, N, N, N-trimethyl chitosan, and tanfloc-based polyelectrolyte multilayers on gellan gum films. Int. J. Biol. Macromol. 2021, 183, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mao, L.; Yao, J.; Zhu, H. Improving the active food packaging function of poly (lactic acid) film coated by poly (vinyl alcohol) based on proanthocyanidin functionalized layered clay. LWT 2023, 174, 114407. [Google Scholar] [CrossRef]
- Koosha, M.; Hamedi, S. Intelligent Chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog. Org. Coat. 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Santos, C.; Ramos, A.; Luís, Â.; Amaral, M.E. Production and Characterization of k-Carrageenan Films Incorporating Cymbopogon winterianus Essential Oil as New Food Packaging Materials. Foods 2023, 12, 2169. [Google Scholar] [CrossRef]
- Diop, C.I.K.; Beltran, S.; Sanz, M.T.; Garcia-Tojal, J.; Trigo-lopez, M. Designing bilayered composite films by direct agar/chitosan and citric acid-crosslinked PVA/agar layer-by-layer casting for packaging applications. Food Hydrocoll. 2023, 144, 108987. [Google Scholar] [CrossRef]
- Wu, L.T.; Tsai, I.L.; Ho, Y.C.; Hang, Y.H.; Lin, C.; Tsai, M.L.; Mi, F.L. Active and intelligent gellan gum-based packaging films for controlling anthocyanins release and monitoring food freshness. Carbohydr. Polym. 2021, 254, 117410. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Qiu, Y.L.; Zhang, G.L.; Hao, H.; Hou, H.M.; Bi, J. Amino carboxymethyl chitosan//dialdehyde starch/polyvinyl alcohol double-layer film loaded with ε-polylysine. Food Chem. 2023, 428, 136775. [Google Scholar] [CrossRef]
- Goudarzi, J.; Moshtaghi, H.; Shahbazi, Y. Kappa-carrageenan-poly (vinyl alcohol) electrospun fiber mats encapsulated with Prunus domestica anthocyanins and epigallocatechin gallate to monitor the freshness and enhance the shelf-life quality of minced beef meat. Food Packag. Shelf Life 2023, 35, 101017. [Google Scholar] [CrossRef]
- Guo, X.; Wu, J.; Meng, X.; Zhang, Y.; Peng, Z. Oxidative characteristics and gel properties of porcine myofibrillar proteins affected by l-lysine and l-histidine in a dose-dependent manner at a low and high salt concentration. Int. J. Food Sci. Technol. 2022, 57, 2556–2567. [Google Scholar] [CrossRef]
- Xu, L.; Xu, X.; Xu, Y.; Huang, M. Oregano essential oil-doped citric acid modified polyvinyl alcohol bio-active films: Properties, bio-functional performance and active packaging application of chicken breast. Food Packag. Shelf Life 2023, 38, 101125. [Google Scholar] [CrossRef]
- Chen, C.W.; Xie, J.; Yang, F.X.; Zhang, H.L.; Xu, Z.W.; Liu, J.L.; Chen, Y.J. Development of moisture-absorbing and antioxidant active packaging film based on poly (vinyl alcohol) incorporated with green tea extract and its effect on the quality of dried eel. J. Food Process. Preserv. 2018, 42, e13374. [Google Scholar] [CrossRef]
- Bao, P.; Chen, L.; Hu, Y.; Wang, Y.; Zhou, C. l-Arginine and l-lysine retard aggregation and polar residue modifications of myofibrillar proteins: Their roles in solubility of myofibrillar proteins in frozen porcine Longissimus lumborum. Food Chem. 2022, 393, 133347. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Liu, J.; Liu, Y.; Zheng, Y.; Pi, R.; Mubango, E.; Tan, Y.; Luo, Y.; Hong, H. Inhibitive effect of cryoprotectants on the oxidative and structural changes in myofibrillar proteins of unwashed mince from silver carp during frozen storage. Food Res. Int. 2022, 161, 111880. [Google Scholar] [CrossRef] [PubMed]
- Da Nóbrega Santos, E.; de Albuquerque Sousa, T.C.; de Santana Neto, D.C.; Grisi, C.V.B.; da Silva Ferreira, V.C.; da Silva, F.A.P. Edible active film based on gelatin and Malpighia emarginata waste extract to inhibit lipid and protein oxidation in beef patties. LWT 2022, 154, 112837. [Google Scholar] [CrossRef]
- Daszykowski, M.; Kula, M.; Stanimirova, I. Quantification and detection of ground garlic adulteration using fourier-transform near-infrared reflectance spectra. Foods 2023, 12, 3377. [Google Scholar] [CrossRef]







| Type | MC (%) | WS (%) | WVP (10−15 g m−1 s−1 pa−1) | OP (10−9 cm3/m2 d·Pa) | TS (MPa) | EAB (%) |
|---|---|---|---|---|---|---|
| SF-0% | 19.61 ± 0.94 e | 32.38 ± 0.51 d | 1.13 ± 0.03 c | 2.46 ± 0.43 bc | 33.71 ± 1.73 a | 33.60 ± 3.49 h |
| SF-1.25% | 22.92 ± 1.27 cd | 34.71 ± 1.75 c | 1.14 ± 0.03 bc | 3.01 ± 0.32 ab | 30.10 ± 2.84 b | 43.63 ± 2.11 fg |
| SF-2.5% | 24.84 ± 0.80 c | 36.62 ± 0.57 c | 1.15 ± 0.04 bc | 3.26 ± 0.13 a | 23.95 ± 0.50 c | 58.43 ± 5.29 e |
| SF-3.75% | 27.76 ± 1.24 b | 39.22 ± 0.66 b | 1.18 ± 0.01 ab | 3.57 ± 0.43 a | 12.74 ± 0.44 d | 81.97 ± 4.45 c |
| SF-5% | 31.04 ± 2.30 a | 42.25 ± 0.63 a | 1.19 ± 0.01 a | 3.52 ± 0.18 a | 11.45 ± 0.73 d | 94.40 ± 2.02 b |
| TF-0% | 18.45 ± 0.70 e | 35.04 ± 1.12 c | 1.03 ± 0.01 ef | 2.01 ± 0.12 c | 35.56 ± 0.99 a | 37.93 ± 0.85 gh |
| TF-1.25% | 20.31 ± 1.71 de | 35.76 ± 1.30 c | 1.05 ± 0.01 def | 2.20 ± 0.26 c | 34.79 ± 2.29 a | 46.63 ± 0.38 f |
| TF-2.5% | 22.72 ± 1.92 cd | 38.98 ± 2.05 b | 1.03 ± 0.01 f | 2.21 ± 0.28 c | 23.28 ± 2.75 c | 72.03 ± 4.91 d |
| TF-3.75% | 24.57 ± 1.60 c | 41.00 ± 1.83 ab | 1.07 ± 0.01 de | 2.29 ± 0.01 bc | 12.39 ± 0.30 d | 91.47 ± 4.09 b |
| TF-5% | 27.40 ± 1.70 b | 42.85 ± 1.80 a | 1.07 ± 0.02 d | 2.29 ± 0.04 bc | 10.07 ± 0.41 d | 112.27 ± 2.28 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Y.; Cheng, X.; Liu, H.; Zang, M.; Zhao, B.; Zhao, X.; Wang, L. Gellan Gum and Polyvinyl Alcohol Based Triple-Layer Films Enriched with Alhagi sparsifolia Flower Extract: Preparation, Characterization, and Application of Dried Shrimp Preservation. Foods 2023, 12, 3979. https://doi.org/10.3390/foods12213979
Yue Y, Cheng X, Liu H, Zang M, Zhao B, Zhao X, Wang L. Gellan Gum and Polyvinyl Alcohol Based Triple-Layer Films Enriched with Alhagi sparsifolia Flower Extract: Preparation, Characterization, and Application of Dried Shrimp Preservation. Foods. 2023; 12(21):3979. https://doi.org/10.3390/foods12213979
Chicago/Turabian StyleYue, Yijing, Xiaoyu Cheng, Haijie Liu, Mingwu Zang, Bing Zhao, Xin Zhao, and Le Wang. 2023. "Gellan Gum and Polyvinyl Alcohol Based Triple-Layer Films Enriched with Alhagi sparsifolia Flower Extract: Preparation, Characterization, and Application of Dried Shrimp Preservation" Foods 12, no. 21: 3979. https://doi.org/10.3390/foods12213979





