Structurally Orientated Rheological and Gut Microbiota Fermentation Property of Mannans Polysaccharides and Oligosaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Subsection
2.2. Preparation of Beta-Mannanases Solution
2.3. Preparation of Mannan Oligosaccharides
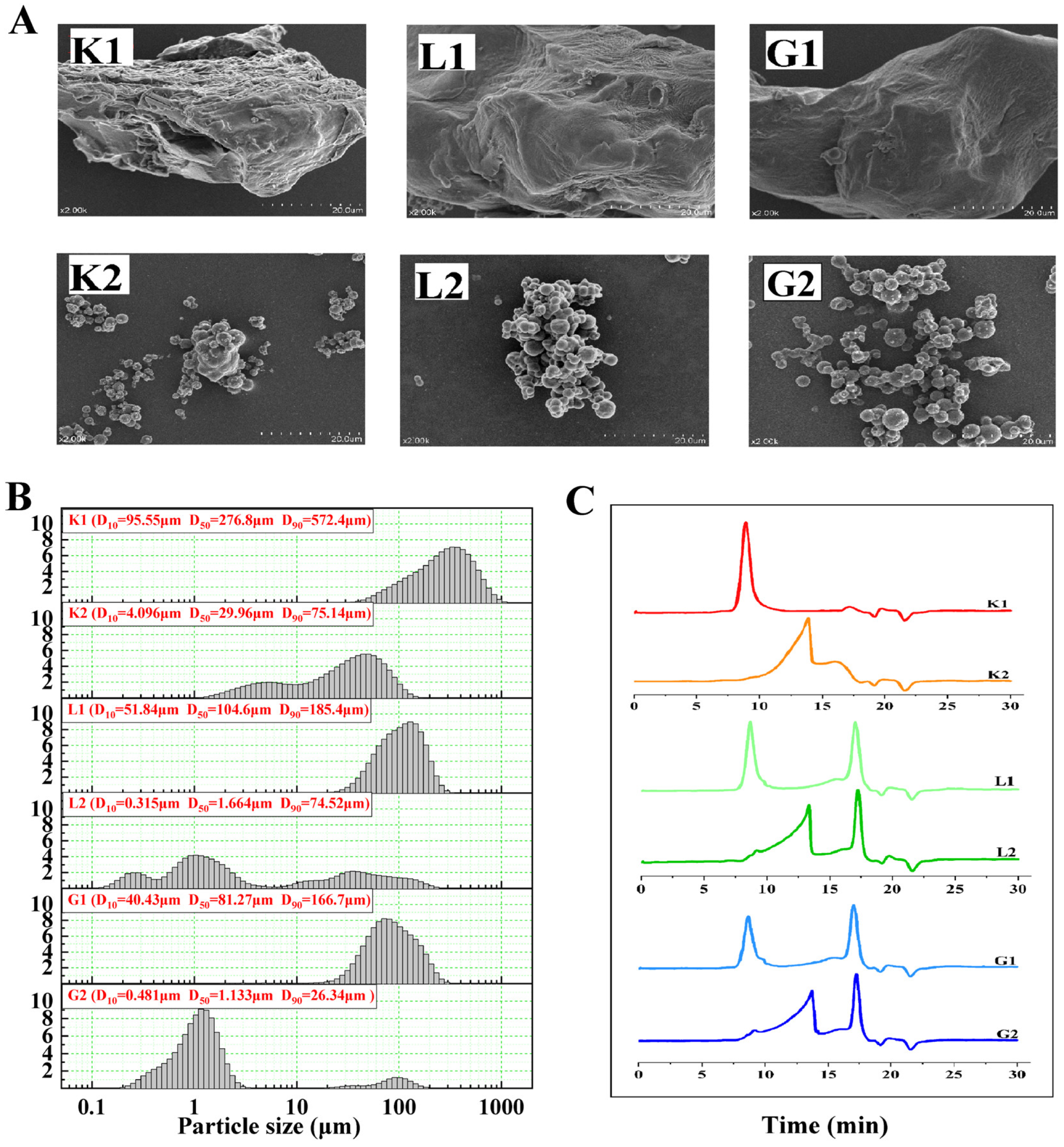
2.4. Scanning Electron Microscopy (SEM) of the Samples
2.5. Particle Size Distribution of the Samples
2.6. Molecular Weight (Mw) Analysis
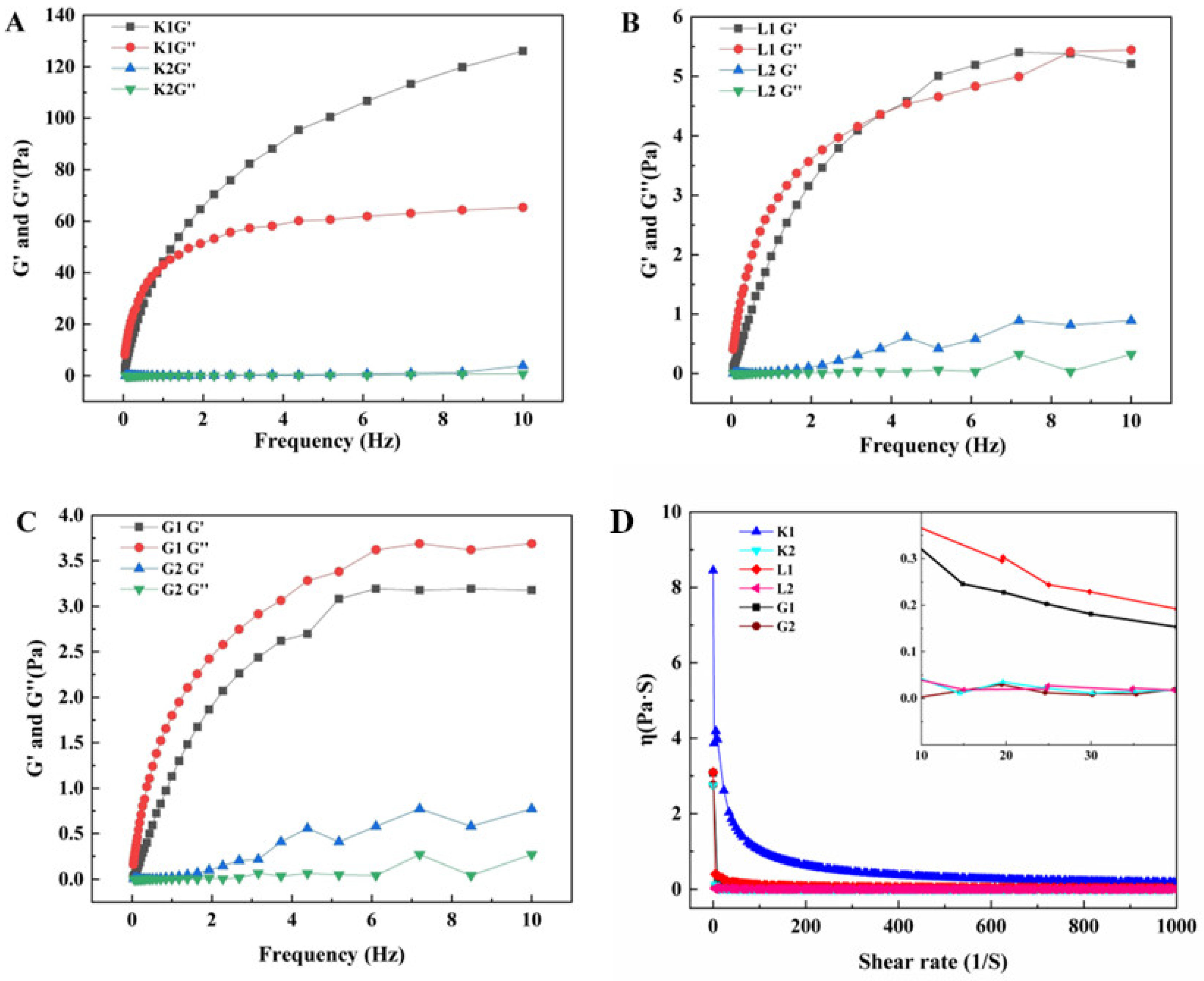
2.7. Rheological Properties of Samples
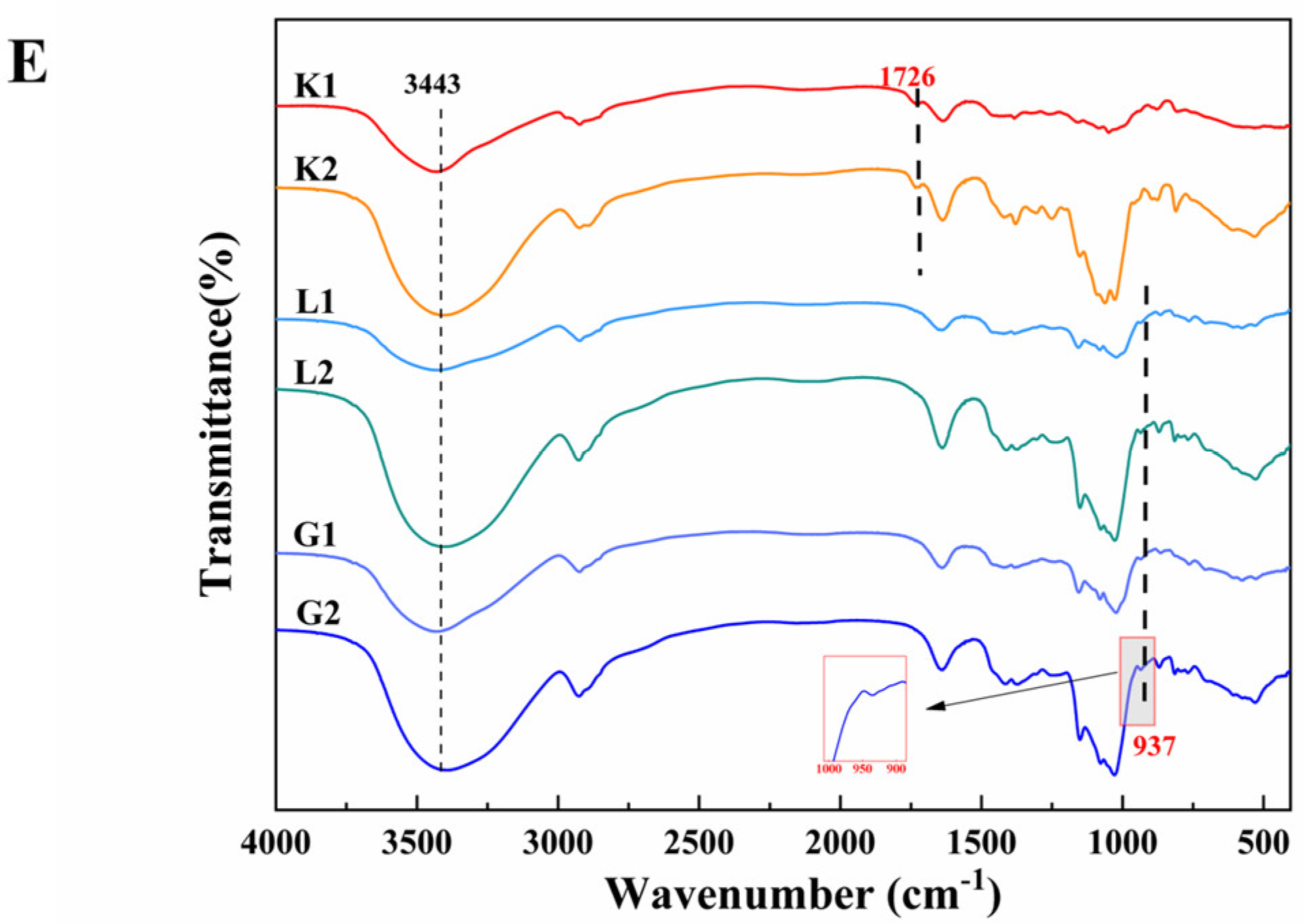
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. In Vitro Gut Microbiota Fermentation
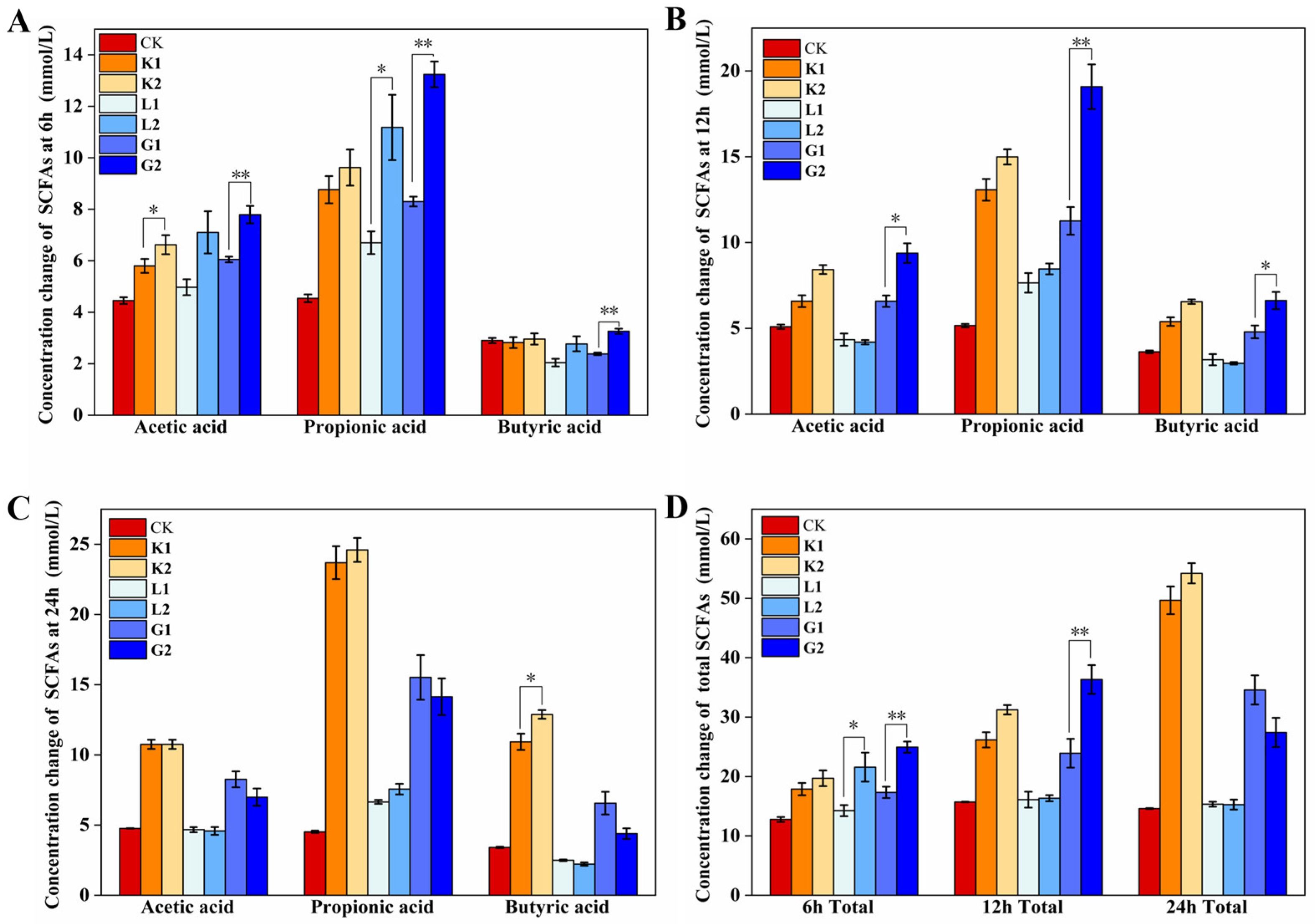
2.10. Determination of SCFAs during the Gut Microbiota Fermentation Progress
2.11. 16S rDNA Gene Sequencing Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Microstructure, Molecular Weight and Particle Size Distribution of Mannan Polysaccharides and Oligosaccharides
3.2. Rheological Properties of Mannans Polysaccharides and Oligosaccharides
3.3. FT–IR Spectra of Polysaccharides and Oligosaccharides
3.4. Production of SCFAs following the Gut Microbiota Fermentation
3.5. Changes in the Gut Microbiota following the Fermentation
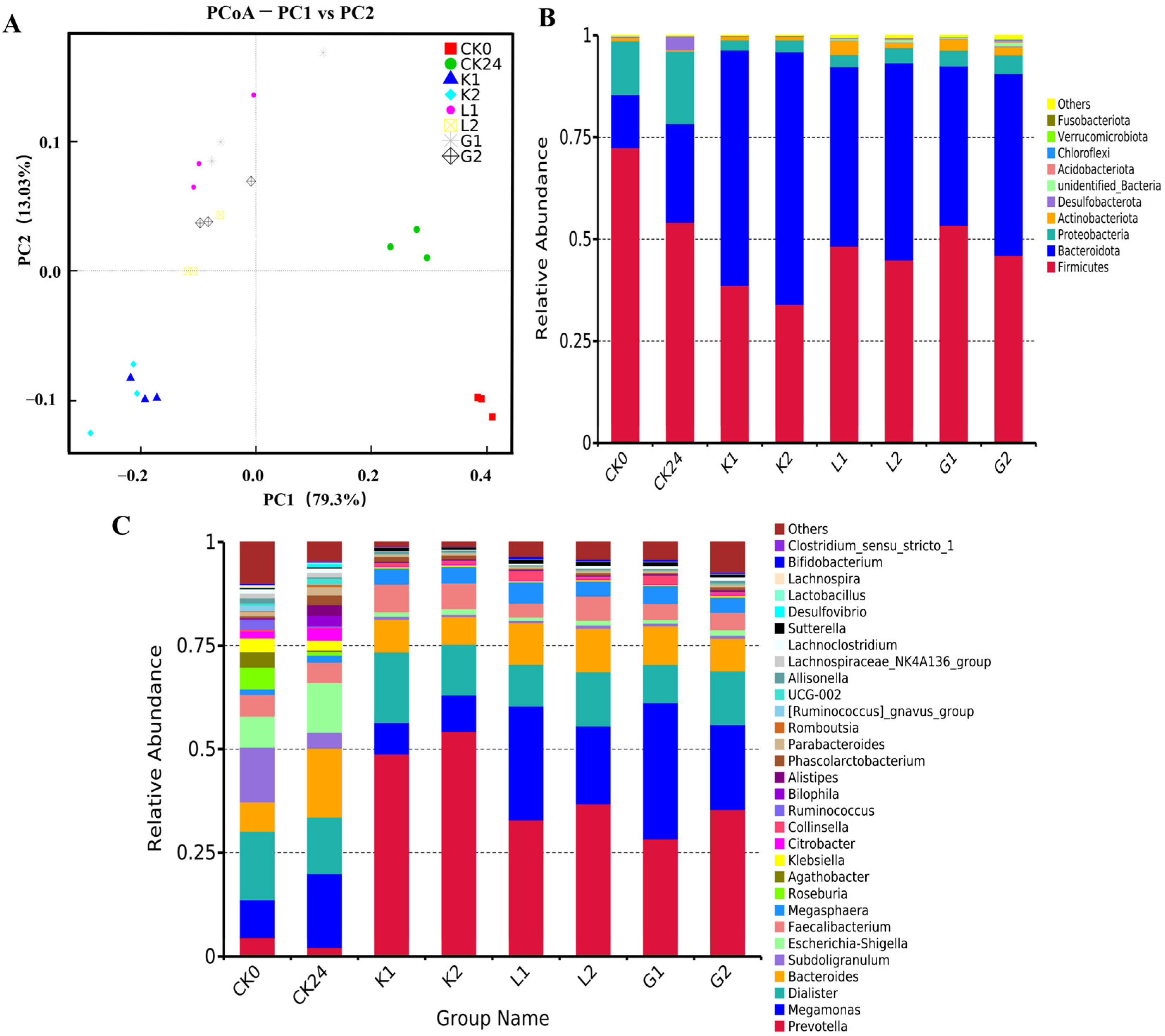
3.5.1. Difference in Alpha and Beta Diversity following the Fermentation
3.5.2. Changes in the Gut Microbiota on Phylum Levels following the Fermentation
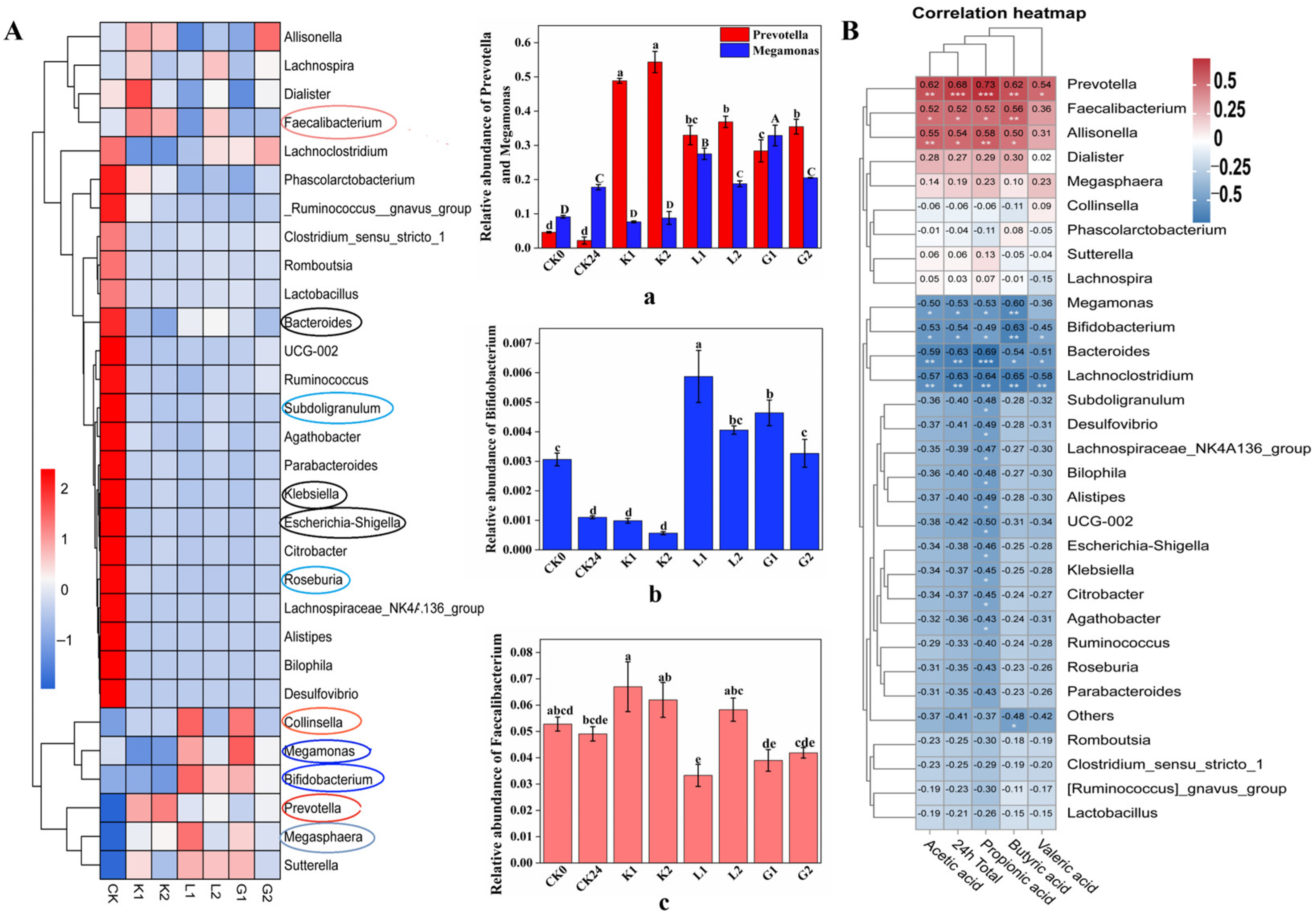
3.5.3. Changes in the Gut Microbiota on Genus Level following the Fermentation
3.5.4. Spearman Correlation Analysis between Flora and SCFAs
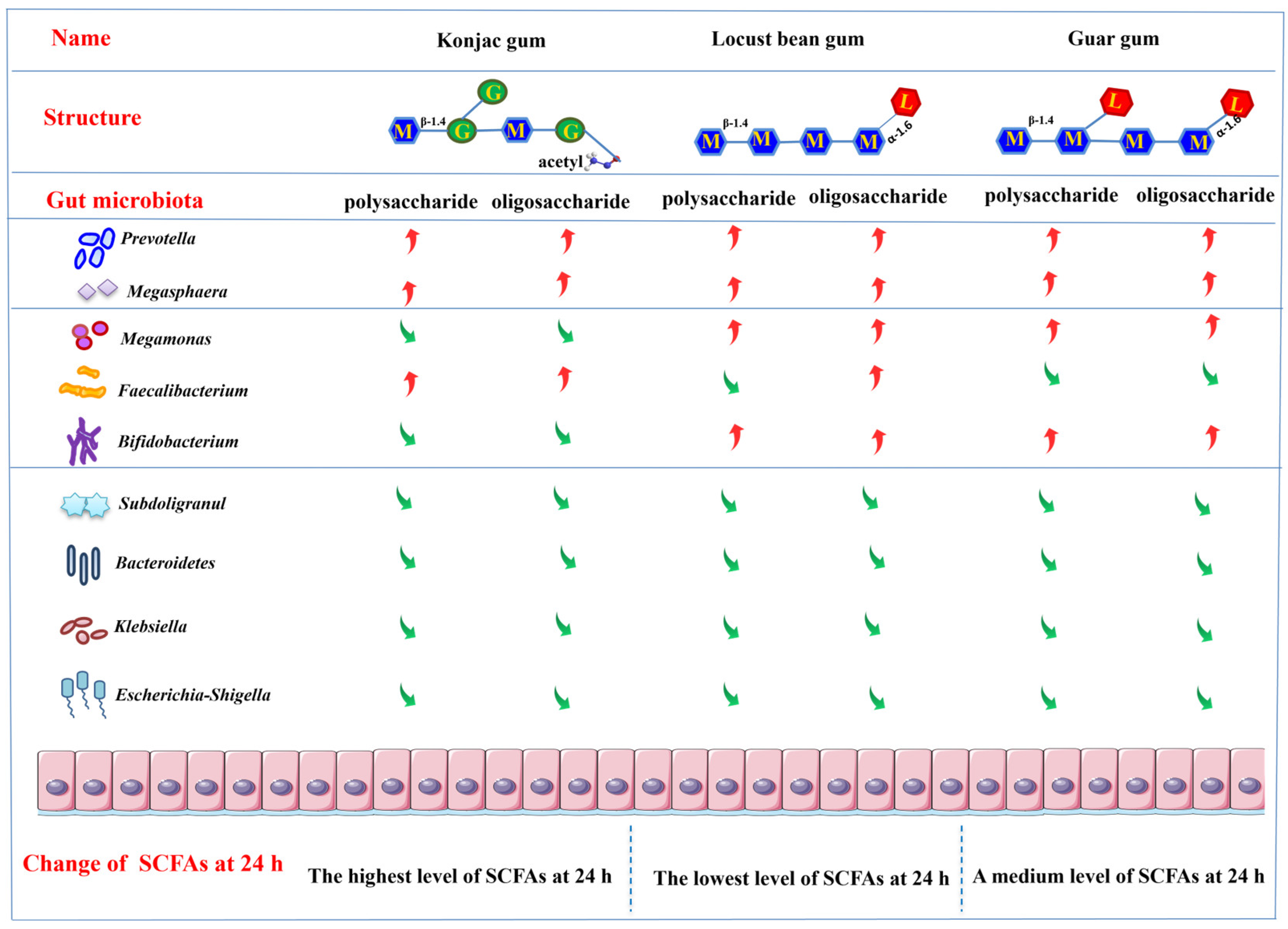
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreira, L.; Filho, E. An overview of mannan structure and mannan-degrading enzyme systems. Appl. Microbiol. Biotechnol. 2008, 79, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C. Konjac glucomannan, a promising polysaccharide of Amorphophallus konjac K. Koch in health care. Int. J. Biol. Macromol. 2016, 92, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, A.M.; Wani, I.A. Guar and Locust bean gum: Composition, total phenolic content, antioxidant and antinutritional characterisation. Bioact. Carbohydr. Diet. Fibre 2017, 11, 53–59. [Google Scholar] [CrossRef]
- Katsuraya, K.; Okuyama, K.; Hatanaka, K.; Oshima, R.; Sato, T.; Matsuzaki, K. Constitution of konjac glucomannan: Chemical analysis and 13C NMR spectroscopy. Carbohydr. Polym. 2003, 53, 183–189. [Google Scholar] [CrossRef]
- La Rosa, S.L.; Leth, M.L.; Michalak, L.; Hansen, M.E.; Pudlo, N.A.; Glowacki, R.; Pereira, G.; Workman, C.T.; Arntzen, M.Ø.; Pope, P.B.; et al. The human gut Firmicute Roseburia intestinalis is a primary degrader of dietary β-mannans. Nat. Commun. 2019, 10, 905. [Google Scholar] [CrossRef]
- Du, G.; Qing, Y.; Wang, H.; Wang, N.; Yue, T.; Yuan, Y. Effects of Tibetan kefir grain fermentation on the physicochemical properties, phenolics, enzyme activity, and antioxidant activity of Lycium barbarum (Goji berry) juice. Food Biosci. 2023, 53, 102555. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, X.; Duan, S.; Li, C.; Hu, B.; Liu, A.; Wu, Y.; Wu, H.; Chen, H.; Wu, W. Effects of in vitro digestion and fecal fermentation on the stability and metabolic behavior of polysaccharides from Craterellus cornucopioides. Food Funct. 2020, 11, 6899–6910. [Google Scholar] [CrossRef]
- del Hierro, J.N.; Cueva, C.; Tamargo, A.; Núñez-Gómez, E.; Moreno-Arribas, M.V.; Reglero, G.J.; Martin, D. In vitro colonic fermentation of saponin-rich extracts from quinoa, lentil, and fenugreek. Effect on sapogenins yield and human gut microbiota. J. Agric. Food Chem. 2019, 68, 106–116. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, X.; Zhen, W.; Li, Z.; Kang, J.; Sun, X.; Wang, S.; Cui, S.W. Rheological properties and stabilizing effects of high-temperature extracted flaxseed gum on oil/water emulsion systems. Food Hydrocoll. 2021, 112, 106289. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Hess, J.M.; Gould, T.J.; Slavin, J.L. Prebiotic dietary fiber and gut health: Comparing the in vitro fermentations of beta-glucan, inulin and xylooligosaccharide. Nutrients 2017, 9, 1361. [Google Scholar] [CrossRef]
- Ma, G.; Xu, Q.; Du, H.; Kimatu, B.M.; Su, A.; Yang, W.; Hu, Q.; Xiao, H. Characterization of polysaccharide from Pleurotus eryngii during simulated gastrointestinal digestion and fermentation. Food Chem. 2022, 370, 131303. [Google Scholar] [CrossRef] [PubMed]
- Kaoutari, A.E.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.L.; Kamm, M.A.; Ng, S.C.; Morrison, M. Proteus spp. as putative gastrointestinal pathogens. Clin. Microbiol. Rev. 2018, 31, 10–1128. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; De Jesus-Laboy, K.M.; Shen, N.; Cox, L.M.; Amir, A.; Gonzalez, A.; Bokulich, N.A.; Song, S.J.; Hoashi, M.; Rivera-Vinas, J.I. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 2016, 22, 250–253. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Dodd, D.; Mackie, R.I.; Cann, I.K. Xylan degradation, a metabolic property shared by rumen and human colonic Bacteroidetes. Mol. Microbiol. 2011, 79, 292–304. [Google Scholar] [CrossRef]
- Takahashi, N.; Yamada, T. Glucose metabolism by Prevotella intermedia and Prevotella nigrescens. Oral Microbiol. Immun. 2000, 15, 188–195. [Google Scholar] [CrossRef]
- Sakon, H.; Nagai, F.; Morotomi, M.; Tanaka, R. Sutterella parvirubra sp. nov. and Megamonas funiformis sp. nov. isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2008, 58, 970–975. [Google Scholar] [CrossRef]
- Heinken, A.; Khan, M.T.; Paglia, G.; Rodionov, D.A.; Harmsen, H.J.; Thiele, I. Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe. J. Bacteriol. 2014, 196, 3289–3302. [Google Scholar] [CrossRef] [PubMed]
- Hillman, E.T.; Kozik, A.J.; Hooker, C.A.; Burnett, J.L.; Heo, Y.; Kiesel, V.A.; Nevins, C.J.; Oshiro, J.M.; Robins, M.M.; Thakkar, R.D. Comparative genomics of the genus Roseburia reveals divergent biosynthetic pathways that may influence colonic competition among species. Microb. Genomics 2020, 6, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Holmstrøm, K.; Collins, M.D.; Møller, T.; Falsen, E.; Lawson, P.A. Subdoligranulum variabile gen. nov. sp. nov. from human feces. Anaerobe 2004, 10, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef]
- Wongkuna, S.; Ghimire, S.; Chankhamhaengdecha, S.; Janvilisri, T.; Scaria, J. Description of Collinsella avium sp. nov. a new member of the Collinsella genus isolated from the ceacum of feral chicken. New Microbes New Infect. 2021, 42, 100902. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ke, S.; Strappe, P.; Ning, M.; Zhou, Z. Structurally Orientated Rheological and Gut Microbiota Fermentation Property of Mannans Polysaccharides and Oligosaccharides. Foods 2023, 12, 4002. https://doi.org/10.3390/foods12214002
Wang J, Ke S, Strappe P, Ning M, Zhou Z. Structurally Orientated Rheological and Gut Microbiota Fermentation Property of Mannans Polysaccharides and Oligosaccharides. Foods. 2023; 12(21):4002. https://doi.org/10.3390/foods12214002
Chicago/Turabian StyleWang, Jing, Sheng Ke, Padraig Strappe, Ming Ning, and Zhongkai Zhou. 2023. "Structurally Orientated Rheological and Gut Microbiota Fermentation Property of Mannans Polysaccharides and Oligosaccharides" Foods 12, no. 21: 4002. https://doi.org/10.3390/foods12214002





